Abstract
A large simultaneous outbreak of respiratory syncytial virus (RSV) and parainfluenza type 3 (PIV-3) infections occurred on an adult hematology unit. Implementation of enhanced infection control was complicated by cocirculation of the two different viruses, with prolonged viral shedding from infected patients, and placed great pressure on health care staff; of 27 infected hematopoietic stem cell transplant patients, 9 died, and the unit was closed for 2 months. Retrospective molecular investigation of the virus strains involved in the outbreak was performed by analyzing part of the fusion gene of PIV-3 and part of the glycoprotein gene of RSV. Reverse transcription-PCR on nasopharyngeal aspirates from patients infected before and during the simultaneous outbreak generated amplicons for sequence analysis. A single strain of RSV and a single strain of PIV-3 had spread from person to person within the unit; 7 patients were infected with RSV, 22 were infected with PIV-3, and 4 were infected with both viruses. The PIV-3 outbreak had started at the beginning of August 3 months before the RSV outbreak; it had arisen when PIV-3 was introduced from the community by a patient and passed to another patient, who became chronically infected with the identical strain and, in spite of being nursed in isolation, was most likely the source from which widespread infection occurred in November. Had these early cases been linked to a common PIV-3 strain at the time of diagnosis, enhanced infection control precautions might have prevented the eventual extensive spread of PIV-3, making it much easier to deal with the later RSV outbreak.
Respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV-3) are members of the Paramyxoviridae family. RSV is numerically the single most important cause of serious respiratory tract infections in young children. PIV-3 is second only to RSV as a cause of pneumonia and bronchiolitis in this age group. These viruses are stable for hours in the environment (3) and spread by direct contact, large-particle aerosols, and fomites (11). RSV and PIV-3 infections recur throughout life despite the presence of specific neutralizing antibodies. There are annual epidemics of these virus infections in the community; in England and Wales, PIV-3 infections occur between May and September each year (16) and RSV infections occur every winter (23).
Clinical features of reinfections are generally mild in immunocompetent individuals, and shedding of the viruses is of relatively short duration. RSV and PIV-3, however, cause serious morbidity with prolonged virus shedding and high mortality in the immunocompromised, especially among hematopoietic stem cell transplant (HSCT) patients (10, 25, 30). RSV pneumonia is reported to have 60% mortality among these patients (25, 27, 29). Similarly, the incidence of PIV-3 infection in HSCT recipients is between 2% and 10% according to different longitudinal studies (2, 9, 16, 17, 21, 28, 30), and the incidence of pneumonia varies between 18% and 44%, with estimates of mortality of between 32% and 75% (9, 17, 21, 28).
This study describes the molecular investigation of a simultaneous outbreak of PIV-3 and RSV in a hematology unit. PCRs were used to generate RSV- and PIV-3-specific amplicons from clinical samples, and direct consensus sequence analysis was applied to determine whether there had been transmission of a single strain each of PIV-3 and RSV or, alternatively, multiple introductions of these viruses from the community.
MATERIALS AND METHODS
Patients. (i) Outbreak cohort.
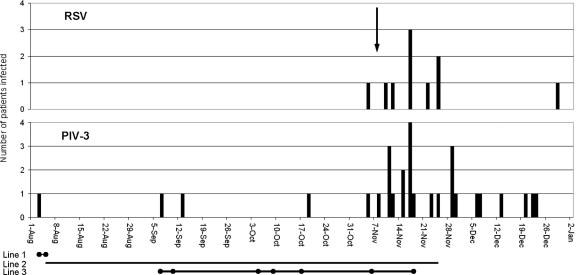
From November to December 1999 (Fig. 1), there was an outbreak of PIV-3 and RSV infections with nosocomial transmission among the inpatients on the adult hematology unit of University College London Hospital (UCH). Thirty patients, aged 17 to 64 years, were affected: 7 patients with various hematological malignancies and 23 HSCT patients (8 autografts, 10 sibling allografts, and 5 matched unrelated allografts). Of these, 19 patients (OB1 to -19) were infected with PIV-3 only and 7 (OB24 to -30) with RSV only. The four remaining patients (OB20 to -23) were infected with both viruses: one patient was infected first with PIV-3 and then 8 days later with RSV (in both cases for 1 day only); the other three patients were first infected with RSV but 13 to 35 days later also became infected with PIV-3. These dual infections lasted 1, 1, and 10 days, respectively, by which time the RSV had cleared, but PIV-3 persisted for some time longer.
FIG. 1.
Incidence of PIV-3 and RSV infections on the hematology unit from August 1999 to January 2000. The vertical scale indicates the number of positive patients; the arrow indicates identification of the outbreak. Line 1, duration of stay of patient Q1 on the unit with laboratory-confirmed shedding of PIV-3. Line 2, duration of stay of patient Q2 on the unit. Line 3, duration of laboratory-confirmed shedding of PIV-3 by patient Q2. •, PIV-3 detected in NPA.
The median length of PIV-3 shedding was 5 days (range, 1 to 103 days), and that of RSV was 12 days (range, 1 to 32 days). Five patients were still shedding virus (four PIV-3 and one RSV) in January 2000 after the outbreak had ended.
(ii) Other hematology patients possibly linked to the outbreak.
Four patients (Q1 to -4) had been hematology unit inpatients before the outbreak was declared. Q1, an autograft recipient, was admitted to the unit in August 1999, was found to be PIV-3 infected 1 day later, and died within a few days. Q2, a matched unrelated allograft recipient admitted to the unit in August 1999, had a dry cough in early September 1999 and was found to be PIV-3 infected. This patient remained symptomatic and died with pneumonia in November 1999 still infected with PIV-3. Once the infection had been diagnosed, the patient was isolated (in a single room with the door closed on her current ward, which had neither negative- nor positive-pressure rooms available). The lengths of her hospital stay and duration of confirmed PIV-3 shedding are shown in Fig. 1. Q3, a patient with multiple myeloma and an upper respiratory tract infection, was found to be PIV-3 infected in early September 1999. Q4, a sibling allograft recipient, was found to be PIV-3 infected in late October 1999 and died 10 days later with pneumonia and a concurrent cytomegalovirus infection.
Q5, a matched unrelated allograft recipient, was found to be PIV-3 infected at another hospital in December 1999 and died soon after but had been on the UCH hematology unit during the outbreak. PX, an autograft recipient found to be PIV-3 infected in November 1999, was an outpatient who had not been recently admitted to the hematology unit. P1, P2, and P3 were two matched unrelated allograft recipients and one autograft recipient; they were found to be PIV-3 infected in May or June 2000, all <4 days after hospital admission, and therefore their infections were most likely community acquired.
Mortality of patients infected with PIV-3 or RSV.
Of all the patients infected in 1999, i.e., outbreak patients and those possibly linked to the outbreak, 11 patients died: 8 had PIV-3 infection alone (a patient with acute lymphatic leukemia who was in relapse and died of pulmonary hemorrhage, an autograft recipient, and 3 sibling and 3 matched unrelated allograft recipients), 2 had both PIV-3 and RSV infection (a patient with acute myeloid leukemia who was first briefly infected with PIV-3 and then briefly infected with RSV and a matched unrelated allograft recipient with sequential infection but who was demonstrated to be dually infected for only 1 day), and 1 had RSV infection alone (an autograft patient in relapse of acute myeloid leukemia).
Setting.
The unit comprised three wards on three consecutive floors of a five-floor building. None of the rooms had the facility for positive- or negative-pressure isolation of patients. Ward 1 had 16 single rooms and 1 double room with shared washing facilities. Ward 2 had seven single rooms, four double rooms, and one four-bed bay with shared washing facilities. Ward 3 had 21 single rooms each with separate washing facilities.
The infectious diseases ward, in a separate building away from the hematology unit, had single rooms with washing facilities for each patient and a high-efficiency particulate air (HEPA)-filtered negative-pressure ventilation system for isolation. At the time of the outbreak the ward had just opened, having been previously transferred from a different hospital.
Course of the outbreak.
The PIV-3 outbreak started on ward 1 at the beginning of November 1999. This ward was immediately closed to new admissions and the PIV-3 infected patients transferred to the infectious diseases ward. The RSV outbreak started at the same time as the PIV-3 outbreak but on a different ward, ward 2, which was also closed to new admissions. Nevertheless, PIV-3 infection spread within a week to ward 3. At this point the RSV-infected patients were transferred to the infectious diseases ward. Despite these precautions, additional patients on wards 1 and 3 became infected with RSV. By mid-November, the whole hematology unit was closed and there were more infected patients than could be accommodated on the infectious diseases ward; almost all patients, who due to ill health could not be discharged, were affected. At the end of December, the remaining PIV-3 infected patients were transferred to the infectious diseases ward. The hematology unit was then deep cleaned and opened for new admissions in January 2000. There were no further cases of PIV-3 in the hematology unit patients until May 2000, but there was one case of RSV in an autograft recipient who was admitted from the community in January 2000 with the infection.
During the outbreak, infection control measures were reviewed, with particular emphasis on hand washing, barrier nursing, isolation of infected patients, stopping access to the unit by staff and visitors with respiratory tract infection, and screening of nasopharyngeal aspirates (NPAs) from all patients in the unit for PIV-3 and RSV twice every week. The criteria to designate a previously infected patient free of infection were that they were asymptomatic and that two NPAs taken a week apart tested negative for the relevant virus. A 5-day course of aerosolized or intravenous ribavirin was recommended for all patients infected with PIV-3 or RSV. In addition, nurses were divided into strictly separate cohorts for each of the wards involved, including the infectious diseases ward, to which nurses were seconded from the hematology unit to care for the infected patients. However, it was not possible similarly to cohort the approximately six doctors who were caring for the patients.
Laboratory diagnosis of PIV-3 and RSV infections.
Laboratory diagnosis of PIV-3 and RSV infections was made by direct immunofluorescence on fixed-cell preparations from NPAs, using fluorescein isothiocynanate-conjugated PIV-3- and RSV-specific monoclonal antibodies (Dako Diagnostics, Ely, Cambridgeshire, United Kingdom; catalogue no. K6104 and K6102, respectively) and virus isolation in human embryonic lung cells and primary rhesus monkey kidney cells (European Cell Culture Collection, Porton, Salisbury, United Kingdom).
Samples for molecular investigation.
The following NPA samples that had been sent for diagnosis and found to contain PIV-3 and/or RSV were stored at −70°C for molecular investigations: PIV-3-infected samples from hematology patients OB1 to -23, Q1, Q2, Q5, PX, and P1 to -3 (see above for further details) and from pediatric patients P4 and P5 (unrelated to the outbreak), whose samples were submitted for testing in October and August 1999, respectively; a PIV-3 reference strain, Wash/47/47885/57 (7); and RSV-infected samples from hematology patients OB20 to -30 and from patients in the community at the time of the outbreak (A1 to A7).
RNA extraction.
The first direct-immunofluorescence-positive NPA from each infected patient was examined. Five hundred microliters of sample was centrifuged at 21,000 × g for 5 min. The supernatant was discarded and the pellet suspended in 140 μl of nuclease-free water (Promega, Madison, WI). RNA was extracted from the suspension using a QIAamp viral RNA kit (catalogue no. 52904) according to the manufacturer's protocol (QIAGEN Ltd., Crawley, West Sussex, United Kingdom) with one modification; RNA was eluted in 50 μl of nuclease-free water. The RNA was stored at −70°C.
Preparation of cDNA for PIV-3 and RSV PCRs.
Reverse transcription was performed using random primers as described previously (31).
PCR for PIV-3.
As described previously (31), the PCR was designed to amplify a portion of the PIV-3 F gene, i.e., the 5′ noncoding and proximal coding region of the F gene of the reference PIV-3 strain Wash/47885/57 (7). The forward primer was 5′-TAACCAATACACCTACATGC-3′ (mRNA sense, nucleotides 296 to 316), and the reverse primer was 5′-GTCAATACCAACAACTATTAGC-3′ (genome sense, nucleotides 38 to 60). The PCR was performed in a 50-μl volume containing 5 μl of cDNA, and 40 cycles of amplification were performed in an Applied Biosystems thermal cycler 480.
PCR for RSV.
Sequences of the glycoprotein G gene and fusion protein gene of genotypes A and B of RSV from GenBank, National Center for Biotechnology Information (www.ncbi.nlm.nih.gov), were aligned using the Sequencher program version 4.0.5 (Gene Codes Corporation, MI). Primers were designed from conserved regions by using the Primer Designer program (Scientific and Educational Software). The forward primer was 5′-TCACTTTGAAGTGTTCAACT-3′ (mRNA sense, from the glycoprotein [G] gene; nucleotides 504 to 523 according to accession no. Z33430). The reverse primer was 5′-GGCAACTCCATTGTTATTTG-3′ (genome sense, from the fusion protein [F] gene; nucleotides, 24 to 5 according to accession no. U31562). PCR amplification was performed in a 50-μl volume containing 5 μl of cDNA, 2.5 mM MgCl2, 5 μl of GeneAmp 10× PCR buffer II, 2.5 units of AmpliTaq Gold (Applied Biosytems), 200 μM of deoxynucleoside triphosphates, and 25 pmol of each primer. One clinical isolate of RSV was used as a positive control and nuclease-free water was used as a negative control for RNA extraction, reverse transcription, and amplification. Samples and controls were denatured at 95°C for 2 min, followed by 40 cycles of amplification in an Applied Biosystems thermal cycler 480. Each cycle consisted of denaturation at 94°C for 30 s, annealing of primers at 40°C for 1 min, and extension at 72°C for 1 min, followed by a final extension at 72°C for 7 min. The sizes of amplicons were ascertained by gel electrophoresis using 1% agarose (Seakems, Flowgen, Lichfield, Staffordshire, United Kingdom).
Sequencing and phylogenetic analysis.
Sequencing and phylogenetic analysis were as described previously (14). The sequences from GenBank, National Center for Biotechnology Information (www.ncbi.nlm.nih.gov), were from nucleotide position 61 to 296 of PIV-3, with reference to accession no. X05303, and from nucleotide position 651 to 918 of RSV, with reference to accession no. Z33430.
RESULTS
PCRs for PIV-3 and RSV.
The relevant amplicons were generated from all 50 NPA samples tested and from the PIV-3 reference strain.
Sequencing and phylogenetic analysis of PIV-3 amplicons.
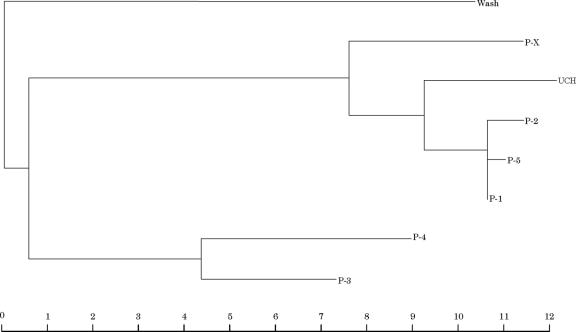
Figures 2 and 3 show the sequence alignments and phylogenetic tree for the PIV-3 amplicons from the NPA samples compared with that from the reference strain. Twenty-six patients (OB1 to -23, Q1, Q2, and Q5) were infected with the same strain of PIV-3 (UCH). Compared to the prototype strain Wash/47885/57 and strains from patients PX and P1 to P5, the UCH PIV-3 strain had a distinctive 6-nucleotide deletion in the noncoding region of the F gene and 17 to 51 base variations (7% to 22%) over the whole length of the amplicon; nucleotide variation in the 5′ noncoding region was higher than that within the proximal coding region. Figure 2 also shows that the UCH PIV-3 strain contains an additional translation initiation codon situated in the 5′ noncoding region; starting from this codon, there is a short open reading frame corresponding to 22 amino acids.
FIG. 2.
Sequence alignments for PIV-3 amplicons (nucleotide positions 61 to 296 of PIV-3; accession number X05303). Nucleotides are numbered with respect to the ATG translation start codon (shaded) of the F gene (5′ noncoding region, −153 to 0; open reading frame, +1 to +75). Note that in the UCH outbreak strain there is another translation start codon (shaded) (see text for discussion). Abbreviations: UCH, amplicons from hematology unit inpatients in 1999 (OB1 to -23, Q1, Q2, and Q5); P-X, amplicon from PX, a hematology outpatient infected with PIV-3 in 1999; P-1 to P-3, amplicons from three hematology inpatients infected with PIV-3 in 2000; P4 and P5, amplicons from two children infected with PIV-3 in 1999; Wash, amplicon from PIV-3 reference strain Wash/47885/57.
FIG. 3.
Phylogenetic tree based on sequence alignments of the PIV-3 amplicons shown in Fig. 2. The scale bar indicates the relative genetic distance according to the Jukes-Cantor algorithm. Abbreviations: UCH, amplicons from hematology unit inpatients in 1999 (OB1 to -23, Q1, Q2, and Q5); P-X, amplicon from PX, a hematology outpatient infected with PIV-3 in 1999; P-1 to P-3, amplicons from three hematology inpatients infected with PIV-3 in 2000; P4 and P5, amplicons from two children infected with PIV-3 in 1999; Wash, amplicon from PIV-3 reference strain Wash/47885/57.
Sequencing and phylogenetic analysis of RSV amplicons.
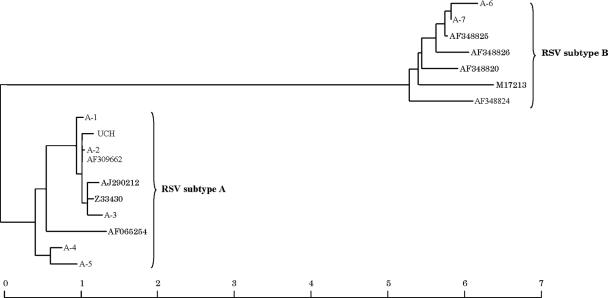
Figures 4 and 5 show the sequence alignments and phylogenetic tree for the RSV amplicons from the NPA samples compared with representative sequences selected from GenBank. Eleven patients (OB20 to -30) were infected with the same strain of RSV subtype A (UCH). Seven other strains were identified from patients A1 to A7 (five subtype A strains and two subtype B strains). The UCH RSV strain differed in the region of the G gene under analysis by 5 to 28 nucleotides (2% to 10%) from the five subtype A strains.
FIG. 4.
Sequence alignments for RSV amplicons (nucleotide positions 651 to 918 of RSV [268 nucleotides from glycoprotein G]; accession number Z33430). Abbreviations: UCH, amplicons from hematology unit inpatients in 1999 (OB20 to -30; A-1 to A-7, amplicons from control patients A1 to A7. Ten representative sequences for RSV subtypes A and B selected from GenBank are indicated by their accession numbers.
FIG. 5.
Phylogenetic tree based on the sequence alignments of RSV amplicons and representative sequences for RSV subtypes A and B selected from GenBank, as shown in Fig. 4. The scale bar indicates relative genetic distance according to the Jukes-Cantor algorithm. Abbreviations: UCH, amplicons from hematology unit inpatients in 1999 (OB20 to -30); A-1 to A-7, amplicons from control patients A1 to A7.
DISCUSSION
In this paper, we describe the molecular investigation of an outbreak of both PIV-3 and RSV in hematology patients. Sequence analysis of the RSV G gene has been extensively used to study genetic diversity among strains in RSV outbreaks (18, 26). Sequence analysis of the PIV-3 hemagglutinin-neuraminidase gene (6, 22) or F gene (31) has also proved useful to investigate the nature of PIV-3 outbreaks. We chose to analyze the nucleotide sequence of a portion of the F gene, which included the noncoding region, because it has a high degree of diversity relative to the other sequenced parts of the PIV-3 genome, including the hemagglutinin-neuraminidase gene (5) and the rest of the F gene (4, 24). This predicted high variability had been previously amply confirmed using sequence analysis of PIV-3 isolates from the United Kingdom (31), which revealed 13 strains that differed as much from each other as they did from strains isolated previously outside Europe (4, 24).
In the present study, retrospective molecular investigation showed that a single strain of RSV and a single strain of PIV-3 spread nosocomially by person-to-person transmission among patients on three different wards in the hematology unit and on the infectious diseases ward. The PIV-3 outbreak strain was of particular interest in that it had a characteristic 6-base deletion along with a number of other distinct nucleic acid changes compared to the other strains. As previously reported (31), there was higher variation in the 5′ coding region of the F gene than in the proximal coding region. Intriguingly, the outbreak strain had an additional open reading frame situated in the 5′ noncoding region which could theoretically be translated in frame with the original PIV-3 F gene open reading frame, possibly reducing translation of the latter. The significance of such a finding in immunocompromised patients is uncertain, although it is clear that the UCH PIV-3 strain circulated in the unit by person-to-person transmission over an extended period of time (about 5 months) without a change in sequence.
Whereas the RSV outbreak was closely temporally clustered and concurrent with the usual winter community outbreak of RSV, molecular investigation showed that the PIV-3 outbreak was protracted, involving at least 26 patients and extending beyond its normal occurrence in spring and summer. At the beginning of August, 3 months before the RSV outbreak, a patient, Q1, had the first PIV-3 infection, presumably importing the infection on admission. Q2 was on the unit at the same time as Q1 and, while still an inpatient a month later, was found to be infected with PIV-3; this, although not realized at the time, turned out to be the same PIV-3 strain as that in patient Q1. Q2 was placed in isolation to prevent transmission of PIV-3, but owing to the conformation of the ward and its toilets, this could not be enforced strictly. The option of transferring the patient to the very recently opened infectious disease ward was discussed but rejected because it would compromise the patient's care, which required nurses with special expertise in HSCT. Patient Q2 is likely to have been the main disseminator of PIV-3, staying in the unit, albeit in isolation, for a long period while still shedding the virus. Two further PIV-3 infections (of inpatients Q3 and Q4) occurred in the subsequent 2 months before the outbreak was recognized, but unfortunately samples were not available for sequencing. At the time, the infections in patients Q1 to Q4 appeared consistent with sporadic introductions into the hospital from the ongoing PIV-3 community outbreak. Notably, had the molecular identity of the viruses in patients Q3 and Q4 been available at the time and had their identity with the virus in patient Q2 been established (which retrospectively seems likely), the potential for a large PIV-3 outbreak would have been obvious. This would have enabled remedial steps to be taken to abort the ongoing transmission before the start of the RSV outbreak.
As regards the other cases possibly linked to the outbreak, patient Q5 was found to be infected with the UCH PIV-3 strain while an inpatient at another hospital, confirming that the virus had been acquired during a recent previous stay in the UCH hematology unit. On the other hand, patient PX, whose infection was community acquired, had a PIV-3 strain that varied by 26 bases (11%) from the outbreak strain and lacked its distinctive 6-nucleotide deletion. Similarly and reassuringly, we were able to show that the PIV-3 strains found in hematology patients P1 to P3 in May and June 2000 were not related to the outbreak strain.
Dual PIV-3 and RSV infections are uncommon, especially in adults (8), and reports of simultaneous nosocomial outbreaks of both viruses are correspondingly rare. Such an outbreak of RSV and PIV-3 was reported in a newborn nursery, in which seven infants were infected with RSV, five with PIV-3, and two with both RSV and PIV-3 (20). Although outbreaks of RSV and PIV-3 on hematology units have been described previously (1, 6, 12, 13, 15, 18, 22, 26, 31), this is the first report of a concurrent PIV-3 and RSV outbreak. Three patients in the outbreak cohort were dually infected. These infections resulted from the unseasonal simultaneous circulation of both viruses in our hematology unit and the inability of infection control measures to limit spread of the viruses. There were four types of patient from the infection control point of view: uninfected, PIV-3 infected, RSV infected, and dually infected. Each of these groups should have been nursed with patients of their own category and by separate groups of staff. Such an ideal situation proved impossible to achieve because of the high number of infected patients, the lack of individual rooms with negative-pressure ventilation except on the newly opened infectious diseases ward, and insufficient staff to divide into enough separate cohorts; indeed, three RSV-infected patients isolated on the infectious diseases ward became infected with PIV-3 while in isolation.
As amply illustrated in the above description of our outbreak, respiratory infections in heavily immunosuppressed adults hospitalized in high-dependency hematology posttransplant units are particularly difficult to manage (19). The symptoms of respiratory virus infection are often diffuse early on, viral shedding may be prolonged, and outbreaks are often not recognized until the pattern of new clinical infections is established, by which time intervention is of limited value. Modern high-occupancy needs and open-plan wards, even if bayed, provide fertile areas for infection. The tendency of health care workers to ignore simple but effective infection control measures such as good hand hygiene may be the most significant factor in the spread of respiratory viruses among hospitalized patients. Ideally, the emphasis of infection control should be on prevention of entry of respiratory viruses to a hematology unit and thus prevention of outbreaks; this can be best achieved by denying access to staff, visitors, and patients if they have a suspected respiratory virus infection. Since the RSV/PIV-3 outbreak, all hematology patients at UCH with features of respiratory virus infection are admitted to the infectious diseases ward, with outreach guidance from hematology nurses as required. The patients are allowed in the hematology unit only after resolution of their infection. Indeed, once the outbreak was over, isolation of the remaining long-term shedders away from the hematology unit proved effective, and there were no further cases.
There was a high morbidity and mortality rate in the UCH outbreak. Of the 35 patients infected, 2 had no signs of respiratory infection, 10 had upper respiratory tract infection alone, and the remaining 23 had various signs and symptoms of lower respiratory tract infection (LRTI) (data not shown). Morbidity was especially high in HSCT patients. Of the 20 patients infected with PIV-3 alone, 15 had LRTI and 7 died (47%); of the 4 infected with RSV alone, 3 had LRTI and 1 died (this patient was also in hematological relapse) (33%); and of the 3 dually infected with RSV and PIV-3, the only patient with LRTI died (100%). However, as with previous reports of high mortality (9, 17, 21, 25, 27-29), the presence of multiple pathological processes in such heavily immunocompromised HSCT patients makes it difficult to be certain of the exact connection between PIV-3 and RSV infections and mortality, especially since postmortems were not performed for any of these patients. There was also a high mortality rate in the eight nontransplant patients with various hematological malignancies, of whom two out of the four with LRTI died, one with PIV-3 infection and one with RSV followed by PIV-3 infection, but the numbers are too small to draw any conclusion, especially since the malignant disease in one patient had relapsed.
In conclusion, as with previous retrospective molecular investigations of RSV (12, 18, 26) and PIV-3 (6, 22, 31) outbreaks on hematology units, our study confirms the ability to differentiate nosocomial person-to-person transmission from multiple reintroductions of virus from the community. Indeed, it is not possible to determine the true nature of a respiratory virus outbreak or to identify lapses in infection control measures without molecular investigations. The use of PCR and sequence analysis for strain identification is ideally suited for the prevention and management of respiratory virus outbreaks and, due to the possibility of automation, will eventually become part of the routine armamentarium of diagnostic virology laboratories.
Acknowledgments
We are grateful to Ian Silver and Maria Erecinska for help in preparing this article. We also thank James Passas and Yvette Sharvell for expert technical assistance.
This work was partly funded by the Leukemia Research Fund (research grant 97/14 to K.N.W.).
Footnotes
Published ahead of print on 28 March 2007.
REFERENCES
- 1.Abdallah, A., K. E. Rowland, S. K. Schepetiuk, L. B. To, and P. Bardy. 2003. An outbreak of respiratory syncytial virus infection in a bone marrow transplant unit: effect on engraftment and outcome of pneumonia without specific antiviral treatment. Bone Marrow Transplant. 32:195-203. [DOI] [PubMed] [Google Scholar]
- 2.Bowden, R. A. 1994. Other viruses after marrow transplantation, p. 443-453. In E. D. Thomas (ed.), Bone marrow transplantation. Blackwell Scientific Publication, Oxford, United Kingdom.7994270
- 3.Brady, M. T., J. Evans, and J. Cuartas. 1990. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am. J. Infect. Control 18:18-23. [DOI] [PubMed] [Google Scholar]
- 4.Coelingh, K. V., and C. C. Winter. 1990. Naturally occurring human parainfluenza type 3 viruses exhibit divergence in amino acid sequence of their fusion protein neutralization epitopes and cleavage sites. J. Virol. 64:1329-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelingh, K. V., C. C. Winter, and B. R. Murphy. 1988. Nucleotide and deduced amino acid sequences of hemagglutinin-neuraminidase genes of human type 3 parainfluenza viruses from 1957-1983. Virology 163:137-143. [DOI] [PubMed] [Google Scholar]
- 6.Cortez, K. J., D. D. Erdman, T. C. Peret, V. J. Gill, R. Childs, A. J. Barrett, and J. E. Bennett. 2001. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J. Infect. Dis. 184:1093-1097. [DOI] [PubMed] [Google Scholar]
- 7.Côté, M. J., D. G. Storey, C. Y. Kang, and K. Dimock. 1987. Nucleotide sequence of the coding and flanking regions of the human parainfluenza virus type 3 fusion glycoprotein gene. J. Gen. Virol. 68:1003-1010. [DOI] [PubMed] [Google Scholar]
- 8.Drews, A. L., R. L. Atmar, W. P. Glezen, B. D. Baxter, P. A. Piedra, and S. B. Greenberg. 1997. Dual respiratory virus infections. Clin. Infect. Dis. 25:1421-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elizaga, J., E. Olavarria, J. Apperley, J. Goldman, and K. Ward. 2001. Parainfluenza virus 3 infection after stem cell transplant: relevance to outcome of rapid diagnosis and ribavirin treatment. Clin. Infect. Dis. 32:413-418. [DOI] [PubMed] [Google Scholar]
- 10.Hall, C. B. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917-1928. [DOI] [PubMed] [Google Scholar]
- 11.Hall, C. B., and R. G. Douglas, Jr. 1981. Modes of transmission of respiratory syncytial virus. J. Pediatr. 99:100-103. [DOI] [PubMed] [Google Scholar]
- 12.Harrington, R. D., T. M. Hooton, R. C. Hackman, G. A. Storch, B. Osborne, C. A. Gleaves, A. Benson, and J. D. Meyers. 1992. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J. Infect. Dis. 165:987-993. [DOI] [PubMed] [Google Scholar]
- 13.Hohenthal, U., J. Nikoskelainen, R. Vainionpaa, R. Peltonen, M. Routamaa, M. Itala, and P. Kotilainen. 2001. Parainfluenza virus type 3 infections in a hematology unit. Bone Marrow Transplant. 27:295-300. [DOI] [PubMed] [Google Scholar]
- 14.Jalal, H., D. F. Bibby, J. W. Tang, J. Bennett, C. Kyriakou, K. Peggs, D. Cubitt, N. S. Brink, K. N. Ward, and R. S. Tedder. 2005. First reported outbreak of diarrhea due to adenovirus infection in a hematology unit for adults. J. Clin. Microbiol. 43:2575-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, B. L., S. Clark, E. T. Curran, S. McNamee, G. Horne, B. Thakker, and J. Hood. 2000. Control of an outbreak of respiratory syncytial virus infection in immunocompromised adults. J. Hosp. Infect. 44:53-57. [DOI] [PubMed] [Google Scholar]
- 16.Laurichesse, H., D. Dedman, J. M. Watson, and M. C. Zambon. 1999. Epidemiological features of parainfluenza virus infections: laboratory surveillance in England and Wales, 1975-1997. Eur. J. Epidemiol. 15:475-484. [DOI] [PubMed] [Google Scholar]
- 17.Lewis, V. A., R. Champlin, J. Englund, R. Couch, J. M. Goodrich, K. Rolston, D. Przepiorka, N. Q. Mirza, H. M. Yousuf, M. Luna, G. P. Bodey, and E. Whimbey. 1996. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clin. Infect. Dis. 23:1033-1037. [DOI] [PubMed] [Google Scholar]
- 18.Mazzulli, T., T. C. Peret, A. McGeer, D. Cann, K. S. MacDonald, R. Chua, D. D. Erdman, and L. J. Anderson. 1999. Molecular characterization of a nosocomial outbreak of human respiratory syncytial virus on an adult leukemia/lymphoma ward. J. Infect. Dis. 180:1686-1689. [DOI] [PubMed] [Google Scholar]
- 19.McCann, S., J. L. Byrne, M. Rovira, P. Shaw, P. Ribaud, S. Sica, L. Volin, E. Olavarria, S. Mackinnon, P. Trabasso, M. T. VanLint, P. Ljungman, K. Ward, P. Browne, A. Gratwohl, A. F. Widmer, and C. Cordonnier. 2004. Outbreaks of infectious diseases in stem cell transplant units: a silent cause of death for patients and transplant programmes. Bone Marrow Transplant. 33:519-529. [DOI] [PubMed] [Google Scholar]
- 20.Meissner, H. C., S. A. Murray, M. A. Kiernan, D. R. Snydman, and K. McIntosh. 1984. A simultaneous outbreak of respiratory syncytial virus and parainfluenza virus type 3 in a newborn nursery. J. Pediatr. 104:680-684. [DOI] [PubMed] [Google Scholar]
- 21.Nichols, W. G., L. Corey, T. Gooley, C. Davis, and M. Boeckh. 2001. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood 98:573-578. [DOI] [PubMed] [Google Scholar]
- 22.Nichols, W. G., D. D. Erdman, A. Han, C. Zukerman, L. Corey, and M. Boeckh. 2004. Prolonged outbreak of human parainfluenza virus 3 infection in a stem cell transplant outpatient department: insights from molecular epidemiologic analysis. Biol. Blood Marrow Transplant. 10:58-64. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson, K. G. 1996. Impact of influenza and respiratory syncytial virus on mortality in England and Wales from January 1975 to December 1990. Epidemiol. Infect. 116:51-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prinoski, K., M.-J. Cote, C. Y. Kang, and K. Dimock. 1992. Evolution of the fusion protein gene of human parainfluenza virus 3. Virus Res. 22:55-69. [DOI] [PubMed] [Google Scholar]
- 25.Sable, C. A., and F. G. Hayden. 1995. Orthomyxoviral and paramyxoviral infections in transplant patients. Infect. Dis. Clin. N. Am. 9:987-1003. [PubMed] [Google Scholar]
- 26.Taylor, G. S., I. B. Vipond, and E. O. Caul. 2001. Molecular epidemiology of outbreak of respiratory syncytial virus within bone marrow transplantation unit. J. Clin. Microbiol. 39:801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wendt, C. H., and M. I. Hertz. 1995. Respiratory syncytial virus and parainfluenza virus infections in the immunocompromised host. Semin. Respir. Infect. 10:224-231. [PubMed] [Google Scholar]
- 28.Wendt, C. H., D. J. Weisdorf, M. C. Jordan, H. H. Balfour, Jr., and M. I. Hertz. 1992. Parainfluenza virus respiratory infection after bone marrow transplantation. N. Engl. J. Med. 326:921-926. [DOI] [PubMed] [Google Scholar]
- 29.Whimbey, E., R. E. Champlin, R. B. Couch, J. A. Englund, J. M. Goodrich, I. Raad, D. Przepiorka, V. A. Lewis, N. Mirza, H. Yousuf, J. J. Tarrand, and G. P. Bodey. 1996. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin. Infect. Dis. 22:778-782. [DOI] [PubMed] [Google Scholar]
- 30.Whimbey, E., S. E. Vartivarian, R. E. Champlin, L. S. Elting, M. Luna, and G. P. Bodey. 1993. Parainfluenza virus infection in adult bone marrow transplant recipients. Eur. J. Clin. Microbiol. Infect. Dis. 12:699-701. [DOI] [PubMed] [Google Scholar]
- 31.Zambon, M., T. Bull, C. J. Sadler, J. M. Goldman, and K. N. Ward. 1998. Molecular epidemiology of two consecutive outbreaks of parainfluenza 3 in a bone marrow transplant unit. J. Clin. Microbiol. 36:2289-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]