Abstract
Multiple-locus variable-number tandem-repeat (VNTR) analysis (MLVA) has been proposed as a means of strain typing for tracking the transmission of leprosy. However, empirical data for a defined population are lacking. To this end, a study was initiated to assess the diversity and distribution of prevalent Mycobacterium leprae strains in Qiubei County, Yunnan Province, People's Republic of China, where the annual detection rate of leprosy is 10-fold higher than the national average rate. Sixty-eight newly diagnosed leprosy patients were included in the study. MLVA at eight M. leprae loci was applied using DNA extracts from skin biopsies. The number of alleles per locus ranged from 4 to 24, providing adequate strain discrimination. MLVA strain typing identified several clusters of patients whose M. leprae specimens shared similar VNTR profiles. Two of these clusters were comprised of patients who resided predominantly in the north and northwest parts of Qiubei County. Furthermore, it was found that multicase families are common in this county: 23 of the 68 patients were from 11 families. Intrafamilial VNTR profiles closely matched within six families, although they were different between the families. Moreover, VNTR patterns related to those found in some multicase families were also detected in patients in the same or adjacent townships, indicating the utility of VNTR strain typing to identify and detect short-range transmission events. Social contact through village markets is proposed as a means of transmission.
Leprosy is a chronic infectious disease caused by Mycobacterium leprae infection. It has been thought that untreated leprosy patients are major sources of infection, and multidrug therapy (MDT) as designed by the World Health Organization (WHO) can interrupt transmission and thereby eventually lead to the elimination of leprosy as a public health concern (23). However, MDT has been implemented for 20 years, but the detection rate of leprosy remains steady in some countries and in some areas of China (6, 24). Since 2002, nearly 1,600 new cases have been detected in China each year (24). The underlying mechanisms of transmission of M. leprae with respect to the source, reservoir, mode of transmission, and interaction with the host are still unclear and may hamper the expected elimination of leprosy.
Wenshan Prefecture in Yunnan Province can be considered an area in China where leprosy is endemic because the new-case detection rate has decreased only moderately from 4.7 to 3.6 per 100,000 during 1985 to 2002. In particular, certain townships of Qiubei County in this prefecture have had detection rates as high as 9.7/100,000 in recent years (6). An epidemiological investigation conducted from 1998 to 2000 based on a serological test for antibodies against phenolic glycolipid I, a dominant M. leprae antigen, showed that over 30% of the general population tested positive. Furthermore, in 28% of household contacts of leprosy patients tested in two villages where leprosy is endemic, Tonghong and Nanqiu in Qiubei County, M. leprae DNA was detectable in nasal swabs (21). This study revealed a significant transmission of leprosy in this area. To further understand the transmission patterns, the present study takes advantage of novel molecular strain typing methods reported since the availability of the M. leprae genome sequence of a strain (TN) isolated from a patient in Tamil Nadu, India (2). Two types of genomic markers have been identified. Three single nucleotide polymorphisms (SNPs) discovered through the partial genome sequencing of an isolate from a patient from Brazil have been useful to track evolution and dispersion of M. leprae at global and species levels (11). On the other hand, the detection of multiple variable-number tandem-repeat (VNTR) loci with a wide range of allelic diversity should be more suitable for the differentiation of isolates within a community and thus useful for detecting recent transmission events (5, 25, 26). To describe M. leprae diversity and assess the value of multiple-locus VNTR analysis (MLVA), we present our findings for Qiubei County.
MATERIALS AND METHODS
Field site, study population, specimen collection, and DNA extraction.
New leprosy cases identified at the Skin Disease Control Stations (SDCS), Qiubei County, from 2002 to 2005 were considered for the epidemiology study. Routine diagnosis was based on clinical, bacterial, and histopathologic examinations, and classification was done in accordance with the Ridley-Joplin scheme (14). After informed consent was obtained from patients, skin biopsies were collected at the SDCS by the punch excision method. The molecular epidemiology studies were approved by the ethics committees of the SDCS and Beijing Friendship Hospital. A portion of each biopsy was fixed in 70% ethanol to enable DNA extraction following transport to the laboratory in Beijing. Ethanol was removed and discarded from the skin biopsy fixed in 70% ethanol. The tissue was rehydrated in 10 mM Tris-1 mM EDTA or phosphate-buffered saline (Promega Corporation, Madison, WI) buffer for 30 min at room temperature prior to DNA extraction using the DNeasy tissue kit (QIAGEN, Valencia, CA). The DNA was eluted in 100 μl of AE elution buffer provided in the kit.
VNTR amplification and DNA sequencing of PCR products.
The M. leprae VNTR locus nomenclature, primers for PCR amplification, and PCR conditions were described previously (5). The primers for the (AC)8a locus were described previously by Zhang et al. (26). During the course of the study, we implemented multiplex PCR in which certain primer sets were combined. The Multiplex PCR kit (QIAGEN) was utilized using 1 μl of a twofold-diluted template and cycled as follows: enzyme activation for 15 min at 95°C, followed by 40 cycles, each comprised of 30 s of denaturation at 94°C, 90 s of annealing at 60°C, and 90 s of extension at 72°C. A final extension step of 10 min at 72°C was performed prior to storage at 10°C. A 5-μl aliquot from the PCR amplification was applied to a 3% agarose gel (Invitrogen Corporation, Carlsbad, CA) in 1× Tris-borate-EDTA buffer and electrophoresed at 80 V for 90 min. PCR products were detected by staining the gel with ethidium bromide. If SYBR Safe DNA gel stain (Invitrogen) was used, it was added to the gel prior to electrophoresis. An EZ Load 20-bp molecular ruler (Bio-Rad, Hercules, CA) was run on lanes 1 and 8 of an eight-well gel. PCR-positive products (1 to 2 μl) were submitted to the BigDye Terminator v 3.1 cycle sequencing method using the forward primer at the Macromolecular Resources Core Facility at Colorado State University or at Boya Bio-Tech Company, Beijing, People's Republic of China. The VNTR allele at each locus was defined as the number of repeat units present for each product. The allele at each of the microsatellites and the 6-7 minisatellite locus were determined by direct sequencing of PCR products.
VNTR data analysis.
The Microsatellite Tool kit (available from the website http://oscar.gen.tcd.ie/∼sdepark/ms-toolkit/) was used to calculate allele frequency. The allele diversity, H, for each locus was calculated by using the equation H = 1 − Σfi2, where f is the frequency of the ith allele. To infer phylogeny, we performed parsimony analysis through the application of PAUP version 4.0 beta (17). All analyses were preformed by heuristic search procedures, with tree bisection-reconnection branch swapping and the MULTREES option. A 50% consensus tree was used to report tree topology. A weighted-step procedure was used based on the assumption that a loss or gain of a repeat consists of a single step. For example, if the number of repeats at a VNTR locus was 6 in one strain and was 9 in a second strain, this constituted three steps.
RESULTS
Characteristics of the field site and the study population.
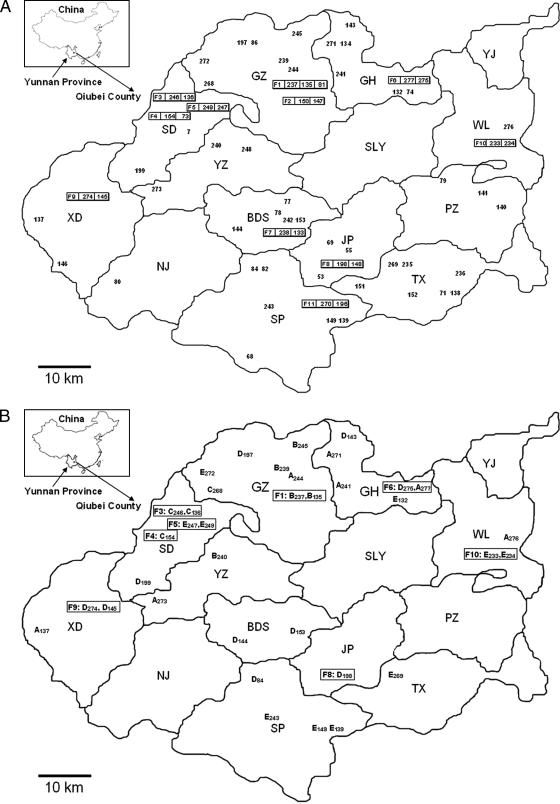
Qiubei County is composed of 14 townships, covering approximately 5,000 km2 of rugged karst terrain with an elevation range of 782 to 2,552 m. The names of the townships have been abbreviated (Fig. 1). The population in 2004 was 452,000. Leprosy control in Qiubei County lagged behind that in other counties in China before the implementation of MDT in 1986 (6). In the last few years, approximately 27 to 30 new leprosy patients were reported annually. From 2002 to 2005, 68 new cases were detected. The majority of the patients are male (n = 55), and the average age is 32.5 years. The patients were clinically classified according to the Ridley-Joplin system as tuberculoid (n = 4), borderline tuberculoid (n = 18), borderline (n = 2), borderline lepromatous (n = 38), lepromatous (n = 5), and unclassified (n = 1). No new cases were found in Shuanglongying and Yangjie townships, and only one case was detected in Nijiao. The distribution of the leprosy patients is represented in Fig. 1A. Uneven distribution of leprosy patients is obvious in the county, with more than one-third (n = 28) from the townships of Gehan (GH), Guanzai (GZ), and Sede (SD), which are located in north and northwestern regions. Furthermore, one-third (n = 23) of the 68 cases came from 11 different multicase families. The families are referred to as F1 to F11, with each comprised of at least two patients from 2003 to 2005 (Fig. 1A).
FIG. 1.
(A) Distribution of patients diagnosed from 2002 to 2005 in Qiubei County, Yunnan Province, People's Republic of China. The capital letters represent the names of the townships of this county. XD, Xindian; NJ, Nijiao; YZ, Yuezhe; BDS, Badaoshao; SP, Shupi; SLY, Shuanglongying; JP, Jinping; TX, Tianxing; PZ, Pingzai; WL, Wenliu; YJ, Yangjie. The inset depicts the Yunnan Province in southern China. Patients are coded by numbers. Multicase families (F1 to F11) are shown as rectangles, and the patient numbers are indicated. (B) The distribution of characteristic strain types A to E identified by MLVA (in Fig. 2) is depicted for some of the patients represented in panel A.
Application of M. leprae VNTR typing in Qiubei County.
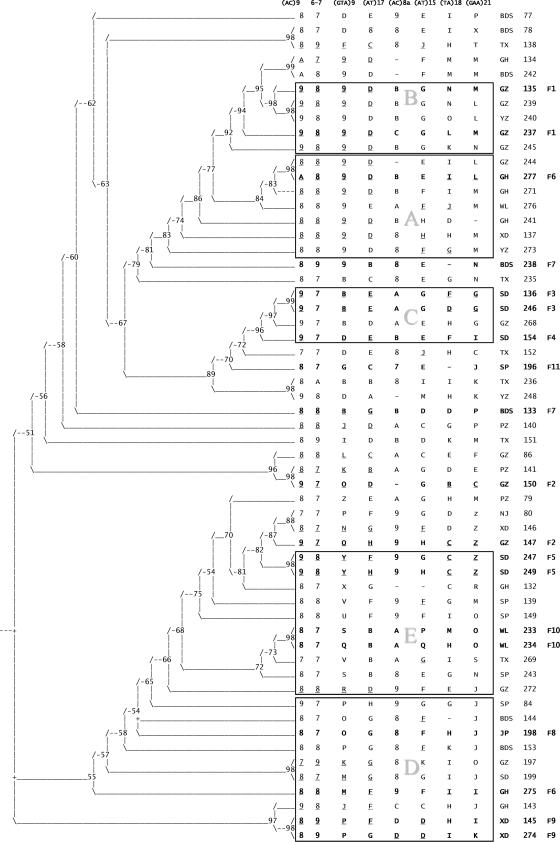
DNA extracts from stored biopsy specimens were utilized for the amplification of M. leprae VNTR loci. Results from a VNTR mapping study of M. leprae present in skin biopsies of leprosy patients from different parts of China, including Yunnan Province, demonstrated that of the loci described previously, (AC)8b, 12-5, 21-3, 18-8, and 27-5, were not polymorphic in China (5, 22). Therefore, we selected microsatellites (AC)9, (GTA)9, and (AT)17 and minisatellite locus 6-7 for strain differentiation in Qiubei County. Subsequently, alleles of four more microsatellite loci, (TA)18, (GAA)21, (AC)8a, and (AT)15, were assessed. The alleles at each locus, defined as the number of tandem repeats, are included in Fig. 2. Of the total alleles shown in Fig. 2, 30% (i.e., 142) were amplified and mapped at the Tropical Medicine Research Institute in Beijing and at Colorado State University. Concordant alleles were obtained for the majority of the loci (137/142). The allelic diversity of the typing markers is shown in Table 1. The allelic index for each locus ranges from 0.5 to 0.9. In some of the isolates, repeated PCR amplifications and sequencing indicated long stretches of repeats at particular loci such as (GAA)21 and (GTA)9, for which the exact end point could not be specified. The maximum numbers of repeats that could be counted before the signal was lost are reported in these instances.
FIG. 2.
Phylogenetic analysis of M. leprae strains isolated from leprosy patients in Qiubei County, Yunnan Province, People's Republic of China. A 50% consensus tree is illustrated, which was obtained with VNTR alleles for eight loci for 57 isolates using parsimony principles as described in Materials and Methods. The numbers at the nodes of branches represent percentages of trees in which the nodes were found. To the right of the tree, the eight locus alleles (repeat number) for each patient are indicated. Alleles 1 to 9 are indicated by the number, while a letter code is used for alleles greater than 9. Alleles 22, 26, 31, 32, 33, 38, 39, 40, 41, and 42 were not found in the data set. Thus, the alleles could be accommodated using 26 letter codes as follows: A to L, 10 to 21, respectively; M to O, 23 to 25, respectively; P to S, 27 to 30, respectively; T to W, 34 to 37, respectively; X and Y, 43 and 44, respectively; and Z, all alleles ≥45. The township of residence is listed after the alleles using capital letters (as described in the legend of Fig. 1), followed by the patient number. The last column indicates the family number for multicase families. The rectangles marked with the letters A to C represent VNTR profiles grouped on the basis of similarity between the strains. The (GTA)9 allele is 9 in clusters A and B and 11 to 13 in cluster C. Strains grouped in clusters D and E are more variable but distinguished by larger (GTA)9 alleles: 15 to 25 in cluster D and >26 in cluster E.
TABLE 1.
Allelic properties of the VNTR loci in M. leprae isolates in leprosy patients from Qiubei Countya
| Locus | Allele | No. of isolates | f (%) |
|---|---|---|---|
| (AC)9 | 7 | 4 | 6.25 |
| 8 | 38 | 59.38 | |
| 9 | 18 | 28.13 | |
| 10 | 4 | 6.25 | |
| 4* | 64^ | 0.56§ | |
| (AC)8a | 7 | 1 | 1.89 |
| 8 | 15 | 28.30 | |
| 9 | 11 | 20.75 | |
| 10 | 12 | 22.64 | |
| 11 | 10 | 18.87 | |
| 12 | 2 | 3.77 | |
| 13 | 2 | 3.77 | |
| 7* | 53^ | 0.79§ | |
| 6-7 | 7 | 35 | 50.72 |
| 8 | 27 | 39.13 | |
| 9 | 6 | 8.70 | |
| 10 | 1 | 1.45 | |
| 4* | 69^ | 0.58§ | |
| (GAA)21 | 12 | 2 | 3.51 |
| 14 | 1 | 1.75 | |
| 15 | 1 | 1.75 | |
| 16 | 3 | 5.26 | |
| 18 | 3 | 5.26 | |
| 19 | 8 | 14.04 | |
| 20 | 3 | 5.26 | |
| 21 | 4 | 7.02 | |
| 23 | 4 | 7.02 | |
| 24 | 4 | 7.02 | |
| 25 | 4 | 7.02 | |
| 26 | 1 | 1.75 | |
| 27 | 2 | 3.51 | |
| 29 | 1 | 1.75 | |
| 30 | 1 | 1.75 | |
| 31 | 1 | 1.75 | |
| 38 | 1 | 1.75 | |
| 50 | 2 | 3.51 | |
| 52 | 1 | 1.75 | |
| 68 | 1 | 1.75 | |
| 100 | 1 | 1.75 | |
| 22* | 57^ | 0.93§ | |
| (GTA)9 | 9 | 16 | 23.53 |
| 11 | 6 | 8.82 | |
| 12 | 2 | 2.94 | |
| 13 | 6 | 8.82 | |
| 15 | 1 | 1.47 | |
| 16 | 2 | 2.94 | |
| 18 | 1 | 1.47 | |
| 19 | 2 | 2.94 | |
| 21 | 1 | 1.47 | |
| 22 | 2 | 2.94 | |
| 24 | 1 | 1.47 | |
| 25 | 4 | 5.88 | |
| 26 | 6 | 8.82 | |
| 28 | 1 | 1.47 | |
| 29 | 2 | 2.94 | |
| 30 | 2 | 2.94 | |
| 31 | 2 | 2.94 | |
| 35 | 1 | 1.47 | |
| 36 | 2 | 2.94 | |
| 37 | 1 | 1.47 | |
| 38 | 1 | 1.47 | |
| 44 | 2 | 2.94 | |
| 45 | 1 | 1.47 | |
| 24* | 68^ | 0.82§ | |
| (AT)17 | 8 | 2 | 3.33 |
| 10 | 1 | 1.67 | |
| 11 | 8 | 13.33 | |
| 12 | 4 | 6.67 | |
| 13 | 19 | 31.67 | |
| 14 | 7 | 11.67 | |
| 15 | 7 | 11.67 | |
| 16 | 9 | 15.00 | |
| 17 | 3 | 5.00 | |
| 9* | 60^ | 0.82§ | |
| (AT)15 | 12 | 3 | 5.36 |
| 13 | 4 | 7.14 | |
| 14 | 10 | 17.86 | |
| 15 | 13 | 23.21 | |
| 16 | 15 | 26.79 | |
| 17 | 4 | 7.14 | |
| 18 | 1 | 1.79 | |
| 19 | 2 | 3.57 | |
| 20 | 1 | 1.79 | |
| 22 | 1 | 1.79 | |
| 26 | 1 | 1.79 | |
| 12* | 56^ | 0.83§ | |
| (TA)18 | 11 | 1 | 1.85 |
| 12 | 4 | 7.41 | |
| 13 | 6 | 11.11 | |
| 14 | 2 | 3.70 | |
| 15 | 2 | 3.70 | |
| 16 | 6 | 11.11 | |
| 17 | 10 | 18.52 | |
| 18 | 12 | 22.22 | |
| 19 | 1 | 1.85 | |
| 20 | 3 | 5.56 | |
| 21 | 1 | 1.85 | |
| 22 | 2 | 3.70 | |
| 23 | 1 | 1.85 | |
| 24 | 2 | 3.70 | |
| 25 | 1 | 1.85 | |
| 15* | 54^ | 0.88§ |
Allele is defined as the number of repeat units found at a VNTR locus in an isolate. The numbers of isolates in which a certain allele was detected are shown.
indicates the total number of isolates in which alleles were mapped. f indicates the frequency of an allele in the population.
indicates the total number of alleles found in the population.
indicates the marker diversity index, calculated as 1 − Σfi2.
Strain diversity and population structure of M. leprae in Qiubei County.
In order to delineate relatedness among the Qiubei isolates, the VNTR data sets were submitted to phylogenetic analysis using parsimony principles in PAUP (17). The 50% consensus tree was evaluated for 57 isolates for which a majority of the loci were mapped. A stepwise mutation model in which a change in one repeat unit per locus is considered to be one mutation event was assumed. With this method, it was possible to discern five groups that we name groups A, B, C, D, and E. This grouping seemed to be dependent on the allele at the (GTA)9 locus. In this population, 24 alleles were detected at the (GTA)9 locus, ranging from those with copy numbers of 9 to those with copy numbers of 45 (Table 1). Allele 9 (i.e., nine GTA repeats) is found in groups A and B, while the alleles are 11 to 13 in group C, 15 to 25 in group D, and 26 or larger in group E. The eight-locus [(AC)9, 6-7, (GTA)9, (AT)17, (AC)8a, (AT)15, (TA)18, and (GAA)21] VNTR signature pattern for group A members tends to be 8-8-9-13-11-[14-17]-[16-19]-[18-21], while it is 9-8-9-13-11-16-[20-24]-[21-24] for group B. A characteristic difference between group A and group B is a one-repeat change at (AC)9. Groups D and E are more heterogeneous than groups A, B, and C.
Distribution of strain types in Qiubei County.
Combining the distribution of patients, as illustrated in Fig. 1, with the phylogenetic analysis (Fig. 2) revealed that more than one strain type can be seen in each township (Fig. 1B). Although the number of patients from each township is limited (1 to 10 patients), it can be seen that group A and group B isolates are distributed mainly in the north part of the county (townships GH, GZ, and YZ) (Fig. 1B). On the other hand, patients in the southern and eastern parts (townships Tianxing, Shupi, Jinping, Wenliu, and Badaoshao) harbor the C, D, and E types of strains.
Transmission scenarios in Qiubei County and the influence of multicase families.
In Qiubei County, intrafamilial transmission appears to be common. In each of groups A to E, there is at least one multicase family. Within intrafamilial patient pairs (F1 [patients 237 and 135], F3, F5, F9, and F10), matching alleles were detected at five or more of the eight loci, indicating a common infectious source and recent transmission within the household. The allelic differences of one to two copies within each pair of the multicase families are seen mostly at the AT-rich loci. Allelic differences across several loci seen within patient pairs of families F6 and F7 suggest that the family members harbor different strains.
Furthermore, we have identified cases in the same or adjoining townships that harbor closely matching genotypes. For example, closely matching M. leprae VNTR patterns were seen in cases 239 and 245 in the GZ township, in the F1 multicase family (patients 237 and 135), and in case 240 in the adjacent township YZ. Similarly, in GH, one member of F6 (patient 277) shared VNTR pattern similarities with two other patients (patients 241 and 271) in the same township as well as with patient 244 in the neighboring GZ township (Fig. 2).
It is worth noting that there are three multicase families in SD: F3 (patients 136 and 246), F4 (patients 73 and 154), and F5 (patients 247 and 249). Families F3 and F4 are similar, both belonging to group C, while F5 is quite different (group E). F3 and F4 cluster with case 268 of the adjoining GZ township.
A trade fair held weekly in the SD township is a meeting place for people from GZ and YZ and serves as a main mode of interaction among the local people. The average distance from the outside villages to SD is 10 km. We postulate that the trade fair may be a means of transmission to other places, although other possibilities must be sought and investigated.
DISCUSSION
Although leprosy rates in China are low enough to satisfy WHO standards of elimination, the continued detection of 20 to 30 cases annually in parts of the southwestern provinces indicates that transmission is ongoing. Molecular methods of strain typing have been applied for several years to study the epidemiology of infectious diseases. The present study has been designed to include new leprosy cases reported to the SDCS over the last 3 years in all 14 townships of Qiubei County. A study population comprised of 68 new cases was thus identified. This first systematic study of a well-defined population, within a specified geographic region and time period, offers unique opportunities for evaluating strain typing as a tool for defining the prevalent population structures of the pathogen and tracking the transmission of leprosy.
With regard to the identification of cluster-related genotypes and close tracking of transmissible isolates of an infectious disease, it has been widely recognized that genetic markers with sufficient heterogeneity within the population are necessary to distinguish multiple transmission clusters yet should be stable enough to link isolates belonging to common recent transmission events (15). Highly stable markers with limited allelic diversity in the study population such as SNPs are not suitable for the study of short-term transmission (7). Recently, three SNPs were used to develop a model of the origin and dispersion of M. leprae (11). The isolates from China belong to one (SNP type 3) of the four major types and represent an alternate eastward branch/route of transmission from the proposed origin in East Africa/Central Asia. In this study, four randomly selected isolates from different townships, 78 (Badaoshao), 79 (Pingzai), 80 (Nijiao), and 84 (Shupi), are all SNP type 3. Additional isolates from prior sample collections in China were also shown to be SNP type 3 (data not shown).
In recent years, short tandem-repeat sequences that generate polymorphisms by the mechanisms of strand slippage and recombination have been widely accepted as being useful markers for epidemiology and transmission surveillance of multiple pathogens. MLVA has been compared with other traditional methods such as restriction fragment length polymorphism, spoligotyping, and multilocus sequence typing and was found to be suitable based on discriminatory potential and convenience in laboratory applications (1, 3, 8, 13, 16). Thus, after the sequence of the M. leprae genome was published, a list of 44 candidate VNTR loci was identified, 9 of which showed polymorphisms in a small panel of reference strains (5). Subsequently, VNTRs were demonstrated in clinical isolates in these and other loci (25, 26). Stability in M. leprae microsatellite loci has been demonstrated during multiple passages in nude mice and armadillo hosts, with an occasional shift by one or two copies in some loci (10, 18).
Although VNTRs are accepted markers for strain typing, the optimal model for the quantification of allelic changes during expansion or contraction of repeats is not known. Furthermore, the process and patterns of mutations at different loci may differ from locus to locus, depending on the sequence and the length of the repeat motif and the array size at each locus (4, 12). We have assumed a stepwise model of mutation for the expansion or contraction of repeat numbers at VNTR loci in order to construe genetic relatedness between isolates. Recent studies of two bacterial species amenable to multiple in vitro passages for measurements of mutation rates have indeed demonstrated that single-repeat insertions and deletions are equally probable and that they constitute a significant proportion of the mutations (∼80%) responsible for strain evolution (9, 19, 20). Furthermore, the mutation rate tends to depend on allele diversity.
A survey of four microsatellites and six minisatellites on 50 M. leprae isolates from China revealed that two of the microsatellites [(AC)8b and (GGT)5] and five minisatellites (12-5, 18-8, 21-3, 23-3, and 27-5) exhibited no or minimal polymorphisms (22). Therefore, in the present study, the polymorphic microsatellites (AC)9 and (GTA)9 were combined with one minisatellite locus (6-7) and five other candidate loci to resolve prevalent strains. In developing this approach, results from previous field studies were taken into consideration (10, 25). When only three microsatellite VNTR loci were applied to investigate the genetic diversity of M. leprae in India, only 2 out of 33 isolates were found to have the same VNTR pattern, with no known epidemiological link. However, within a known multicase family, these three loci were informative and identical (25). Therefore, it was clear that several loci are required to discern strain identity, but which loci would prove to be informative was not known. The study reported previously by Zhang et al. utilized isolates collected mainly from Japan and a few others from Thailand, Korea, and Indonesia (26). Those strains were collected at different times and maintained in mice by multiple passages. Although many loci were mapped, they were not ideally suited for finding relationships relevant to local transmission. However, striking strain identity was detected when multiple patients in households were studied, indicating infection with the same strain.
Our present study was therefore focused on newly diagnosed leprosy patients residing in the small area of Qiubei County over a 3-year period. MLVA was shown to be more meaningful than strain typing with a single locus or a few loci. We illustrate that if only loci with many alleles, such as (GAA)21 and (TA)18, are used as first-level strain-typing markers, it is likely that related strains may not be recognized. Furthermore, microevolution at microsatellite loci is evident in Qiubei, even in strains infecting patients in the same household. However, by including six other loci, even some with limited allelic diversity, major groupings were discernible. Therefore, in addition to having a sufficient number of loci, it may be necessary to pay attention to the allelic diversity of loci in the prevalent population.
The study depicts several characteristic features of M. leprae from patients in Qiubei County. First, the clustering of patients within families is severe in Qiubei County. Multicase families, sharing closely matching VNTR genotypes, indicate ongoing transmission prior to detection and treatment with MDT. One or two unit differences in copy number are expected in loci prone to high rates of mutation, such as the AT-rich regions, due to the long incubation period and unknown number of transmission cycles before the onset of disease. We propose that multicase families may constitute epidemic foci and a source of M. leprae in villages; therefore the predominant strain or cluster(s) tends to be that found in multicase families (Fig. 1 and 2). The high level of transmission involving families is indicated by the finding that three other multicase households (F12, F13, and F14) have been identified in the GZ township since early 2006. Of these, F13 and F14 arose from previously defined single-case families. Furthermore, new cases were found in existing multicase families (as in F1).
A long term-study is required to monitor the evolution and transmission of M. leprae in Qiubei and to verify the trends in data from 2002 to 2005 deduced via this MLVA molecular epidemiology approach. The mechanism of expansion of the (GTA)9 locus in isolates with large allele sizes as seen in group E deserves further study.
Acknowledgments
We gratefully acknowledge the contributions of Xiaohua Chen, Xiujun Tian, and Yan Wen at BTMRI and support of the Qiubei Skin Disease Control Station.
We acknowledge NIH/NIAID grants NO1 AI-25469, RO3-AI-59644, and RO1-AI-63457.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Arricau-Bouvery, N., Y. Hauck, A. Bejaoui, D. Frangoulidis, C. C. Bodier, A. Souriau, H. Meyer, H. Neubauer, A. Rodolakis, and G. Vergnaud. 2006. Molecular characterization of Coxiella burnetii isolates by infrequent restriction site-PCR and MLVA typing. BMC Microbiol. 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honoré, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M.-A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 3.Danin-Poleg, Y., L. A. Cohen, H. Gancz, Y. Y. Broza, H. Goldshmidt, E. Malul, L. Valinsky, L. Lerner, M. Broza, and Y. Kashi. 2007. Vibrio cholerae strain typing and phylogeny study based on simple sequence repeats. J. Clin. Microbiol. 45:736-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckert, K. A., and G. Yan. 2000. Mutational analyses of dinucleotide and tetranucleotide microsatellites in Escherichia coli: influence of sequence on expansion mutagenesis. Nucleic Acids Res. 28:2831-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groathouse, N. A., B. Rivoire, H. Kim, H. Lee, S.-N. Cho, P. J. Brennan, and V. D. Vissa. 2004. Multiple polymorphic loci for molecular typing of Mycobacterium leprae. J. Clin. Microbiol. 42:1666-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huan-Ying, L., R. Shun-Peng, and Y. Rong-De. 2002. Leprosy control in a prefecture of Yunnan Province in the Peoples' Republic of China, using intensive health education of the public and primary health care workers for the detection of cases, 1998-1999. Lepr. Rev. 73:84-87. [PubMed] [Google Scholar]
- 7.Keim, P., M. N. Van Ert, T. Pearson, A. J. Vogler, L. Y. Huynh, and D. M. Wagner. 2004. Anthrax molecular epidemiology and forensics: using the appropriate marker for different evolutionary scales. Infect. Genet. Evol. 4:205-213. [DOI] [PubMed] [Google Scholar]
- 8.Malachowa, N., A. Sabat, M. Gniadkowski, J. Krzyszton-Russjan, J. Empel, J. Miedzobrodzki, K. Kosowska-Shick, P. C. Appelbaum, and W. Hryniewicz. 2005. Comparison of multiple-locus variable-number tandem-repeat analysis with pulsed-field gel electrophoresis, spa typing, and multilocus sequence typing for clonal characterization of Staphylococcus aureus isolates. J. Clin. Microbiol. 43:3095-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh, J. W., M. M. O'Leary, K. A. Shutt, A. W. Pasculle, S. Johnson, D. N. Gerding, C. A. Muto, and L. H. Harrison. 2006. Multilocus variable-number tandem-repeat analysis for investigation of Clostridium difficile transmission in hospitals. J. Clin. Microbiol. 44:2558-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka, M., L. Zhang, T. Budiawan, K. Saeki, and S. Izumi. 2004. Genotyping of Mycobacterium leprae on the basis of the polymorphism of TTC repeats for analysis of leprosy transmission. J. Clin. Microbiol. 42:741-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monot, M., N. Honore, T. Garnier, R. Araoz, J. Y. Coppee, C. Lacroix, S. Sow, J. S. Spencer, R. W. Truman, D. L. Williams, R. Gelber, M. Virmond, B. Flageul, S. N. Cho, B. Ji, A. Paniz-Mondolfi, J. Convit, S. Young, P. E. Fine, V. Rasolofo, P. J. Brennan, and S. T. Cole. 2005. On the origin of leprosy. Science 308:1040-1042. [DOI] [PubMed] [Google Scholar]
- 12.Noller, A. C., M. C. McEllistrem, K. A. Shutt, and L. H. Harrison. 2006. Locus-specific mutational events in a multilocus variable-number tandem repeat analysis of Escherichia coli O157:H7. J. Clin. Microbiol. 44:374-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oelemann, M. C., R. Diel, V. Vatin, W. Haas, S. Rüsch-Gerdes, C. Locht, S. Niemann, and P. Supply. 2006. Assessment of an optimized mycobacterial interspersed repetitive-unit-variable-number tandem-repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J. Clin. Microbiol. 45:691-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridley, R. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity—a five group system. Int. J. Lepr. 34:225-273. [PubMed] [Google Scholar]
- 15.Savine, E., R. M. Warren, G. D. van der Spuy, N. Beyers, P. D. van Helden, C. Locht, and P. Supply. 2002. Stability of variable-number tandem repeats of mycobacterial interspersed repetitive units from 12 loci in serial isolates of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:4561-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schouls, L. M., A. van der Ende, M. Damen, and I. van de Pol. 2006. Multiple-locus variable-number tandem repeat analysis of Neisseria meningitides yields groupings similar to those obtained by multilocus sequence typing. J. Clin. Microbiol. 44:1509-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swofford, D. 1999. PAUP: phylogenetic analysis using parsimony (and other methods), version 4.0 beta21. Sinauer, Sunderland, MA.
- 18.Truman, R., A. B. Fontes, A. B. de Miranda, P. Suffys, and T. Gillis. 2004. Genotypic variation and stability of four variable-number tandem repeats and their suitability for discriminating strains of Mycobacterium leprae. J. Clin. Microbiol. 42:2558-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogler, A. J., C. E. Keys, C. Allender, I. Bailey, J. Girard, T. Pearson, K. L. Smith, D. M. Wagner, and P. Keim. 2007. Mutations, mutation rates, and evolution at the hypervariable VNTR loci of Yersinia pestis. Mutat. Res. -158612:145. [DOI] [PubMed] [Google Scholar]
- 20.Vogler, A. J., C. Keys, Y. Nemoto, R. E. Colman, Z. Jay, and P. Keim. 2006. Effect of repeat copy number on variable-number tandem repeat mutations in Escherichia coli O157:H7. J. Bacteriol. 188:4253-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng, X. M., S. Y. Chen, S. P. Ran, C. H. Zhang, and H. Y. Li. 2000. Immuno-histopathology in the diagnosis of early leprosy. Int. J. Lepr. Other Mycobact. Dis. 68:426-433. [PubMed] [Google Scholar]
- 22.Weng, X. M., Y. Wen, X. J. Tian, H. B. Wang, X. J. Tan, and H. Y. Li. 2006. Preliminary VNTR genotyping on 50 M. leprae isolates in China. J. Chin. Epidemiol. 27:402-405. [PubMed] [Google Scholar]
- 23.World Health Organization. 2002. Leprosy. Global situation. Wkly. Epidemiol. Rec. -877:1. [PubMed] [Google Scholar]
- 24.World Health Organization. 2006. Global leprosy situation, 2006. Wkly. Epidemiol. Rec. 81:309-316. [PubMed] [Google Scholar]
- 25.Young, S. K., G. M. Taylor, S. Jain, L. M. Suneetha, S. Suneetha, D. N. Lockwood, and D. B. Young. 2004. Microsatellite mapping of Mycobacterium leprae populations in infected humans. J. Clin. Microbiol. 42:4931-4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, L., T. Budiawan, and M. Matsuoka. 2005. Diversity of potential short tandem repeats in Mycobacterium leprae and application for molecular typing. J. Clin. Microbiol. 43:5221-5229. [DOI] [PMC free article] [PubMed] [Google Scholar]