Free-living amebae of the genus Acanthamoeba are causal agents of granulomatous amebic encephalitis in humans, almost exclusively in immunocompromised hosts. The prognosis is poor, and the mortality is high. Because the symptoms are nonspecific, diagnosis can be difficult and is often made postmortem. We previously reported a 70-year-old female with systemic lupus erythematosus who was diagnosed postmortem with Acanthamoeba encephalitis following hematoxylin-eosin staining, immunostaining, and peroxidase staining of brain tissue sections obtained at autopsy (1). As further confirmation, the ameba was isolated from a sample of unfixed brain tissue and identified as Acanthamoeba polyphaga (1). In this letter, we describe the application of PCR to samples of the patient's cerebrospinal fluid (CSF), brain tissue, and serum to evaluate its effectiveness as a diagnostic tool.
DNA was extracted from the patient's brain and from centrifuged sequential CSF and serum samples obtained by lumbar puncture over a 2-month period (June to August 2003) as previously described (7). The following primer set, which was used to identify Acanthamoeba mitochondrial 16S rRNA gene DNA, gave an amplicon of 161 bp (3): Aca16Sf1010 (5′-TTATATTGACTTGTACAGGTGCT-3′) and Aca16Sr1180 (5′-CATAATGATTTGACTTCTTCTCCT-3′).
Positive controls consisted of DNA extracted from two strains of Acanthamoeba polyphaga: one strain isolated from the patient's brain tissue and a second isolated from a corneal biopsy sample from a patient with Acanthamoeba keratitis (CDC:V029). Water (Sigma) was used as a negative control.
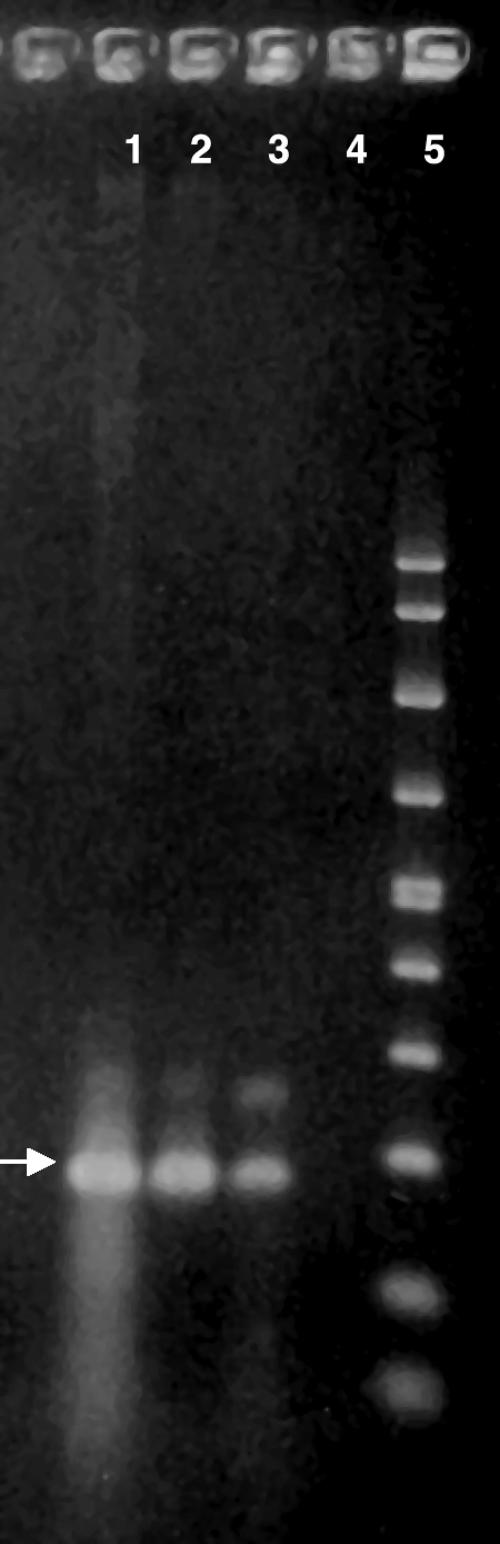
A band at ∼161 bp for Acanthamoeba mitochondrial DNA was seen in unfixed brain tissue (Fig. 1, lane 1) and in sections of the patient's formalin-fixed brain tissue (Fig. 1, lane 3).
FIG. 1.
PCR gel showing DNA extracts from brain tissue and Acanthamoeba controls, amplified with a 161-bp primer set for Acanthamoeba spp. An arrow indicates 161 bp. Lane 1, DNA sample extracted from unfixed brain tissue of the patient; lane 2, DNA extract from A. polyphaga (strain CDC:V029); lane 3, DNA extract from A. polyphaga isolated from the patient's brain tissue; lane 4, negative control, pure water (Sigma); lane 5, molecular size marker.
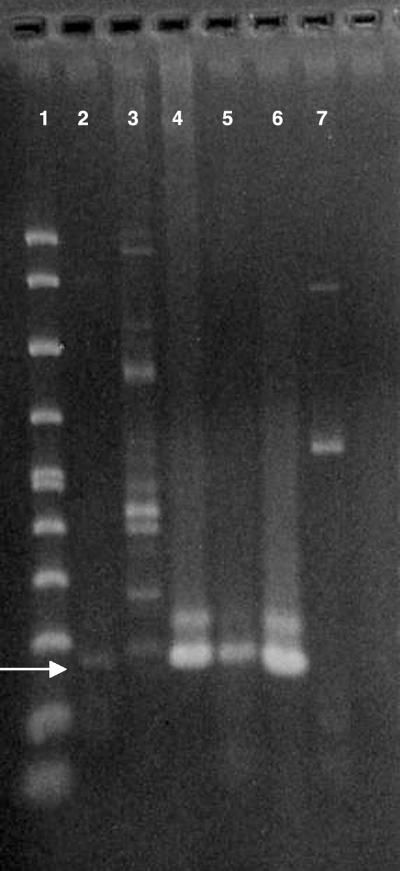
DNA bands were also seen in all CSF samples tested. The earliest samples (Fig. 2, lanes 1 and 2) produced lighter bands than the later samples. Lane 2 contains multiple bands, perhaps from DNA of host cells present in the sample. Ameba DNA in the CSF may be the result of extensive necrosis of brain tissue and release of intact amebae or of DNA from lysed amebae.
FIG. 2.
PCR gel with DNA extracted from centrifuged CSF samples over a 2-month period, amplified with a 161-bp primer set for Acanthamoeba. An arrow indicates 161 bp. Lane 1, molecular size marker; lane 2, CSF sample (June 2003) showing a weak DNA band; lane 3, CSF sample from ∼10 days later also showing a weak DNA band, along with multiple bands, probably from leukocytes present in the sample; lane 4, CSF sample from ∼3 weeks later; lane 5, CSF sample from ∼4 weeks later (August) at the time of the patient's death; lane 6, Acanthamoeba polyphaga, positive control; lane 7, negative control, pure water (Sigma).
Two samples of patient serum, one acute phase and the second obtained shortly before the patient's death, were also tested for ameba DNA (data not included). Neither sample, however, showed evidence of a band. CSF from ∼20 other encephalitis patients negative for amebic encephalitis (based on immunofluorescent antibody staining of sera), showed no evidence of a band comparable to Acanthamoeba DNA (data not included).
In the present case, the etiologic ameba had been isolated from unfixed brain tissue (Fig. 1, lane 3). With some exceptions, amebae are not seen in or isolated or identified from CSF (2, 4-6). Attempts at culturing amebae from the patient's CSF were not successful (1), suggesting that intact organisms were not present in the CSF samples(s) or were present in such low numbers that they were missed in sampling.
This is the first report of detection of Acanthamoeba DNA in the CSF of an acanthamoebiasis patient. The data presented in this report suggest that PCR of CSF samples may have made premortem diagnosis and treatment possible.
Acknowledgments
The research was supported in part by a grant from the Emerging Infectious Diseases Program of the Centers for Disease Control and Prevention (U50/CCU915548-09) to Carol A. Glaser of the California Department of Health Services, Richmond, CA, whom we thank for her interest and encouragement of this research.
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Bloch, K. C., and F. L. Schuster. 2005. Inability to make a premortem diagnosis of Acanthamoeba species infection in a patient with fatal granulomatous amebic encephalitis. J. Clin. Microbiol. 43:3003-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callicott, J. H., E. C. Nelson, M. M. Jones, J. G. dos Santos, J. P. Utz, R. J. Duma, and J. V. Morrison, Jr. 1968. Meningoencephalitis due to pathogenic free-living amoebae. Report of two cases. JAMA 206:579-582. [PubMed] [Google Scholar]
- 3.Foreman, O., J. Sykes, L. Ball, N. Yang, and H. De Cock. 2004. Disseminated infection with Balamuthia mandrillaris in a dog. Vet. Pathol. 41:506-510. [DOI] [PubMed] [Google Scholar]
- 4.Petry, F., M. Torzewski, J. Bohl, T. Wilhelm-Schwenkmezger, P. Scheid, J. Walochnik, R. Michel, L. Zöller, K. J. Werhahn, S. Bhakdi, and K. J. Lackner. 2006. Early diagnosis of Acanthamoeba infection during routine cytological examination of cerebrospinal fluid. J. Clin. Microbiol. 44:1903-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma, P. P., P. Gupta, M. V. Murali, and V. G. Ramachandran. 1993. Primary amebic meningoencephalitis caused by Acanthamoeba: successfully treated with cotrimoxazole. Indian Pediatr. 30:1219-1222. [PubMed] [Google Scholar]
- 6.Singhal, T., A. Bajpai, V. Kalra, S. K. Kabra, J. Samantary, G. Satpathy, and A. K. Gupta. 2001. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr. Infect. Dis. J. 20:623-627. [DOI] [PubMed] [Google Scholar]
- 7.Yagi, S., G. C. Booton, G. S. Visvesvara, and F. L. Schuster. 2005. Detection of Balamuthia mitochondrial 16S rRNA gene DNA in clinical specimens by PCR. J. Clin. Microbiol. 43:3192-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]