Abstract
Nosocomial Candida bloodstream infections rank among infections with highest mortality rates. A retrospective cohort analysis was conducted at Catholic University Hospital to estimate the risk factors for mortality of patients with candidemia. We reviewed records for patients with a Candida bloodstream infection over a 5-year period (January 2000 through December 2004). Two hundred ninety-four patients (42.1% male; mean age ± standard deviation, 65 ± 12 years) were studied. Patients most commonly were admitted with a surgical diagnosis (162 patients [55.1%]), had a central venous catheter (213 [72.4%]), cancer (118 [40.1%]), or diabetes (58 [19.7%]). One hundred fifty-four (52.3%) patients died within 30 days. Of 294 patients, 168 (57.1%) were infected by Candida albicans, 64 (21.7%) by Candida parapsilosis, 28 (9.5%) by Candida tropicalis, and 26 (8.8%) by Candida glabrata. When fungal isolates were tested for biofilm formation capacity, biofilm production was most commonly observed for isolates of C. tropicalis (20 of 28 patients [71.4%]), followed by C. glabrata (6 of 26 [23.1%]), C. albicans (38 of 168 [22.6%]), and C. parapsilosis (14 of 64 [21.8%]). Multivariable analysis identified inadequate antifungal therapy (odds ratio [OR], 2.35; 95% confidence interval [95% CI], 1.09 to 5.10; P = 0.03), infection with overall biofilm-forming Candida species (OR, 2.33; 95% CI, 1.26 to 4.30; P = 0.007), and Acute Physiology and Chronic Health Evaluation III scores (OR, 1.03; 95% CI, 1.01 to 1.15; P < 0.001) as independent predictors of mortality. Notably, if mortality was analyzed according to the different biofilm-forming Candida species studied, only infections caused by C. albicans (P < 0.001) and C. parapsilosis (P = 0.003) correlated with increased mortality. Together with well-established factors, Candida biofilm production was therefore shown to be associated with greater mortality of patients with candidemia, probably by preventing complete organism eradication from the blood.
Nosocomial Candida bloodstream infections (BSIs), with an estimated annual incidence in the United States of 6 to 10 candidemia episodes per 100,000 population, have become a considerable public health concern (24, 25, 29). Although antifungal treatment appears to reduce mortality and excess hospitalization, the rate of candidemia-attributable mortality remains high (19% to 49%) (8, 25). Risk factors for candidemia are associated with modern therapeutics, including broad-spectrum antibiotics, hyperalimentation fluids, cancer chemotherapy, immunosuppressive agents following organ transplantation, and indwelling medical devices (17, 19).
Devices, such as stents, shunts, prostheses, endotracheal tubes, and vascular catheters, have been shown to support colonization and biofilm formation by Candida (17). The Candida biofilm lifestyle results in antifungal drug resistance and protection of the fungus from host defenses (20, 32), which carry important clinical repercussions. Device-related infections are difficult to treat, and affected devices often need to be removed (23), which can be hazardous for some patients (39).
Central venous catheters (CVCs) appear to be the most common risk factor for candidemia development in patients without neutropenia or major immunodeficiencies. More than half of BSIs, including candidemia, in intensive care unit (ICU) patients are catheter related (34, 35).
Parenteral nutrition has also been associated with increased risk of candidemia, particularly in outbreak settings (4). High-glucose media promote the formation of biofilms, particularly of Candida parapsilosis, by enhancing the microorganism's capacity to colonize indwelling CVCs and cause infections in patients receiving intravenous hyperalimentation nutrition (37).
Inadequate antifungal therapy has been shown to be an important predictor of adverse outcome for patients with Candida BSIs (9, 10, 12). Interestingly, two recent studies addressing the effects of delayed treatment for patients with candidemia found that receipt of an antifungal agent >24 h after the blood sample was obtained for culture was associated with an increased risk of mortality (6, 26).
To evaluate risk factors for mortality in hospitalized patients with Candida BSIs, a retrospective cohort analysis was conducted at a large Italian university hospital. In particular, we wanted to determine whether the formation of biofilms by Candida species, which is intrinsically associated with its infectiousness (3, 32), influenced the clinical outcomes in patients with candidemia.
MATERIALS AND METHODS
Study design, patient population, and data collection.
This retrospective cohort study was conducted from January 2000 to December 2004 at the Catholic University Hospital in Rome, Italy, which admits about 60,000 patients annually, and we used mortality as the outcome measure. Accordingly, two groups of patients (survivors and nonsurvivors) were compared.
Eligible patients who were hospitalized during the study period and had at least one positive blood culture for Candida species were identified by electronically querying the microbiology laboratory historical database. A standardized case report form was used to extract the following data from the patients' hospital records: age, sex, past medical and surgical history, Candida species type and date of culture, presence of a CVC and/or indwelling urinary catheter, total parenteral nutrition (TPN) administration, immunosuppressive therapy (antineoplastic agents and corticosteroids) within 30 days before infection, degree and duration of neutropenia, Acute Physiology and Chronic Health Evaluation III (APACHE III) score (16), type of hospital admission (medical or surgical), location of patient at time of blood culture (ICU or non-ICU), and duration of hospitalization. The type, dose, and duration of antifungal therapy were also identified.
Definitions.
Candidemias were defined as hospital acquired if patients developed positive blood cultures after 48 h of hospital admission (7). Other episodes of candidemia were classified as health care associated or community acquired according to definitions suggested by Friedman et al. (5). Criteria for defining health care-associated candidemia include the following: intravenous therapy received at home; wound care or specialized nursing care through a health care agency, family, or friends or self-administered intravenous medical therapy within 30 days prior to the onset of candidemia; hospital or hemodialysis clinic attendance or intravenous chemotherapy received within 30 days prior to the onset of candidemia; hospitalization in an acute care hospital for 2 or more days within 90 days prior to the onset of candidemia; and residence in a nursing home or long-term care facility (5). Candidemia was defined as CVC related if (i) the semiquantitative catheter tip culture yielded more than 15 CFU of the same Candida species or (ii) simultaneous quantitative cultures showed a ratio of ≥5:1 in CFU of blood samples obtained through the catheter and a peripheral vein (23). The outcome variable was defined as survival or death within 30 days of the first documented candidemia episode.
Inadequate therapy of candidemia was defined as the lack of antifungal treatment, administration of antifungal treatment 48 h after drawing the first positive blood sample for culture, administration of antifungal treatment with isolation of an organism found resistant to the antifungal agent in vitro, or antifungal administration for less than 5 days (1, 2, 26).
Microbiological methods.
To detect candidemia, blood samples were obtained after sterile disinfection of a peripheral vein or CVC, inoculation into aerobic media, and processing by BACTEC 9240 automated system (Becton Dickinson Microbiology Systems). Species identification of Candida isolates was performed by micromorphology analysis (40) and biochemical tests (Vitek 2 Yeasts Identification; bioMérieux). For all isolates, MICs of amphotericin B, fluconazole, itraconazole, ketoconazole, voriconazole, flucytosine, and caspofungin were determined using the microdilution broth procedure recommended by the Clinical and Laboratory Standards Institute (CLSI) (formerly National Committee for Clinical Laboratory Standards) (27). According to published criteria (27, 31), isolates were classified as susceptible, susceptible dose dependent, or resistant to flucytosine, fluconazole, and voriconazole. Isolates were maintained as frozen stocks and subcultured on Sabouraud dextrose agar plates at 37°C.
Biofilm formation assays.
Biofilm formation was determined according to well-established protocols (19, 33, 37), with slight modifications. To induce in vitro biofilm production by Candida isolates, Sabouraud dextrose broth (SDB) containing 8% glucose was used. Candida albicans ATCC 90028 and C. parapsilosis ATCC 96142 served as controls in each experiment. Testing of isolates was performed on three different occasions in quadruplicate. Briefly, organisms were grown for 24 h at 37°C on Sabouraud dextrose agar plates, and saline-washed suspensions of each isolate were used to prepare 3 × 107 CFU/ml inocula in SDB. Each well of polystyrene microtiter plates (Nunclon; Nalgene) was inoculated with 20 μl of yeast cell suspension and 180 μl of SDB. After 24 h of incubation, planktonic cells were discarded by washing the wells twice with 0.15 M phosphate-buffered saline (PBS), and 200 μl of PBS was added to each well. The remaining cells adhering to the plastic surface (biofilm) were quantified by two methods. First, biofilm was measured directly by spectrophotometric readings at 405 nm with a microtiter plate reader (microplate reader model 550; Bio-Rad). The percent transmittance (%T) was calculated by subtracting the %T value for each test sample from the %T value for the reagent blank to obtain a measure of the amount of light blocked passing through the wells (%Tbloc). Biofilm production by each isolate was scored as either negative (%Tbloc, <10), 1+ (%Tbloc, 10 to 20), 2+ (%Tbloc, 20 to 35), 3+ (%Tbloc, 35 to 50), or 4+ (%Tbloc, ≥50). On the basis of these scores, biofilm-producing isolates were further categorized as low-biofilm (1+) or high-biofilm producers (2+, 3+, or 4+). In the second method, biofilm was quantified biochemically using the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT; Sigma) reduction assay. In this assay, XTT is reduced by mitochondrial dehydrogenase into a water-soluble formazan product that results in a colorimetric change. A 100-μl aliquot of the XTT salt solution (1 mg/ml in PBS) and 1 μM menadione solution (Sigma; prepared in acetone) were added to each prewashed biofilm and to control wells (for measurement of background XTT reduction levels). The plates were incubated in the dark at 37°C for 5 h, and the amount of XTT formazan was measured in the microtiter plate reader at 490 nm.
To ascertain whether different yeast isolates in the planktonic phase metabolized XTT in an equal fashion, preliminary experiments were carried out on 80 isolates (35 C. albicans isolates, 25 C. parapsilosis isolates, 10 Candida tropicalis isolates, and 10 Candida glabrata isolates) that were selected from the pool of 294 isolates as described previously (14).
Statistical analysis.
Intercooled Stata software (version 8) (Stata Corporation) was used for statistical analysis. Categorical variables were compared using the χ2 test or Fisher's exact test. Continuous variables were compared using Student's t test. The relationship between the outcome variable and independent variables were measured by the odds ratios (OR) and their 95% confidence intervals (95% CI) derived from univariate and multivariable analyses. Variables with P values of <0.2 in the univariate analysis were included in a multivariable logistic regression model. One-way analysis of variance was used to compare the optical density values from individual biofilms. All tests were two sided. The significance level was set at a P value of <0.05.
RESULTS
Species distribution, biofilm production, and in vitro antifungal susceptibility of Candida isolates.
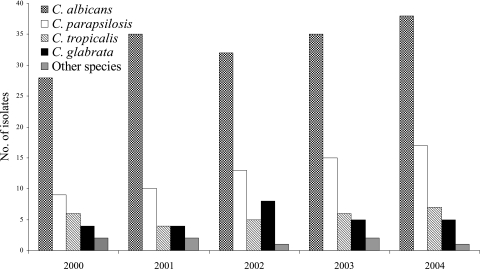
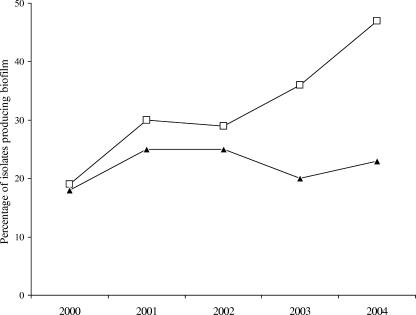
The annual distribution of Candida species causing BSIs at our institution was similar between 2000 and 2004 (Fig. 1), whereas the proportion of candidemia cases due to biofilm-producing isolates increased from 19% to 47% for non-C. albicans Candida species and remained fairly constant (17% to 20%) for C. albicans (Fig. 2). A total of 294 patients with Candida BSIs were evaluated during the study period. Organisms causing infection included C. albicans (57.1%; 168 patients), C. parapsilosis (21.7%; 64 patients), C. tropicalis (9.5%; 28 patients), C. glabrata (8.8%; 26 patients), and other Candida species (2.7%; 8 patients).
FIG. 1.
Annual distribution of Candida species causing BSIs during a 5-year study period.
FIG. 2.
Annual distribution of biofilm-producing Candida albicans (▴) and non-C. albicans Candida species (□) isolated from BSIs between 2000 and 2004. The amount of biofilm produced was determined by the %T and XTT reduction methods as described in Materials and Methods.
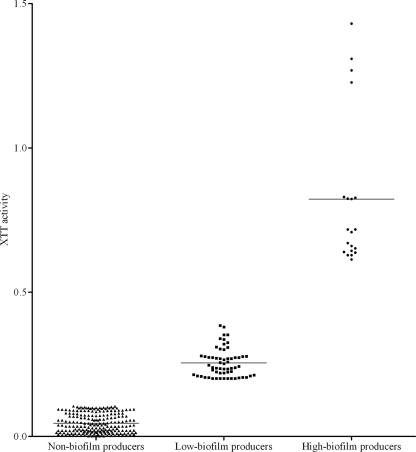
Eighty (27.2%) of 294 patients studied were infected by biofilm-forming isolates, as assessed by the %T method. Of these, 20 isolates were high-biofilm producers and 60 low-biofilm producers. Biofilm production by C. albicans was significantly less frequent (22.6%) than that by all other non-C. albicans species (33.3%; P = 0.04). Among the species most commonly isolated from Candida BSIs (C. albicans, C. parapsilosis, C. tropicalis, and C. glabrata), biofilm production was most frequently observed for isolates of C. tropicalis (71.4% [20 of 28]), followed by C. glabrata (23.1% [6 of 26]), C. albicans (22.6% [38 of 168]), and C. parapsilosis (21.8% [14 of 64]). Among biofilm-positive isolates, the highest relative intensity of biofilm formation (%Tbloc, >35) was seen for C. tropicalis isolates (30% [6 of 20]), followed by C. parapsilosis (21% [3 of 14]). To confirm the validity of these results, we also measured biofilm growth by using the XTT reduction assay. For all the isolates tested, %T determinations correlated well with XTT absorbance measurements, as seen in Fig. 3. These findings were in accord with previous works indicating that XTT assay absorbance readings are proportional to the cellular density in Candida biofilms (13, 21, 33). Importantly, Kuhn et al. (18) reported that, even if the XTT reduction assay remains a valuable tool for examining the yeast biofilm form, its use to make direct comparisons between Candida isolates may be sometimes limited, since different Candida species or strains metabolize XTT with different capabilities. This is of particular importance in the absence of correlation with other methods. To rule out the possibility that the different abilities of Candida species to metabolize tetrazolium salts may account for differences in XTT reduction rates in our isolates, we compared, in preliminary experiments, the XTT activity of 80 randomly selected isolates (from the pool of 294 isolates). Similar values were obtained, and no significant difference was noted between the XTT activities of the 80 isolates (data not shown). Therefore, differences seen in the XTT signals from the 294 isolates we tested for biofilm formation were assumed to be a reflection of changes in cell number.
FIG. 3.
XTT formazan signal produced by 294 Candida isolates examined for biofilm formation ability. According to the %T results (see the text for details), isolates were classified as nonbiofilm producers or low- or high-biofilm producers. Differences in XTT activity among groups were statistically significant (P < 0.0001). Each symbol depicts the result for one Candida isolate; the black lines show the mean values.
Isolates of Candida species were all found susceptible in vitro to amphotericin B, flucytosine, caspofungin, and, except for C. glabrata and Candida krusei, to azole antifungal agents. Ten isolates of C. glabrata showed decreased susceptibility to fluconazole and voriconazole (MICs, ≥16 μg/ml and ≥2 μg/ml, respectively). Four isolates were classified as resistant (MICs, ≥64 μg/ml and ≥4 μg/ml, respectively) to these drugs and six isolates as susceptible-dose dependent (MICs, 16 to 32 μg/ml and 2 μg/ml, respectively). The two isolates of C. krusei were resistant to fluconazole and susceptible to voriconazole.
Patient demographic, clinical characteristics, and outcomes.
Of 294 patients with Candida BSIs (mean age ± standard deviation, 65 ± 12 years; 42.1% male), 262 cases (89.1%) were hospital-acquired candidemias, 27 (9.1%) were health care-associated candidemias, and 5 (1.7%) were community-acquired candidemias. Among underlying conditions or risk factors for candidemia, solid-organ cancer was the most common (40.1%), followed by diabetes (19.7%) and liver disease (10.2%). Variables present at the onset of infection included the presence of an indwelling urinary catheter (76.8%) and/or CVC (72.4%), TPN administration (46.9%), immunosuppressive therapy (40.8%), and neutropenia (6.1%). One hundred two patients (34.7%) were in the ICU at the time of candidemia, and 162 (55.1%) were admitted with a surgical diagnosis. There were 60 (20.4%) catheter-related candidemia cases. The 30-day mortality rate for this cohort of hospitalized patients was 52.3% (154 of 294 patients).
Patients infected with C. albicans were more likely to have solid-organ cancer (45.2% versus 33.3% [P = 0.03]) or indwelling urinary catheters (80.9% versus 71.4% [P = 0.05]) and more likely to have received immunosuppressive therapy (48.8% versus 30.1% [P = 0.001]) compared to patients infected with non-C. albicans Candida species. The mortality rate in patients with BSIs due to C. albicans was significantly higher than in patients with BSIs due to non-C. albicans Candida species (57.7% versus 45.2% [P = 0.03]) (Table 1).
TABLE 1.
Epidemiological characteristics and outcomes of 294 patients with candidemia by type of fungal isolate
| Variable (no. of patients [%])a | Patients infected with:
|
Pb | |
|---|---|---|---|
| Candida albicans (n = 168) | Non-C. albicans Candida species (n = 126) | ||
| Age (yr) (mean ± SD) | 64.9 ± 15.4 | 65.1 ± 13.7 | 0.90 |
| Male sex | 74 (44) | 50 (39.6) | 0.45 |
| Hospital-acquired candidemia | 154 (91.6) | 108 (85.7) | 0.10 |
| Duration of hospitalization (no. of days) (mean ± SD)c | 47.7 ± 38.2 | 50.1 ± 42.6 | 0.60 |
| Diabetes | 32 (19) | 26 (20.6) | 0.73 |
| Liver disease | 16 (9.5) | 14 (11.1) | 0.65 |
| Solid-organ cancer | 76 (45.2) | 42 (33.3) | 0.03 |
| Hematological malignancy | 12 (7.1) | 14 (11.1) | 0.23 |
| Neutropeniad | 10 (5.9) | 8 (6.3) | 0.88 |
| Immunosuppressive therapy | 82 (48.8) | 38 (30.1) | 0.001 |
| Surgical admission | 100 (59.5) | 62 (49.2) | 0.07 |
| In the ICU at diagnosis | 57 (33.9) | 45 (35.7) | 0.75 |
| Indwelling urinary catheter | 136 (80.9) | 90 (71.4) | 0.05 |
| CVC | 120 (71.4) | 93 (73.8) | 0.65 |
| TPN | 72 (42.8) | 66 (52.3) | 0.10 |
| CVC-related candidemia | 30 (17.8) | 30 (23.8) | 0.21 |
| Mortality rate | 97 (57.7) | 57 (45.2) | 0.03 |
Values are presented as the number (percentage) of patients with the specified characteristic, unless otherwise indicated.
P value comparing the value for patients infected with C. albicans to the value for patients infected with non-C. albicans Candida species.
For patients transferred from another hospital, the duration of hospitalization was calculated by the date of the first hospital admission.
White blood cell count of less than 0.5 × 109 cells/liter.
Two hundred fifty-one patients (85.4%) received adequate systemic antifungal therapy for candidemia for a median length of 16 days (range, 1 to 29). Therapy consisted of fluconazole in 136 (54.1%) cases, amphotericin B (conventional or lipidic formulations) in 83 (33%) cases, caspofungin in 22 (8.6%) cases, voriconazole in 9 (3.6%) cases, and itraconazole in 2 (0.7%) cases. Therapy was considered inadequate for 43 patients. Of these, 31 patients received antifungal treatment after 48 h of the time that a positive blood sample for culture was drawn, 6 patients (treated with fluconazole) were infected by either C. krusei or C. glabrata which was found to be resistant to azoles in vitro, and the remaining six patients were never treated.
Univariate and multivariable analyses.
From the univariate analysis of risk factors for mortality in patients with candidemia, age (P < 0.001), hospital-acquired candidemia (P = 0.01), immunosuppressive therapy (P = 0.003), APACHE III score (P < 0.001), presence of CVC (P = 0.003), infection by C. albicans (P = 0.03), biofilm production by overall Candida species (P < 0.001), and inadequate antifungal therapy (P = 0.01) were variables significantly associated with increased mortality. In contrast, infection by C. parapsilosis was associated with decreased mortality (P = 0.007) (Table 2).
TABLE 2.
Variables associated with mortality in 294 patients with candidemia
| Characteristic (no. of patients [%])a | No. of patients who:
|
OR (95% CI) | Pb | |
|---|---|---|---|---|
| Died (n = 154) | Survived (n = 140) | |||
| Age (yr) (mean ± SD) | 69 ± 12 | 59 ± 16 | <0.001 | |
| Male sex | 70 (45.4) | 54 (38.5) | 1.32 (0.81-2.17) | 0.23 |
| Hospital-acquired candidemia | 144 (93.5) | 118 (84.2) | 2.68 (1.16-6.59) | 0.01 |
| Neutropeniac | 10 (6.5) | 8 (5.7) | 1.14 (0.39-3.44) | 0.78 |
| Immunosuppressive therapy | 75 (48.7) | 45 (32.1) | 2.00 (1.21-3.31) | 0.003 |
| Duration of hospitalization (no. of days) (mean ± SD)d | 46 ± 39 | 51 ± 40 | 0.27 | |
| Surgical admission | 87 (56.4) | 75 (53.5) | 1.12 (0.69-1.83) | 0.61 |
| In the ICU at diagnosis | 52 (33.7) | 50 (35.7) | 0.91 (0.55-1.52) | 0.72 |
| TPN | 72 (46.7) | 66 (47.1) | 0.98 (0.60-1.59) | 0.94 |
| CVC | 123 (79.8) | 90 (64.2) | 2.20 (1.26-3.86) | 0.003 |
| Catheter-related candidemia | 26 (16.8) | 34 (24.2) | 0.63 (0.34-1.16) | 0.11 |
| APACHE III score (mean ± SD) | 38 ± 16 | 27 ± 17 | <0.001 | |
| Species isolated | ||||
| Candida albicans | 97 (62.9) | 71 (50.7) | 1.65 (1.01-2.70) | 0.03 |
| Candida parapsilosis | 24 (15.5) | 40 (28.5) | 0.46 (0.25-0.84) | 0.007 |
| Candida tropicalis | 12 (7.8) | 16 (11.4) | 0.65 (0.27-1.54) | 0.28 |
| Candida glabrata | 15 (9.7) | 11 (7.8) | 1.26 (0.52-3.16) | 0.57 |
| Other Candida species | 6 (3.8) | 2 (1.4) | 1.44 (0.95-2.18) | 0.19 |
| Biofilm production by fungal isolatee | 56 (36.3) | 24 (17.1) | 2.76 (1.54-5.00) | <0.001 |
| Inadequate antifungal therapy | 30 (19.4) | 13 (9.3) | 2.36 (1.13-5.16) | 0.01 |
Values are presented as the number (percentage) of patients with the specified characteristic, unless otherwise indicated.
P value comparing the value for patients who died to the value for patients who survived.
White blood cell count of less than 0.5 × 109 cells/liter.
For patients transferred from another hospital, the duration of hospitalization was calculated by the date of the first hospital admission.
As assessed by %T and XTT absorbance determinations (see Materials and Methods for details).
By multivariable analysis, we found that inadequate antifungal therapy (OR, 2.35; 95% CI, 1.09 to 5.10; P = 0.03), infection by biofilm-forming Candida species (OR, 2.33; 95% CI, 1.26 to 4.30; P = 0.007), and APACHE III score (OR, 1.03; 95% CI, 1.01 to 1.15; P < 0.001) were independent risk factors for mortality.
Relationship between mortality and biofilm production by Candida species.
The mortality rate in patients with BSIs due to biofilm-positive isolates was significantly higher than in patients with BSIs due to biofilm-negative isolates (70% [56 of 80] versus 45.7% [98 of 214]; P < 0.001). When the patients were stratified for severity of illness, patients infected with a biofilm-forming isolate also had a higher risk of mortality than patients infected with a nonbiofilm producer for patients with APACHE III scores less than or equal to 15 (n = 50) (66.7% versus 13.6% [P = 0.002]) and for patients with APACHE III scores greater than 15 (n = 244) (70.3% versus 54.1% [P = 0.01]). The relationship between biofilm positivity of Candida isolates and mortality for the 294 patients with candidemia is displayed in Table 3. Among infections by biofilm-producing isolates, only those due to C. albicans (P < 0.001) or C. parapsilosis (P = 0.003) correlated with increased mortality. No statistically significant differences in mortality between patients infected by biofilm-forming isolates and patients infected by biofilm-negative isolates were noted for the other Candida species. When mortality was analyzed in relation to the amount of biofilm produced by overall Candida species isolates, we found that mortality was lowest if isolates were nonbiofilm producers (45.8%) and significantly increased to 66.6% (P = 0.009) and 75% (P = 0.002) if isolates exhibited a low and high biofilm-forming ability, respectively.
TABLE 3.
Crude OR and 95% CI for mortality according to biofilm production by isolates of Candida species
| Candida species | Patients infected by biofilm-positive isolate
|
Patients infected by biofilm-negative isolate
|
OR (95% CI) | Pa | ||
|---|---|---|---|---|---|---|
| Total no. | No. (%) who died | Total no. | No. (%) who died | |||
| C. albicans | 38 | 32 (84.2) | 130 | 65 (50) | 3.90 (1.72-8.83) | <0.001 |
| C. parapsilosis | 14 | 10 (71.4) | 50 | 14 (28) | 4.16 (1.46-11.82) | 0.003 |
| C. tropicalis | 20 | 8 (40) | 8 | 4 (50) | 0.88 (0.54-1.45) | 0.62 |
| C. glabrata | 6 | 4 (66.6) | 20 | 11 (55) | 1.46 (0.32-6.62) | 0.61 |
| Otherb | 2 | 2 (100) | 6 | 4 (66.6) | 0.34 | |
| Total | 80 | 56 (70) | 214 | 98 (45.7) | 2.76 (1.55-5.00) | <0.001 |
P value comparing the number of patients who were infected with a biofilm-positive isolate and died to the number of patients infected with a biofilm-negative isolate and died.
Includes Candida guilliermondii (three patients), Candida krusei (two patients), and Candida spp. (three patients).
DISCUSSION
Candida species are now the fourth most common cause of nosocomial BSIs worldwide (30). Variables, such as patient age, underlying disease, location in hospital (ICU or non-ICU), and antifungal drug exposure may influence the frequency and rank order of Candida species causing BSIs (28). In this study, the distribution of Candida species was similar to reports from other European countries (1, 36, 38), with C. albicans being the fungal species most frequently isolated from blood but there were significant increases found in the prevalence of non-C. albicans Candida species invading the bloodstream. An exception is the frequency of C. glabrata, which was the fourth most common species in our study but the second most common in Switzerland (22), the United Kingdom (15), and the United States (9). A similar trend was observed in our study for BSI isolates of biofilm-forming non-C. albicans Candida species, whose frequency increased compared to biofilm-forming C. albicans isolates. The explanation for this observation is not clear and warrants further study, but it is possible that some medical procedures might consistently impact the risk of developing candidemia caused by a fungal isolate capable of forming biofilm over the years.
Candidemia ranks among the infections with highest mortality rates (10). Estimated rates of crude mortality due to candidemia can reach almost 60%, and attributable mortality rates of 49% have been reported in patients with candidemia (8). Candidemia has been associated with mortality rate increases of 10% and 14.5% in hospitalized pediatric and adult patients, respectively (41). The impact of candidemia on mortality suggested a compelling need for clinical prediction rules to identify subsets of hospitalized patients at particularly high risk for candidemia. In accord with these findings, a high mortality rate (52.3%) was also seen in our patient population.
In the multivariable analysis of factors that may influence clinical outcome, we found, not surprisingly, that inadequate antifungal therapy was an independent predictor of mortality. Previous studies have shown that early and appropriate antifungal treatment correlated with improved candidemia outcomes (1, 6). Antifungal resistance was rarely found in our study and was restricted to a few isolates. With the exception of C. krusei, 15% of our C. glabrata isolates were resistant to fluconazole. It is interesting to note that 31 (10.5%) of our patients did not receive antifungal therapy within 48 h after a blood sample was obtained for culture. According to previous observations (6, 26), this finding should induce clinicians to consider the use of empirical antifungal therapy in patients at high risk for candidemia. Our analysis also showed that a high APACHE III score was independently associated with an increased risk of mortality, thereby confirming the results of previous studies (2, 6). Notably, multiple logistic regression analysis identified infection by biofilm-forming Candida isolates as another independent predictor of mortality. In fact, we found death rates differed significantly between patients infected by biofilm-positive Candida species and biofilm-negative organisms (70% versus 45.7%). Moreover, among patients with low APACHE III scores, the deaths of patients with candidemia caused by biofilm-forming isolates were greater than those of patients with candidemia caused by biofilm-negative isolates.
Our study extends and, in part, confirms the findings of Shin et al. (37) by showing similar results in a retrospective cohort study using a heterogeneous hospital population. Shin and colleagues examined, for the first time, biofilm production among bloodstream Candida isolates obtained from 101 nonneutropenic patients. They found no significant association between biofilm formation and outcome of fungemia (in terms of cleared or uncleared infection) by C. albicans or non-C. albicans Candida species, suggesting that factors besides biofilm formation are involved in clearing Candida from the bloodstream. The small numbers of bloodstream isolates obtained for each Candida species in that study, though, underscored the need for a larger clinical study.
Candida species and strain type were shown to affect biofilm formation in vitro (3, 11, 19, 37). Hawser and Douglas (11) demonstrated that isolates of C. parapsilosis, Candida pseudotropicalis, and C. glabrata made significantly less biofilm than C. albicans, which is more pathogenic. The finding that C. albicans isolates consistently produce more biofilm in vitro than non-C. albicans isolates was confirmed recently (19). On the other hand, non-C. albicans Candida species, particularly C. tropicalis and C. parapsilosis, appear to form biofilms readily when grown in SDB medium containing 8% glucose (considered very similar to the in vivo milieu) (37). This capability might derive from the potential of these isolates to cause candidemia in patients receiving TPN, where the solution administered usually has a high glucose concentration. Of the 59 patients with CVC-related candidemia studied by Shin et al. (37), 46 (78%) received TPN via a CVC, and the non-C. albicans Candida species recovered most frequently from blood samples was C. parapsilosis, which had a high frequency of biofilm production. In our study, C. tropicalis was the species with the highest percentage of biofilm positivity (71.4%), whereas only 22.6% of the C. albicans isolates tested could form biofilms in high-glucose SDB medium. Similar percentages were shown by isolates of C. glabrata (23.1%) and C. parapsilosis (21.8%).
Another remarkable finding of our study was the association between biofilm-forming ability of C. albicans and C. parapsilosis isolates and increased mortality rates for patients with BSIs due to these organisms, thus suggesting that the capability to form biofilms may be important in contributing to the virulence of C. albicans or enhancing the pathogenic potential of C. parapsilosis. However, Hawser and Douglas (11) failed to reveal any correlation between pathogenicity and in vitro biofilm formation in C. albicans isolates (all 15 isolates in their study were biofilm producers), even though the authors did not report any criteria or assay to test the pathogenicity of their isolates. Similarly, Shin et al. (37) did not find any significant association between biofilm production and clinical outcome of candidemia caused by C. albicans, since only 2 of 30 blood isolates of C. albicans in their study were biofilm positive when tested for the capacity to form biofilms using the same assay as that in the present study. Discrepancies between our results and those of the Shin's group support the concept that biofilm formation is a stable characteristic of Candida strains that varies greatly among clinical isolates, as recently stated by Jain et al. (13).
In this study, we used polystyrene plates to grow biofilms. Although a valid assay for biofilm formation in C. albicans based on polystyrene microtiter plates has now been established and standardized (33), it is possible that use of other materials, such those used for indwelling devices, including catheters (e.g., silicone elastomer), may give results different from those we obtained here, perhaps because the polystyrene may not reflect exactly the ability to form a biofilm in vivo. In addition to the substrate material, it is important to note that other environmental parameters, such as substrate preconditioning solutions (i.e., those mimicking physiologic conditions, such as the presence of serum or saliva) and growth media can affect biofilm production. Again, our biofilm assays did not include an initial step, where nonadherent cells are removed after 90-min incubation with the standardized Candida suspension (adhesion phase) and adherent cells are incubated for prolonged periods in the presence of media (biofilm formation phase). This step would hamper the coadhesion of planktonic cells to adherent cells and ensure uniform biofilm formation on plastic surfaces (19). In this context, our study has some limitations. However, our analyses showed that the amount of biofilm formation was highly correlated between the two methods we employed to indirectly measure it. This correlation suggested that the different abilities exhibited by our Candida strains to adhere and grow on polystyrene surfaces were really a reflection of intrinsic physiological differences among the various clinical isolates being compared.
In conclusion, we showed that, together with inadequate antifungal therapy and high APACHE III scores, Candida biofilms adversely affect the health of hospitalized patients with candidemia and are thus a significant predictor of mortality. Our findings provide a foundation for future studies examining the correlation between biofilm formation and clinical outcome in high-risk patient populations.
Acknowledgments
This work was supported by “FIRB” project 2001, grant RBNE01PUB5 008, and the Università Cattolica del Sacro Cuore, Fondi Ateneo Linea D1-2006.
We thank Paul Kretchmer at San Francisco Edit for his assistance in editing the manuscript.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Almirante, B., D. Rodriguez, B. J. Park, M. Cuenca-Estrella, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, P. Saballs, S. K. Fridkin, J. Morgan, J. L. Rodriguez-Tudela, D. W. Warnock, A. Pahissa, and the Barcelona Candidemia Project Study Group. 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43:1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng, J. R., L. C. Lin, T. G. Young, C. E. Liu, C. H. Chen, and R. W. Tsay. 2006. Risk factors for candidemia-related mortality at a medical center in central Taiwan. J. Microbiol. Immunol. Infect. 39:155-161. [PubMed] [Google Scholar]
- 3.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 4.Eggimann, P., J. Garbino, and D. Pittet. 2003. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 3:685-702. [DOI] [PubMed] [Google Scholar]
- 5.Friedman, N. D., K. S. Kaye, J. E. Stout, S. A. McGarry, S. L. Trivette, J. P. Briggs, W. Lamm, C. Clark, J. MacFarquhar, A. L. Walton, L. B. Reller, and D. J. Sexton. 2002. Health-care associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 137:791-797. [DOI] [PubMed] [Google Scholar]
- 6.Garey, K. W., M. Rege, M. P. Pai, D. N. Mingo, K. J. Suda, R. S. Turpin, and D. T. Bearden. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25-31. [DOI] [PubMed] [Google Scholar]
- 7.Garner, J. S., W. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control. 16:128-140. [DOI] [PubMed] [Google Scholar]
- 8.Gudlaugsson, O., S. Gillespie, K. Lee, J. Vande Berg, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172-1177. [DOI] [PubMed] [Google Scholar]
- 9.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbarth, S., K. Ferriere, S. Hugonnet, B. Ricou, P. Suter, and D. Pittet. 2002. Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch. Surg. 137:1353-1359. [DOI] [PubMed] [Google Scholar]
- 11.Hawser, S. P., and L. J. Douglas. 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 62:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 13.Jain, N., R. Kohli, E. Cook, P. Gialanella, T. Chang, and B. C. Fries. 2007. Biofilm formation by and antifungal susceptibility of Candida isolates from urine. Appl. Environ. Microbiol. 73:1697-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin, Y., H. K. Yip, Y. H. Samaranayake, J. Y. Yau, and L. P. Samaranayake. 2003. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J. Clin. Microbiol. 41:2961-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kibbler, C. C., S. Seaton, R. A. Barnes, W. R. Gransden, R. E. Holliman, E. M. Johnson, J. D. Perry, D. J. Sullivan, and J. A. Wilson. 2003. Management and outcome of bloodstream infections due to Candida species in England and Wales. J. Hosp. Infect. 54:18-24. [DOI] [PubMed] [Google Scholar]
- 16.Knaus, W. A., D. P. Wagner, E. A. Draper, J. E. Zimmerman, M. Bergner, P. G. Bastos, C. A. Sirio, D. J. Murphy, T. Lotring, A. Damiano, and F. E. Harrell, Jr. 1991. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100:1619-1636. [DOI] [PubMed] [Google Scholar]
- 17.Kojic, E. M., and R. O. Darouiche. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn, D. M., M. Balkis, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2003. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 41:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn, D. M., J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 70:878-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumamoto, C. A. 2002. Candida biofilms. Curr. Opin. Microbiol. 5:608-611. [DOI] [PubMed] [Google Scholar]
- 21.Li, X., Z. Yan, and J. Xu. 2003. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 149:353-362. [DOI] [PubMed] [Google Scholar]
- 22.Marchetti, O., J. Bille, U. Fluckiger, P. Eggiman, C. Ruef, J. Garbino, T. Calandra, M. P. Glausser, M. G. Tauber, and D. Pittet. 2004. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991-2000. Clin. Infect. Dis. 38:311-320. [DOI] [PubMed] [Google Scholar]
- 23.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 24.Morgan, J. 2005. Global trends in candidemia: review of reports from 1995-2005. Curr. Infect. Dis. Rep. 7:429-439. [DOI] [PubMed] [Google Scholar]
- 25.Morgan, J., M. I. Meltzer, B. D. Plikaytis, A. N. Sofair, S. Huie-White, S. Wilcox, L. H. Harrison, E. C. Seaberg, R. A. Hajjeh, and S. M. Teutsch. 2005. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect. Control. Hosp. Epidemiol. 26:540-547. [DOI] [PubMed] [Google Scholar]
- 26.Morrell, M., V. J. Fraser, and M. H. Kollef. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 28.Pfaller, M. A., and D. J. Diekema. 2004. Twelve-years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, S. A. Messer, and The SENTRY Participant Group. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., D. J. Diekema, J. H. Rex, A. Espinel-Ingroff, E. M. Johnson, D. Andes, V. Chaturvedi, M. A. Ghannoum, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, P. Troke, T. J. Walsh, and D. W. Warnock. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramage, G., S. P. Saville, D. P. Thomas, and J. L. Lopez-Ribot. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van der Horst, J. E. Edwards, R. G. Washburn, W. M. Scheld, A. W. Karchmer, A. P. Dine, M. J. Levenstein, and C. D. Webb for The Candidemia Study Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]
- 35.Richards, M. J., J. R. Edwards, D. H. Culver, and M. P. Gaynes. 2000. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21:510-515. [DOI] [PubMed] [Google Scholar]
- 36.Richet, H., P. Roux, C. Des Champs, Y. Esnault, A. Andremont, and the French Candidemia Study Group. 2002. Candidemia in French hospitals: incidence rates and characteristics. Clin. Microbiol. Infect. 8:405-412. [DOI] [PubMed] [Google Scholar]
- 37.Shin, J. H., S. J. Kee, M. G. Shin, S. H. Kim, D. H. Shin, S. K. Lee, S. P. Suh, and D. W. Ryang. 2002. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 40:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tortorano, A. M., E. Biraghi, A. Astolfi, C. Ossi, M. Tejada, C. Farina, S. Perin, C. Bonaccorso, C. Cavanna, A. Ravallo, A. Grossi, and the FIMUA Candidemia Study Group. 2002. European Confederation of Medical Mycology (ECMM) prospective survey of candidaemia: report from one Italian region. J. Hosp. Infect. 51:297-304. [DOI] [PubMed] [Google Scholar]
- 39.Walsh, T. J., and J. H. Rex. 2002. All catheter-related candidemia is not the same: assessment of the balance between the risks and benefits of removal of vascular catheters. Clin. Infect. Dis. 34:600-602. [DOI] [PubMed] [Google Scholar]
- 40.Warren, N. G., and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184-1199. In R. P. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, DC.
- 41.Zaoutis, T. E., J. Argon, J. Chu, J. A. Berlin, T. J. Walsh, and C. Feudtner. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 41:1232-1239. [DOI] [PubMed] [Google Scholar]