Abstract
A serological enzyme-linked immunosorbent assay was developed using a synthetic peptide from the VP0 protein of human parechoviruses (HPeVs). Seroprevalence for HPeVs was 70% in children of ≤5 years of age and 95% in adults. For children from whom serial sera were sampled, seropositivity increased from 22% to 88% between 2 and 24 months of age.
The Picornaviridae family contains single-stranded positive-sense RNA viruses whose genomes are packaged into a nonenveloped icosahedral capsid (9). This family is divided into nine genera, with five of them (Enterovirus, Hepatovirus, Rhinovirus, Kobuvirus, and Parechovirus) containing human pathogens (9, 12). The parechovirus genus originally contained human parechovirus 1 (HPeV-1) and HPeV-2, which were formerly known as echoviruses 22 and 23, respectively. This genus was subsequently expanded by inclusion of a third human type (HPeV-3) (4, 6) as well as a species isolated from rodents (Ljungan virus) (11). More recently, on the basis of genetic analyses, the existence of two new HPeV genotypes (HPeV-4 and -5) has also been suggested (2). HPeV-1, -2, and -3 are associated with infections that occur predominantly in young children (1, 3, 9). HPeV-1 and HPeV-2 cause illnesses also associated with enterovirus, i.e., gastroenteritis, aseptic meningitis, encephalitis, and respiratory syndromes (7, 9, 13). HPeV-3 infections have been associated with transient paralysis (6) and neonatal sepsis-like illnesses (1, 3, 4). The RNA genome of HPeV includes a single open reading frame flanked by 5′ and 3′ untranslated regions (12). This open reading frame encodes a polyprotein which is processed by viral proteinases into three capsid (VP0, VP3, and VP1) and seven nonstructural (2A, 2B, 2C, 3A, 3B, 3C, and 3D) proteins (12). In a previous study on the immunological properties of HPeV-1 capsid proteins, important antigenic sites were identified in the N-terminal region of the VP0 protein (8). In particular, two 12-amino-acid synthetic peptides, P208 (including residues 79 to 90 of the HPeV-1 polyprotein) and P209 (including residues 88 to 99), were found to be highly immunogenic, as demonstrated by an enzyme-linked immunosorbent assay (ELISA) (8). In this study, we sought to develop and evaluate an ELISA using a synthetic peptide containing residues 79 to 99 of the VP0 protein as an antigen for the detection of HPeV-1, -2, and -3 antibodies.
A 21-amino-acid synthetic peptide (PepVP0-21; residues 79 to 99 of the HPeV-3/Can82853-01 polyprotein; GenBank number AJ998818) (Fig. 1) was synthesized with an Applied Biosystems 433A peptide synthesizer using 9-fluorenylmethoxy carbonyl chemistry, followed by reverse-phase liquid chromatography purification and analysis of the correct molecular mass. Antisera specific to HPeV-1 (VR-1063AS/horse), HPeV-2 (VR-1064AS/horse), coxsackievirus A1 (VR-1005AS/horse), coxsackievirus B2 (VR-1033AS/horse), poliovirus 1 (VR-1001AS/horse), and rhinovirus type 100 (VR-1300AS/rabbit) were purchased from the American Type Culture Collection (ATCC). Because no antiserum specific for HPeV-3 was available from the ATCC, two BALB/c mice were immunized subcutaneously with three sequential 50-μg doses of HPeV-3/Can82853-01 virus (grown on Vero cells and purified by ultracentrifugation) or PepVP0-21 at 2-week intervals using Freund's complete adjuvant with the first dose (8). Human sera from 48 Canadian children (10 months to 5 years old) and 20 adults were randomly selected. Serial sera collected at 2, 7, 13, and 24 months of age from nine patients from Israel were also available. The PepVP0-21-based ELISA was performed under the following conditions. Ninety-six-microwell plates were coated with 5 μg/ml of PepVP0-21 in 0.1 M sodium bicarbonate buffer, pH 9.6 (100 μl/well), and then incubated overnight at 4°C. The plates were washed three times with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (washing buffer [WB]) and blocked with 0.1% bovine serum albumin in PBS for 45 min. The wells were washed again three times with WB, and then sera, diluted in PBS containing 1% bovine serum albumin, 1% fetal calf serum, and 0.1% Tween 20, were added into the wells. The plates were incubated for 1 h at 37°C and washed as described above. Horseradish peroxidase-conjugated anti-human, anti-horse, anti-mouse, or anti-rabbit immunoglobulins (depending on the origin of the host serum) were added to the wells, and the plates were incubated at 37°C for 1 h. After five washes with the WB, antibodies were detected by adding 100 μl of 3,3′,5,5′-tetramethyl benzidine substrate (Research Diagnostics Inc., Flanders, NJ), and the reaction was stopped after 30 min with 1 M H2SO4. The absorbance was measured at 450 nm in a spectrophotometer.
FIG. 1.
Comparison of amino acid sequences of the synthetic peptide (PepVP0-21; residues 79 to 99 of the polyprotein of HPeV-3/Can82853-01; GenBank number AJ998818) with the corresponding region of HPeV-1/Harris, S45208; HPeV-2w/Williamson, AJ005695; HPeV-2c/Connecticut, AF055846; HPeV-4, AM235749; and HPeV-5, NC_008286. Asterisks denote residues identical to those of PepVP0-21, whereas residues that differ are shaded.
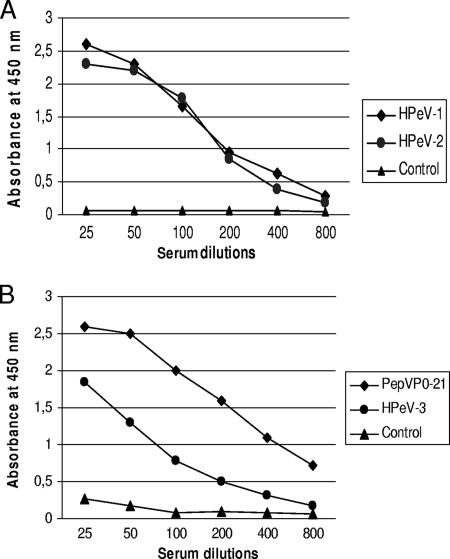
A preliminary ELISA was designed by using reference antisera from horses immunized with HPeV-1 or HPeV-2 and a serum from a nonimmunized horse as a control. PepVP0-21 was clearly recognized by HPeV-1 and HPeV-2 antisera, as shown in Fig. 2A. Antisera against other picornaviruses (coxsackievirus A1, coxsackievirus B2, poliovirus 1, and rhinovirus type 100) did not react with the peptide (data not shown). Sera from mice immunized with PepVP0-21 or with the purified HPeV-3 strain were also found to react with the peptide, in contrast to the preimmune mouse serum (Fig. 2B). The ELISA was further evaluated with human sera randomly selected from our virology laboratory. At the 1/100 dilution, serum samples could be divided into two groups. Group A sera (n = 26) had absorbance values ranging from 0.08 to 0.24, with a mean absorbance of 0.19 and a standard deviation (SD) of 0.03, whereas group B sera (n = 42) had absorbance values between 0.25 and 0.94. An absorbance value of 0.25 (which corresponds to the mean absorbance of group A plus 2 standard deviations) was chosen as the cutoff value for the assay. Using this threshold, HPeV seroprevalences were found to be 70% (34/48) in Canadian children of ≤5 years of age and 95% (19/20) in adults. The test was further evaluated using serial sera collected at 2, 7, 13, and 24 months of age from nine children from Israel. As shown in Table 1, the percentage of HPeV seropositivity was 22% (2/9) at 2 months of age and increased thereafter to reach 88% (8/9) at the age of 24 months.
FIG. 2.
(A) Reactivity of reference HPeV-1 (ATCC, VR-1063AS/HO) and HPeV-2 (VR-1064AS/HO) antisera raised in horses against PepVP0-21 in ELISA. The plates were coated with 500 ng of the peptide, and sera were used at the indicated dilutions. A serum sample from a nonimmunized horse was used as a control. (B) Reactivity of HPeV-3/Can82853-01 (GenBank number AJ998818) and PepVP0-21 antisera raised in mice against PepVP0-21 in ELISA. The plates were coated as described above, and sera were used at the indicated dilutions. A preimmune mouse serum was used as a control.
TABLE 1.
Evolution of HPeV serological status of nine children of ages from 2 months to 2 years as determined by PepVP0-21 ELISA
| Patient no. | HPeV serological status at indicated age
|
|||
|---|---|---|---|---|
| 2 mo | 7 mo | 13 mo | 24 mo | |
| 1 | − | + | + | + |
| 2 | + | + | + | + |
| 3 | − | − | + | + |
| 4 | − | + | + | + |
| 5 | + | + | + | + |
| 6 | − | − | NAa | + |
| 7 | − | − | NA | − |
| 8 | − | − | + | + |
| 9 | − | − | NA | + |
NA, not available.
Although HPeV infections are usually associated with mild gastrointestinal or respiratory symptoms, it has been shown that HPeV-1 can also cause more-severe conditions such as encephalitis (10) and flaccid paralysis (5). We also reported that HPeV-3 infections could be associated with sepsis-like syndromes in neonates (1, 4). The diagnostic procedure generally used to identify HPeV infections consists of virus isolation followed by neutralization assays or reverse transcriptase PCR. However, for comprehensive epidemiological studies, there is a need for a simple serological test. In this report, we describe the development and evaluation of an ELISA for the detection of HPeV antibodies. The antigen used in this test (PepVP0-21) is located in a region which has previously been shown to be immunogenic in HPeV-1 (8). In addition, amino acid sequence alignment revealed that the PepVP0-21 sequence, in particular residues 79 to 88, was highly conserved among HPeV-1, -2, and -3 strains as well as in the two recently described HPeV genotypes 4 and 5 (2) (Fig. 1). Thus, although our assay was formally validated with HPeVs 1 to 3, we suggest that it could detect all known HPeV types. Importantly, the PepVP0-21 ELISA was specific, as antisera raised against other related picornaviruses (coxsackievirus A1, coxsackievirus B1, poliovirus 1, and rhinovirus type 100) yielded negative results.
Seroprevalence rates for HPeV-1 and -3 were previously determined using serum samples from different age groups (6, 7). These reports showed that the rates of HPeV-1 and -3 seropositivity increased with age to reach 97% (7) and 87% (6), respectively, in adults. Our ELISA confirmed the high level of HPeV antibodies in Canadian adults (95%). In addition, we studied serial sera from nine young children from a different country (Israel). Our results show that a significant proportion of these children lack antibodies at 2 months of age (which may explain the sepsis-like presentation described for neonates [1, 3, 4]) and suggest that seroconversion occurs mainly during the first year of life. This last point is in agreement with an HPeV-1 study in which 8 of 9 children (88.8%) were found to have HPeV-1 antibodies by the age of 1 to 2 years (7). However, since our assay is not type specific, we cannot distinguish between seroconversion patterns for the different HPeV types. Additional serological studies are warranted to further define the epidemiology of HPeV infections caused by new genotypes.
Footnotes
Published ahead of print on 18 April 2007.
REFERENCES
- 1.Abed, Y., and G. Boivin. 2006. Human parechovirus infections in Canada. Emerg. Infect. Dis. 12:969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Sunaidi, M., C. H. Williams, P. J. Hughes, D. P. Schnurr, and G. Stanway. 2007. Analysis of a new human parechovirus allows the definition of parechovirus types and the identification of RNA structural domains. J. Virol. 81:1013-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benschop, K. S. M., J. Schinkel, R. P. Minnaar, D. Pajkrt, L. Spanjerberg, H. C. Kraakman, B. Berghout, H. L. Zaajer, M. G. H. M. Beld, and K. C. Wolthers. 2006. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin. Infect. Dis. 42:204-210. [DOI] [PubMed] [Google Scholar]
- 4.Boivin, G., Y. Abed, and F. D. Boucher. 2005. Human parechovirus-3 and neonatal infections. Emerg. Infect. Dis. 11:103-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueroa, J. P., D. Ashley, D. King, and B. Hull. 1989. An outbreak of acute flaccid paralysis in Jamaica associated with echovirus type 22. J. Med. Virol. 29:315-319. [DOI] [PubMed] [Google Scholar]
- 6.Ito, M., T. Yamashita, H. Tsuzuki, N. Takeda, and K. Sakae. 2004. Isolation and identification of a novel human parechovirus. J. Gen. Virol. 85:391-398. [DOI] [PubMed] [Google Scholar]
- 7.Joki-Korpela, P., and T. Hyypia. 1998. Diagnosis and epidemiology of echovirus 22 infections. Clin. Infect. Dis. 26:129-136. [DOI] [PubMed] [Google Scholar]
- 8.Joki-Korpela, P., M. Roivainen, T. Poyry, and T. Hyypia. 2000. Antigenic properties of human parechovirus 1. J. Gen. Virol. 81:1709-1718. [DOI] [PubMed] [Google Scholar]
- 9.Joki-Korpela, P., and T. Hyypia. 2001. Parechoviruses, a novel group of human picornaviruses. Ann. Med. 33:466-471. [DOI] [PubMed] [Google Scholar]
- 10.Koskiniemi, M., R. Paeteau, and K. Linnavuori. 1989. Severe encephalitis associated with disseminated echovirus 22 infection. Scand. J. Infect. Dis. 21:463-466. [DOI] [PubMed] [Google Scholar]
- 11.Niklasson, B., L. Kinnunen, B. Hornfeldt, J. Horling, C. Benemar, K. O. Hedlund, L. Matskova, T. Hyypia, and G. Winberg. 1999. A new picornavirus isolated from bank voles (Clethriomys glareolus). Virology 255:86-93. [DOI] [PubMed] [Google Scholar]
- 12.Stanway, G., and T. Hyypiä. 1999. Parechoviruses. J. Virol. 73:5249-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanway, G., P. Joki-Korpela, and T. Hyypiä. 2000. Human parechoviruses—biology and clinical significance. Rev. Med. Virol. 10:57-69. [DOI] [PubMed] [Google Scholar]