Abstract
This cost minimization analysis investigated the financial impact of the treatment of fungemias due to Candida glabrata from a hospital perspective using three competing alternatives: (i) performing in-house susceptibility testing on all C. glabrata isolates and changing patients to less expensive fluconazole therapy for isolates that test susceptible; (ii) susceptibility testing at outside laboratories with delayed deescalation to fluconazole if isolates test susceptible; and (iii) no routine susceptibility testing with full echinocandin treatment course. Sensitivity analyses and Monte Carlo simulation enhanced the robustness of the model through variation of all assumptions and costs. In the base case, the use of in-house testing displayed a cost advantage over the options of send-out testing and no susceptibility testing ($2,226 versus $2,410 versus $3,136, respectively). Sensitivity analyses determined that the cost of echinocandin therapy and the turnaround time for send-out testing had the potential to impact the base case model. The decision model indicated that in-house susceptibility testing of C. glabrata isolates should result in lower overall treatment costs in patients with documented C. glabrata fungemias.
Antimicrobial susceptibility testing is an integral tool in the management of bacterial infections, and it is utilized by clinicians to help determine the optimal drug regimens for patients. Regarding antifungal susceptibility testing, several institutions have reported on their experience with testing and revealed that these data are both appreciated by clinicians and utilized to change therapy (4, 10). However, institution-based antifungal susceptibility testing remains a relatively new concept which has not yet been widely incorporated into many hospital microbiology laboratories. Interestingly, the number of laboratories that perform antifungal susceptibility testing is increasing, from 50 in 1997 to over 100 today (22). This increase in testing has been spurred mainly by two progressive trends.
First, prior to the azole antifungal agents, amphotericin B was the only reliable agent available to treat systemic infection. As such, susceptibility testing would have offered no true benefit to clinicians. However, since that time the azoles and triazoles have been introduced, expanding the antifungal armamentarium. Most recently, the echinocandins, displaying superb activity against most Candida spp., have come into routine use (6). This increased use is not without a financial impact on health systems, as echinocandins have a substantially greater acquisition cost than fluconazole.
Second, a shift has taken place in the composition of Candida spp. causing infections over the past decade. Infections due to Candida spp. other than C. albicans have increased and now comprise approximately 50% of all Candida bloodstream isolates (4). Some of these non-C. albicans isolates, such as Candida parapsilosis and C. tropicalis, are traditionally very sensitive to fluconazole. Candida krusei, however, is intrinsically resistant to fluconazole. Isolation of Candida glabrata, which comprises about 20% of all Candida isolates in the United States, presents a conundrum to clinicians, since the organism displays a variable susceptibility pattern to fluconazole (23). Table 1 showcases the institution-to-institution variability in both incidence of and fluconazole resistance to C. glabrata. Also of interest is the predilection for fluconazole-resistant C. glabrata to display cross-resistance to other azole antifungals (23). As such, many experts now recommend that patients with invasive infection due to C. glabrata be empirically treated with agents other than the azoles. The utility of susceptibility testing would be to allow for appropriate deescalation in therapy once results are known (13, 21). The financial impact of deescalating echinocandin therapy is not without precedence. Other studies have investigated the cost savings by using peptide nucleic acid fluorescence in situ hybridization to rapidly identify C. albicans in order to decrease echinocandin use (1, 8).
TABLE 1.
C. glabrata fungemias in the United States
| Site (reference) | No. of isolates | % of all candidemias | Time period | % Sa | % S-DDb | % Rc |
|---|---|---|---|---|---|---|
| University of Michigan (16) | 103 | 17 | 1995-2002 | NAf | NA | 60 |
| MD Anderson (2) | 34 | 24 | 1998-2001 | 59 | 24 | 17 |
| Johns Hopkins (15) | 52 | 34 | June 2003-July 2004d | 57 | 25 | 18 |
| North America (23) | 331 | 20e | During 2001 and 2002 | 64 | 26 | 10 |
S, susceptible. MIC ≤ 8 mg/liter.
S-DD, susceptible-dose dependent. MIC, 16 to 32 mg/liter.
R, resistant. MIC ≥ 64 mg/liter.
Excludes August and September 2003.
Global assessment program; collected isolates from all types of invasive candidiasis.
NA, not applicable.
Given the above developments, antifungal susceptibility testing can be of utility to practitioners. Microbiology laboratories have the option of performing susceptibility analysis internally or sending isolates to outside facilities for testing. The cost, turnaround time, and required labor may differ greatly between the two. We have undertaken a cost minimization analysis to investigate the financial impact of alternative susceptibility testing strategies for patients with C. glabrata blood isolates.
MATERIALS AND METHODS
A decision analytic model was developed from a hospital perspective based on three testing and treatment options for patients with documented C. glabrata fungemias. The three competing alternatives investigated are as follows: (i) performing in-house susceptibility testing on all C. glabrata isolates and changing patients to less-expensive fluconazole therapy for isolates that test susceptible; (ii) susceptibility testing at outside laboratories with delayed deescalation to fluconazole if susceptible; and (iii) no routine susceptibility testing with full echinocandin treatment course.
The decision tree was created using decision analysis software (DATA; TreeAge, Inc.). Data collected from literature reports, reference material, and expert opinion were used to populate the model (Table 2). Medication acquisition cost was added to labor cost and the price of testing to determine overall treatment cost. Univariate and multivariate sensitivity analysis (Monte Carlo simulation) enhanced the robustness of the model through variation of all probabilities and costs that populated the model.
TABLE 2.
Decision analytic model variables
| Model variable (reference) | Value for base case | Sensitivity range
|
|
|---|---|---|---|
| Low | High | ||
| Medication cost/day | |||
| Echinocandin | |||
| AWP (2) | $224 | 0 | $224 |
| FSS (27) | $118 | 0 | $224 |
| Fluconazole | |||
| AWP (2) | $36 | 0 | $72 |
| FSS (27) | $16 | 0 | $72 |
| Labor cost | |||
| In-house (26) | $34 | 0 | $68 |
| Send-out (26) | $5 | 0 | $10 |
| Susceptibility testing cost | |||
| Institutionala | $71 | $36 | $107 |
| Send-outa,b | $59 | 0 | $301 |
| Turn-around time (days) | |||
| In-housea | 5 | 2 | 6 |
| Send-outa,b | 7 | 4 | 11 |
| Treatment days | 14 | 6 | 28 |
| C. glabrata susceptibility to fluconazole (1, 15, 23) | 60% | 25% | 90% |
Internal estimates.
Data taken from http://strl.uthscsa.edu/fungus/ (accessed 7 January 2007).
The base case model made the following assumptions: (i) echinocandin treatment was initiated when yeast was detected on Gram stain; (ii) laboratories had the ability to identify Candida isolates to the species level; (iii) identification of C. glabrata to the species level would take 2 days and in-house susceptibility testing or send-out would begin immediately following definitive identification; and (iv) if isolates tested susceptible, patients were switched to fluconazole to complete the 14-day course. C. glabrata isolates for which the fluconazole MIC was ≤8 mg/liter as tested by broth microdilution according to CLSI standards were considered susceptible to standard doses of fluconazole (7). A mean susceptibility rate of 60% was obtained from literature reports and was used in the base case. Susceptibility rates were varied between 90% and 25% (3, 15, 16, 23).
Realizing that not all institutions begin echinocandin therapy after identification of yeast isolates, a second model was developed under the assumption that patients would receive 2 days of fluconazole after identification of yeast by Gram staining. Patients were then changed to an echinocandin on day 3 after identification of C. glabrata. Depending on susceptibility of the isolate, patients received either 14 days (fluconazole to echinocandin and then back to fluconazole) or 16 days (fluconazole to echinocandin) of total therapy.
Institution and send-out turnaround times for completion of susceptibility testing after identifying yeast on Gram stain was estimated at 5 and 7 days, respectively. In order to investigate the financial effects of early discharges and longer treatment courses of other disease states, we varied the total treatment duration between 4 and 28 days in the sensitivity analysis.
The base case utilized the average wholesale price (AWP) or Federal Supply Schedule (FSS) price of fluconazole and micafungin (2, 27). Recent trials have demonstrated that all three echinocandins (anidulafungin, caspofungin, and micafungin) are effective in the treatment of candidemia (5, 24, 25). Micafungin was used in this review because it had the lowest listed echinocandin price (2). A daily dose of 400 mg intravenous (i.v.) fluconazole was chosen based on guideline recommendations (21). The impact of dosing fluconazole at 800 mg i.v. daily was considered in the sensitivity analyses. For micafungin, a daily dose of 100 mg was based on a recent, double-blind, noninferiority trial (25). Medication cost was varied between $0 and AWP in the sensitivity analyses.
The cost of in-house susceptibility testing was determined by adding the cost of commercially prepared broth microdilution plates (YeastOne; Trek Diagnostics, Cleveland, OH), inoculation broth, and demineralized water. A cost per test of $71 was used in the base case and varied by ±50% in the sensitivity analysis. The cost of labor for in-house susceptibility testing was determined by summing the times to set up, read, and enter results of testing into the computer system. Total testing time was estimated at 99 min. Laboratory technologist hourly wage was obtained from the 2005 U.S. Bureau of Labor Statistics and valued at $18.90 per hour for a total cost of $34 for in-house testing (26).
The cost of commercially available send-out testing used in the base case was $59 and was obtained from the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio (http://strl.uthscsa.edu/fungus/; accessed 7 January 2007). Labor time was estimated at 15 min to subculture and complete paperwork, totaling $5 in labor cost. The cost of labor ranged between $0 and twice the estimated cost in the sensitivity analysis.
RESULTS
Model results.
The results of the decision analytic model are displayed in Table 3. In the base case, internal susceptibility testing displayed the lowest overall treatment cost. Treatment cost was $2,226 when susceptibility was performed in-house, $2,410 with send-out testing, and $3,136 for no testing whatsoever. This resulted in a mean cost difference of $184 for in-house testing over send-out testing and a $910 advantage over no testing.
TABLE 3.
Summary of model results
| Order of administration and pricing (reference) | Treatment cost ($)
|
Difference ($; institutional advantage over no testing) | ||
|---|---|---|---|---|
| Institution testing | Send-out testing | No testing | ||
| Echinocandin initially | ||||
| AWP pricing (2) | 2,226 | 2,410 | 3,136 | 910 |
| FSS pricing (27) | 1,206 | 1,288 | 1,652 | 446 |
| Fluconazole initially | ||||
| AWP pricing (2) | 1,916 | 2,214 | 3,208 | 1,292 |
| FSS pricing (27) | 1,035 | 1,178 | 1,684 | 649 |
Sensitivity analyses.
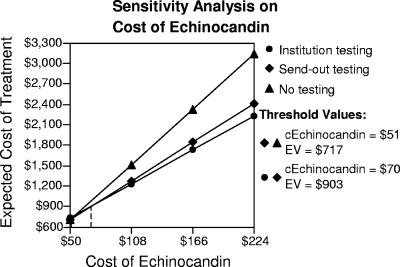
Univariate sensitivity analyses determined that the cost of echinocandin therapy and the turn-around time for send-out testing had the potential to impact the base case model. The sensitivity analysis of the cost of echinocandin therapy is displayed in Fig. 1. The echinocandin break-even costs per day for institutional testing over send-out testing and no testing at all were $70 and $51, respectively. Susceptibility results in the send-out group would need to be reported in 5.4 days or fewer after identification of yeast from the blood sample for send-out testing to provide the same economic benefit as in-house testing. The Monte Carlo simulation evaluated all variables over 10,000 iterations of our model. The simulation determined in-house susceptibility testing to be the optimal path in 60% of cases.
FIG. 1.
Sensitivity analysis of the daily cost of echinocandin therapy. Send-out testing becomes the preferred option with a daily echinocandin cost (cEchinocandin) of ≤$70. Any form of susceptibility testing loses the financial advantage with an echinocandin cost of ≤$51 per day. EV, expected treatment cost.
The results of our model were not altered with higher doses of fluconazole. When an 800-mg daily dose of fluconazole was used and the rate of susceptibility to fluconazole was changed to 85%, the cost was $2,078 for institutional testing versus $2,296 for send-out and $3,136 for no testing. Our model also showed that it was advantageous for the hospital to test patients discharged in less than 14 days to complete their treatment courses at home. Testing was financially advantageous if the patient received more than 6 days of therapy while in the hospital.
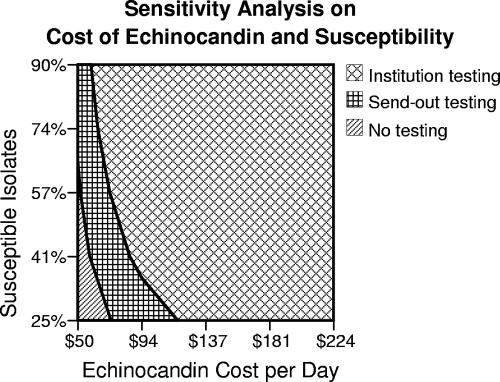
A two-way sensitivity analysis was performed on the cost of echinocandin therapy and rates of susceptibility to fluconazole (Fig. 2). Susceptibility testing, whether in-house or sent out, maintained an advantage over no testing in a majority of situations. The difference in institutional testing versus send-out testing was variable depending on the cost of echinocandin and the rates of susceptible agents.
FIG. 2.
Two-way sensitivity analysis on the cost of echinocandin therapy and varying susceptibility rates to fluconazole.
The analysis using FSS costs ($118/day for echinocandin and $16/day for fluconazole) favored in-house testing, though differences were less pronounced. The total treatment costs were $1,206, $1,288, and $1,652 for the in-house, send-out, and no-testing arms, respectively. Again, the cost of echinocandin therapy and turnaround time for send-out testing had the potential to impact the analysis. The break-even points for cost of echinocandin therapy and turnaround time were $32 per day and 5.7 days in this second analysis.
The difference in treatment cost between institutional testing and no testing was greatest when fluconazole was initiated after identification of yeast by Gram staining and prior to identification of C. glabrata ($1,292). The total costs of therapy were $1,916, $2,214, and $3,208 for the groups using in-house, send-out, and no testing, respectively. The use of FSS pricing and fluconazole initially resulted in the lowest treatment cost in all models for institutional testing ($1,035).
DISCUSSION
The primary objective of this study was to investigate the financial impact of three testing and treatment options in a select group of patients. We performed a cost minimization analysis where fluconazole and echinocandin were considered equivalent in terms of clinical efficacy and side effect profiles for the treatment of candidemias. The results of our model indicate a cost advantage in performing susceptibility testing, either internally or by sending cultures to an outside laboratory, over not testing at all. The model also indicates a small advantage for performing susceptibility tests internally versus send-out testing. We report a $910 savings in our base case with institutional testing over no testing. Knowing that institutions have reported identifying up to 52 isolates annually, this could lead to substantial health care savings depending on the incidence of C. glabrata fungemias in a particular institution (15). These results are particularly important in today's cost-conscious health system environment, given the high cost of antifungal therapy and emerging patterns of resistant organisms.
One limitation was the use of AWP in the base case. The daily cost of both fluconazole and echinocandin is likely lower than AWP at most institutions. The cost of echinocandin therapy declined in 2006 after the introduction of competing class alternatives. Despite this, the acquisition price of echinocandins remains high. The choice of agent and contract price may vary depending on the institution. We feel the sensitivity analyses enhance the generalizability of this study. Results displayed in Fig. 2 and the results of the analysis using FSS pricing make it possible for individual institutions to predict the best alternative based on echinocandin pricing structure and their own susceptibility patterns. At the same time, some clinicians may feel more comfortable using higher dosing of fluconazole. When we used an 800-mg daily dose of i.v. fluconazole, results still favored testing.
As with the cost of medication therapy, the cost of competing testing methods, susceptibility turnaround time, time and ability to deescalate therapy once testing results are known, and cost of labor may be highly variable depending on the institutional laboratory or send-out facility. Changes in the cost of testing and labor did not impact the results of our model, and the turnaround time of send-out laboratories must be very near to that of an internal laboratory to display a cost advantage. Institution susceptibility testing was preferred in most scenarios. Of interest, the availability of fluconazole disks for susceptibility testing by disk diffusion will result in additional cost reductions for susceptibility testing and will enhance the benefit of performing this testing in-house as applied in this model.
We solely utilized the treatment of candidemia caused by C. glabrata in the base case of our analysis. This was chosen because of the standard treatment duration of 14 days after a positive blood culture (21). However, we believe our results may translate to other infections requiring longer durations of therapy. Our findings support the idea that deescalation of therapy from an echinocandin to fluconazole would provide an even more pronounced economic benefit when treatment continues for weeks or months. When total treatment time was increased to 28 days, the difference between testing and no testing was $2,489 per patient.
Although our study focused on the financial incentives of antifungal susceptibility testing, it is even more important to review the clinical implications. A crucial question is whether in vitro susceptibility testing affects clinical outcomes. If it does not, then most clinicians would find it to be of little utility in practice. Logically, one would think that clinical outcomes would correlate well with the results of in vitro susceptibilities. Surprisingly, the literature does not always support this logic as it relates to antifungal susceptibility testing. Indeed, several studies have found that the acute physiology and chronic health evaluation score at the time of fungemia, not in vitro susceptibility test results, was the most important factor in predicting mortality (16). The reason for this discrepancy more likely reflects the severely ill condition of most patients with fungemia rather than refuting the validity of susceptibility testing (16). Regarding the echinocandins, one study showed that patients with Candida isolates having higher caspofungin MICs (>2 μg/ml) had superior clinical outcomes compared to those with isolates displaying caspofungin MICs of <1 μg/ml (14). However, since only three patients treated with caspofungin were infected with isolates with MICs of >2 μg/ml, this study does not supply enough data to make any conclusions regarding the effect of elevated echinocandin MICs on outcome. Conversely, a recent review by Pfaller and colleagues provides ample evidence that fluconazole MIC does correlate clinically. Analysis of MIC correlation to clinical outcome in studies of patients with mucosal and invasive candidiasis showed that higher doses (meaning a dose/MIC ratio of >25:1) are more often associated with successful outcomes than are lower doses (22). In addition, a recent study found that the fluconazole dose/MIC ratio was significantly higher in survivors of candidemia (13.3 ± 10.5) than in nonsurvivors (7.0 ± 8.0) (20). As such, fluconazole susceptibility testing provides crucial information that can be utilized to optimize both clinical and financial outcomes.
Another potential clinical advantage of susceptibility testing (especially when done in-house) is in facilitating quicker interventions. In the base case model of our study, echinocandin treatment was initiated when yeast was detected on Gram stain. The decision to initiate fluconazole or an echinocandin empirically is a source of controversy and depends largely on local susceptibility patterns. Recent literature showing a correlation between time to initiation of appropriate antifungal therapy and in-hospital mortality due to candidemia has exemplified the importance of this decision (9, 18). More rapid susceptibility results would shorten the time to make appropriate therapy adjustments in the case of inappropriate empirical therapy.
This study supports the assumption that antifungal susceptibility testing is a necessity in today's world of resistant organisms and expensive agents. As such, institutions are left with two options: in-house testing and send-out testing. Both methods increase laboratory costs while decreasing pharmacy costs to a greater extent. Instituting this practice into hospital microbiology laboratories, which are often already financially constrained, is a practical barrier that must be addressed.
Conclusion.
The decision model indicates that susceptibility testing of C. glabrata isolates should result in lower overall treatment costs for patients with documented C. glabrata fungemias.
Acknowledgments
We report no conflicts of interest.
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Alexander, B. D., E. D. Ashley, L. B. Reller, and S. D. Reed. 2005. Cost savings with implementation of PNA FISH testing for identification of Candida albicans in blood cultures. Diagn. Microbiol. Infect. Dis. 54:277-282. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2006. Drug topics red book 2006. Medical Economics Co., Montvale, NJ.
- 3.Antoniadou, A., H. A. Torres, R. E. Lewis, J. Thornby, G. P. Bodey, J. J. Tarrand, X. Han, K. V. I. Rolston, A. Safdar, I. I. Raad, and D. P. Kontoyiannis. 2003. Candidemia in a tertiary care center. In vitro susceptibility and its association with outcome of initial antifungal therapy. Medicine 82:309-321. [DOI] [PubMed] [Google Scholar]
- 4.Baddley, J. W., M. Patel, M. Jones, G. Cloud, A. C. Smith, and S. A. Moser. 2004. Utility of real-time antifungal susceptibility testing for fluconazole in the treatment of candidemia. Diagn. Microbiol. Infect. Dis. 50:119-124. [DOI] [PubMed] [Google Scholar]
- 5.Betts, R. F., C. Rotstein, D. Talwar, M. Nucci, J. Dewaele, L. Arnold, L. Kovanda, C. Wu, and D. Buell. 2006. Comparison of micafungin and caspofungin for candidemia or invasive candidiasis, abstr. M-1308a. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 6.Boucher, H. W., A. H. Groll, C. C. Chiou, and T. J. Walsh. 2004. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs 64:1997-2020. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard second edition. Approved standard M27-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Forrest, G. N., K. Mankes, M. A. Jabra-Rizk, E. Weekes, J. K. Johnson, D. P. Lincalis, and R. A. Venezia. 2006. Peptide nucleic acid fluorescence in situ hybridization-based identification of Candida albicans and its impact on mortality and antifungal therapy costs. J. Clin. Microbiol. 44:3381-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garey, K. W., M. Rege, M. P. Pai, D. E. Mingo, K. J. Suda, R. S. Turpin, and D. T. Bearden. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25-31. [DOI] [PubMed] [Google Scholar]
- 10.Hadley, S., J. A. Martinez, L. McDermott, B. Rapino, and D. R. Snydman. 2002. Real-time antifungal susceptibility screening aids management of invasive yeast infections in immunocompromised patients. J. Antimicrob. Chemother. 49:415-419. [DOI] [PubMed] [Google Scholar]
- 11.Reference deleted.
- 12.Reference deleted.
- 13.Hospenthal, D. R., C. K. Murray, and M. G. Rinaldi. 2004. The role of antifungal susceptibility testing in the therapy of candidiasis. Diagn. Microbiol. Infect. Dis. 48:153-160. [DOI] [PubMed] [Google Scholar]
- 14.Kartsonis, N., J. Killar, L. Mixson, C. M. Hoe, C. Sable, K. Bartizal, and M. Motyl. 2005. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob. Agents Chemother. 49:3616-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magill, S. S., C. Shields, C. L. Sears, M. Choti, and W. G. Merz. 2006. Triazole cross-resistance among Candida spp.: case report, occurrence among bloodstream isolates, and implications for antifungal therapy. J. Clin. Microbiol. 44:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malani, A., J. Hmoud, L. Chiu, P. L. Carver, A. Bielacyzc, and C. A. Kauffman. 2005. Candida glabrata fungemia: experience in a tertiary care center. Clin. Infect. Dis. 41:975-981. [DOI] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Morrell, M., V. J. Fraser, and M. H. Kollef. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.Pai, M. P., R. S. Turpin, and K. W. Garey. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 51:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for the treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., D. J. Diekama, and D. J. Sheehan. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19:435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS antifungal surveillance program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reboli, A., C. Rotstein, P. Pappas, J. Schranz, D. Krause, and T. Walsh. 2005. Anidulafungin versus fluconazole for treatment of candidemia and invasive candidiasis (c/ic), p. 418, abstr. M-718. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 25.Ruhnke, M., E. Kuse, P. Chetchotisakd, C. Arns Da Cunha, and H. Diekmann-Berndt. 2005. Comparison of micafungin and liposomal amphotericin B for invasive candidiasis, abstr. M-722c. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 26.U.S. Department of Labor. Accessed 20 November 2006. National compensation survey: occupational wages in the United States, July 2005. http://www.bls.gov/ocs/.
- 27.U.S. Department of Veterans Affairs, Pharmacy Benefits Management Strategic Healthcare Group. Accessed 20 November 2006. Drug and pharmaceutical prices. http://www.pbm.va.gov/PBM/prices.htm.