Abstract
Rare cases of leukoencephalitis have been reported in infants with documented enterovirus (EV) central nervous system (CNS) infections. A case of fatal encephalitis with white matter lesions caused by echovirus 18 is described, and it highlights the role of EV CNS infection as a potential cause of leukoencephalitis in infants.
CASE REPORT
The patient described herein was an 18-month-old male infant who was the eldest child in a three-child family and had no particular medical history. Twelve hours before his hospital admission, his parents reported fever, vomiting, and a general tonic and clonic seizure. On hospital admission, he was afebrile (36.5°C), conscious, feverish, and without any particular symptoms on physical and neurological examination. Lumbar puncture revealed the presence of 184 white cells per μl of cerebrospinal fluid (CSF) with 98% lymphocytes. CSF protein and glucose concentrations were 2.08 g/liter and 3.77 mmol/liter, respectively, and the blood concentration was 5.74 mmol/liter. No bacterial counts were observed after classical staining of CSF. Moreover, electroencephalogram findings revealed diffuse and excessive slow-wave activity. Initial treatment included ceftriaxone (100 mg/kg/day), amoxicillin (100 mg/kg/day), and acyclovir (250 mg/m2). Following a new episode of febrile general seizure, the infant was transferred to the pediatric department of the regional university hospital (Champagne Ardenne, France). On admission, his child-modified Glasgow score was 13/15, with eyes opening in response to a voice and consolable crying; he was afebrile, tachycardic, and normotensive. A second lumbar puncture revealed the presence of 81 white cells per μl of CSF with 100% mononuclear cells. CSF protein and glucose concentrations were 3.2 g/liter and 3.1 mmol/liter, respectively, associated with an alpha interferon (IFN-α) level of 12 IU/ml (the IFN-α level was <2 IU/ml of blood plasma). Three days after the beginning of febrile illness, the Glasgow score remained the same (13/15) and was not associated with high levels of C-reactive protein (<3 mg/liter). Classical biochemical parameters reflecting metabolic disorders were examined and found to be normal, indicating the absence of an inborn metabolic disorder.
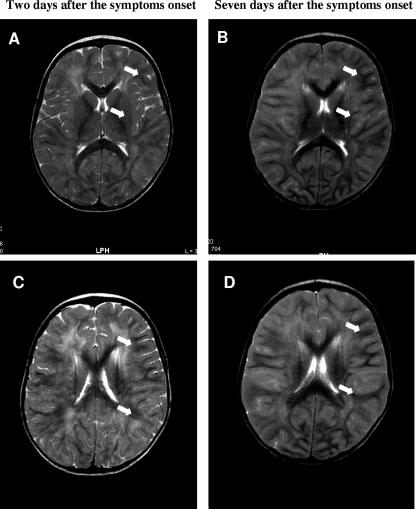
Two days after the admission of this infant to the university hospital, brain magnetic resonance imaging (MRI) studies with and without gadolinium revealed a diffuse hyperintense signal in the white matter. MRI T2 showed modifications, symmetrically involving the white matter of both cerebral hemispheres, that were predominantly located in the periventricular and subcortical white matter (Fig. 1A and C). Seven days after his initial admission to the university hospital, a second brain MRI study revealed an extensive and diffuse hyperintense signal in the subcortical and deep white matter of both cerebral hemispheres (Fig. 1B and D). At that time, several images showed extension of the hyperintense signal into the cortical gray matter (not shown).
FIG. 1.
Brain MRI studies (axial, T2 weighted). (A and C) Axial, T2-weighted, diffuse, hyperintense signals with particular predominance in the periventricular and subcortical white matter of both cerebral hemispheres on day 2 after symptom onset (arrows). (B and D) Seven days after symptom onset, an axial, T2-weighted study revealed the extension of diffuse, hyperintense signals into the subcortical and deep white matter of both cerebral hemispheres (arrows).
Testing of CSF, peripheral blood, throat, and urine samples by classical bacterial culture assays demonstrated an absence of pathogens. These serum samples demonstrated an absence of specific immunoglobulin M (IgM) and IgG antibodies directed against Mycoplasma and Chlamydia pneumoniae by enzyme-linked immunosorbent assay. The presence of protective postvaccination levels of IgG against mumps, measles, and rubella associated with an absence of anti-hepatitis B virus-, anti-human immunodeficiency virus type 1- and 2-, anti-influenza type A- and B-, anti-adenovirus-, anti-varicella-zoster virus-, anti-human cytomegalovirus-, and anti-coxsackievirus B4 antibodies was demonstrated in the same serum samples tested by enzyme-linked immunosorbent assay, whereas the presence of anti-Epstein-Barr virus antibodies was assessed by Western blot assay, demonstrating a profile compatible with an old infection (not shown). An echovirus (EV) strain was isolated from a throat sample by classical viral cell culture techniques and identified by a microseroneutralization technique as a nontypeable EV strain (not shown).
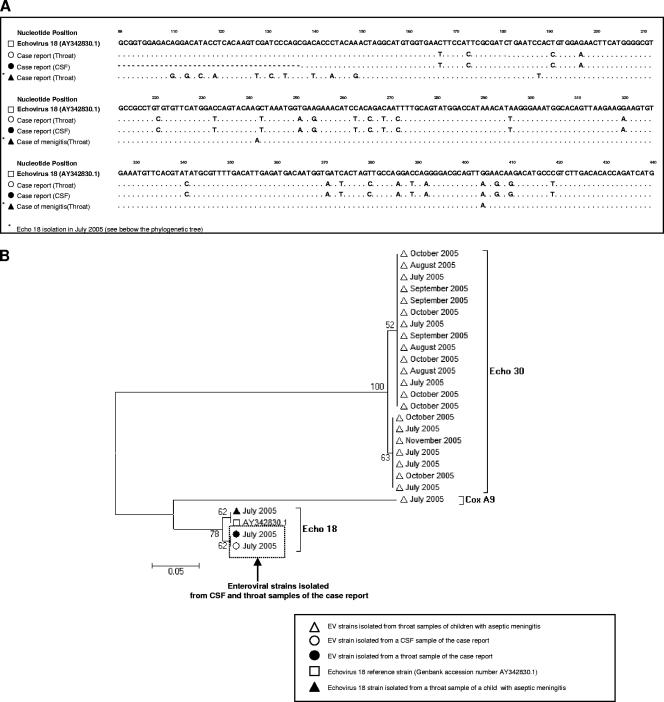
PCR analysis for herpesviruses did not show any amplification and thus excluded the possibility of herpesvirus encephalitis. The use of a standardized reverse transcription (RT)-PCR assay for EV, followed by microwell hybridization, assessed the presence of EV genomic RNA in throat and CSF samples (3). Genotyping of these EV strains by partial amplification and sequencing of the VP1 gene revealed 100% sequence homology in both throat and CSF samples from this infant. The phylogenetic analysis placed them closest to EV-18, which was circulating during the same epidemic season (Fig. 2) (2).
FIG. 2.
Comparison of the VP1 gene sequences of the two EV-18 strains isolated from samples from the patient described in this case report with those obtained from other, cocirculating, EV strains isolated from the throats of infants with classical aseptic meningitis during the same epidemic season.
Four days after the beginning of the febrile illness, a high intravenous bolus dose of solumedrol (1 g/1.73 m2/day) was delivered over 3 days, with no improvement of the clinical symptoms or neurological signs. Within the 2 days after the initiation of this corticosteroid therapy, the infant developed generalized seizures that were successively treated by intravenous administration of clonazepam (0.2 mg/kg/day) and oral administration of valproic acid (24 mg/kg/day). The appearance of dysautonomic troubles associated with a Glasgow score to 8/15 led to the rapid admission of this infant to the pediatric intensive care unit. The patient died 8 days after the beginning of the infectious syndrome because of major cerebral edema inducing bilateral cerebellar tonsil herniation. No autopsy was carried out.
EVs (Picornaviridae), specifically EV and coxsackievirus A or B, are usually responsible for aseptic meningitis occurring in the summer and fall in young infants (7). Moreover, EV can induce acute cerebral lesions and accounts for 11% of the total number of viral encephalitis cases in the general population (4). In contrast to aseptic meningitis, encephalitis due to EV can cause focal gray matter lesions in infants and may be responsible for neurological impairment (4, 7). Rare cases of encephalitis with white matter lesions have been reported in infants with documented EV central nervous system (CNS) infections (1, 5, 6, 8). EV-related leukoencephalitis has been previously reported by David et al., who described a potential case of acute disseminated encephalomyelitis (ADEM) after coxsackievirus B infection, and more recently by Mariotti et al. and Saitoh et al., who described positive detection of EV by PCR in the CSF of children with radiological features of ADEM (1, 6, 8). In the present case report, the radiological findings on the patient and detection of EV genomic RNA are highly suggestive of infective meningoencephalitis (8). Brain MRI findings in a T2-weighted study showing symmetrical diffuse hyperintense signals located predominantly in the periventricular and subcortical white matter are not suggestive of ADEM (Fig. 1) (1). Moreover, serological results and vaccine management do not favor recent postinfectious or postvaccination events that may have potentially induced immune-mediated mechanisms responsible for ADEM in this infant (5).
The use of a standardized PCR assay for EV RNA, followed by a hybridization assay, allowed us to determine the presence of EV genomic RNA in a CSF sample from the infant studied (3). This positive EV molecular detection associated with high levels of IFN-α was compatible with viral CSF infection in this study (3, 7). Moreover, RT-PCR amplification of the VP1 gene and amplicon sequencing have been used to identify genetically identical EV-18 strains in paired CSF and throat samples (Fig. 2) (2). This documented EV-18 CNS infection appeared as the only evident cause of the development of the patient's neurological syndrome, thus demonstrating that EV infection can cause meningoencephalitis with diffuse and extensive white matter lesions in infants more than 12 months old. EVs are neurotropic agents which are also able to infect in vitro-cultured glial cells (9). Moreover, in mouse models, it has been demonstrated that Theiler's virus (Picornaviridae) could not only replicate in glial cells but also induce viral immune-mediated mechanisms responsible for the destruction of noninfected white matter cells, producing myelin-specific mRNAs (10). Therefore, in the present case we hypothesized that an active EV CNS infection may have initiated infective meningoencephalitis with white matter involvement.
PCR-based assays have been demonstrated to have distinct advantages over culture-based methods for the diagnosis of encephalitis due to EV; therefore, these techniques should help to define more precisely the significance of these viruses in infants with encephalitis (7). However, it is likely that the frequency of acute encephalitis with EV infection is underestimated because it is not usual to perform cranial MRI scans associated with a PCR assay for EV detection in the CSF of all infants with acute or subacute onset of general or multifocal neurological symptoms (8).
This report highlights the role of EV CNS infections as a potential cause of leukoencephalitis in infants. Moreover, our findings suggest that the use of RT-PCR techniques for the detection of viral infection in the CSF may be valuable for the medical management of infants with leukoencephalitis.
Acknowledgments
This work was supported in part by a grant for clinical and virological research (EA-3798: DAT/PPCIDH) from the Medical University and School of Medicine of Reims, France.
None of us has a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy).
Footnotes
Published ahead of print on 18 April 2007.
REFERENCES
- 1.David, P., D. Baleriaux, W. O. Bank, D. Amrom, D. De Temmerman, C. Babusiaux, C. Matos, C. Van Steenwinckel, C. Lloret-Pastor, and H. B. Szliwowski. 1993. MRI of acute disseminated encephalomyelitis after coxsackie B infection. J. Neuroradiol. 20:258-265. [PubMed] [Google Scholar]
- 2.Iturriza-Gómara, M., B. Megson, and J. Gray. 2006. Molecular detection and characterization of human enteroviruses directly from clinical samples using RT-PCR and DNA sequencing. J. Med. Virol. 78:243-253. [DOI] [PubMed] [Google Scholar]
- 3.Jacques, J., J. Carquin, V. Brodard, H. Moret, D. Lebrun, M. Bouscambert, J. Motte, G. Rémy, and L. Andréoletti. 2003. New reverse transcription-PCR assay for rapid and sensitive detection of enterovirus genomes in cerebrospinal fluid specimens of patients with aseptic meningitis. J. Clin. Microbiol. 41:5726-5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koskiniemi, M., T. Rantalaiho, H. Piiparinen, C. H. von Bonsdorff, M. Farkkila A. Jarvinen, E. Kinnunen, S. Koskiniemi, L. Mannonen, M. Muttilainen, K. Linnavuori, I. Porras, M. Puolakkainen, K. Raiha, E. M. Salonen, P. Ukkonen, A. Vaheri, and V. Valtonen. 2001. Infection of the central nervous system of suspected viral origin: a collaborative study from Finland. J. Neurovirol. 7:400-408. [DOI] [PubMed] [Google Scholar]
- 5.Leake, J. A. D., S. Albani, A. S. Kao, M. O. Senac, G. F. Billman, M. P. Nespeca, A. D. Paulino, E. R. Quintela, M. H. Sawyer, and J. S. Bradley. 2004. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr. Infect. Dis. J. 23:756-764. [DOI] [PubMed] [Google Scholar]
- 6.Mariotti, P., A. P. Batocchi, C. Colosimo, et al. 2004. Positive PCR for enterovirus in the cerebrospinal fluid of a child with acute disseminated encephalomyelitis. J. Neurol. 251:1267-1269. [DOI] [PubMed] [Google Scholar]
- 7.Rotbart, H. A. 1995. Enteroviral infection of the central nervous system. Clin. Infect. Dis. J. 20:971-981. [DOI] [PubMed] [Google Scholar]
- 8.Saitoh, A., M. H. Sawyer, and J. A. D. Leake. 2004. Acute disseminated encephalomyelitis associated with enteroviral infection. Pediatr. Infect. Dis. J. 23:1174-1175. [PubMed] [Google Scholar]
- 9.Wen, Y. Y., T. Y. Chang, S. T. Chen, C. Li, and H. S. Liu. 2003. Comparative study of enterovirus 71 infection of human cell lines. J. Med. Virol. 70:109-118. [DOI] [PubMed] [Google Scholar]
- 10.Yamada, M., A. Zurbriggen, and R. S. Fujinami. 1990. The relationship between viral RNA, myelin-specific mRNAs, and demyelination in central nervous system disease during Theiler's virus infection. Am. J. Pathol. 137:1467-1479. [PMC free article] [PubMed] [Google Scholar]