Abstract
A number of epidemiological studies have shown human metapneumovirus (hMPV) to be one of the most important viral agents associated with acute respiratory infections in humans. However, due to the difficulty in growing the virus, all epidemiological studies of hMPV infection have been performed on the basis of the molecular method. Thus, the development of a cell line suitable for the isolation of hMPV from clinical specimens is a crucial step for further research. Using the Vero E6 cell line, which could be stably maintained for 1 month without passage or medium change, we succeeded in isolating 79 strains from 4,112 specimens obtained in Yamagata, Japan, in 2004 and 2005. The total isolation rate was 1.9% (79/4,112). The monthly distribution revealed that hMPV infections occurred between February and April in 2004 and throughout most of the year in 2005. Phylogenetic analysis indicated that subgenogroup B2 was predominant in 2004, whereas three subgenogroups, A2, B1, and B2, had cocirculated in 2005. Although multiple subgenogroups cocirculated in 2005, each individual subgenogroup strain was found to predominate at specific sites. An infectivity assay of hMPV strains also indicated that the infection efficiency in Vero E6 cells was better than that in LLC-MK2 cells. Finally, we found that Vero E6 cells are useful for the isolation of hMPVs and that this utility might aid further research into hMPVs beyond the epidemiological data shown in this study.
In 2001, a new respiratory virus, human metapneumovirus (hMPV), was first isolated from nasopharyngeal specimens from children with acute respiratory infection (ARI) (31). Based on genetic and phylogenetic analyses, hMPV has been categorized as a member of the genus Metapneumovirus of the subfamily Pneumovirinae of the family Paramyxoviridae (31). It has been recognized as a common cause of respiratory infections, ranging from upper respiratory infections to severe bronchiolitis and pneumonia, in both children and adults worldwide (3, 31). The seasonality of hMPV infections resembles that of human respiratory syncytial virus infections, and the epidemic season of hMPV is generally reported to be from winter to early spring (2, 3, 30, 31, 35). hMPVs are separated into two groups, serotypes A and B, with each serotype divided into genetic sublineages 1 and 2 on the basis of genetic differences and neutralization assays (12, 25, 32, 33). However, insufficient epidemiological data for hMPV have been accumulated to date.
With regard to the isolation of hMPV, van den Hoogen et al. first reported that the virus isolates replicated slowly in tertiary monkey kidney (tMK) cells and very poorly in African green monkey kidney (Vero) and human lung adenocarcinoma (A549) cells and could not be propagated in Madin-Darby canine kidney or chicken embryo fibroblast (CEF) cells (31). Since then, hMPV isolation has commonly been performed using tMK and rhesus monkey kidney (LLC-MK2) cell lines, especially LLC-MK2 (3, 4, 5, 6, 10, 12, 13, 14, 15, 23, 24, 35). However, only strains 1 to 38 have been isolated in previous studies using tMK and LLC-MK2 cell lines (3, 4, 10, 14, 24, 25, 35). Furthermore, Døllner et al. detected 50 hMPV-positive cases by reverse transcription-PCR (RT-PCR) but failed to find any cytopathic effect (CPE) using the LLC-MK2 cell line (8). Of course, after 5 to 10 passages, the hMPV titers reach 107 or more tissue culture infective doses per ml, and these viruses show CPEs within 5 days postinfection (24). However, it is still quite difficult to isolate hMPVs directly from clinical specimens, and RT-PCR has since been widely used for the detection and laboratory diagnosis of hMPV. Therefore, if a cell line suitable for the isolation of hMPV can be developed, it will be of great benefit for further research on hMPV.
Historically, the Vero E6 cell line was chosen from seven clones for use in the recovery of the Lassa virus, as it could easily be maintained for a long period (7 to 10 days at that time) without changing of the medium and was found to be superior for use in the replication and plaque production of slow-growing noncytolytic agents infecting humans and rodents (9). This cell line has been further used for the isolation and growth of measles virus, Ebola virus, Crimean-Congo hemorrhagic fever virus, severe acute respiratory syndrome (SARS) coronavirus, and so on (20, 26, 27, 29), and has potential application for the isolation of other viruses.
After the emergence of SARS coronavirus, we had the opportunity to use the Vero E6 cell line, primarily to isolate SARS coronavirus, in our role as members of a public health laboratory (20). Fortunately, it was not necessary for us to isolate SARS coronavirus. However, by accident, we found that the Vero E6 cell line was sensitive to hMPV when we applied this cell line to the isolation of common respiratory viruses from clinical specimens from children with ARI. Using this Vero E6 cell line, we succeeded in isolating 79 hMPV strains, which could be the highest number of strains yet isolated, after an observation of 4 weeks. In this paper, we describe the effectiveness of this Vero E6 cell line for hMPV isolation as well as our epidemiological findings and further phylogenetic analysis, which we believe to be the first epidemiological data based on virus isolation. On the basis of the results presented herein, it is hoped that this method will be of great use in the further study of hMPV isolation.
MATERIALS AND METHODS
Collection of specimens.
Between January 2004 and December 2005, 4,112 nasopharyngeal swab specimens were obtained from patients with ARI at pediatric clinics collaborating with the local health authority of the Yamagata Prefecture for the surveillance of viral diseases in Japan. Among them, 3,958 (96.3%) were from patients ≤15 years old, 108 (2.6%) were from patients of unknown ages, and 46 (1.1%) were from patients >15 years old. The specimens were collected and placed immediately in tubes containing a transport medium and transported to the Department of Microbiology, Yamagata Prefectural Institute of Public Health, for virus isolation.
Virus isolation and identification.
Virus isolation was carried out using a modified microplate method. Briefly, human embryonic lung fibroblast, human laryngeal carcinoma (HEp-2), Vero, Madin-Darby canine kidney, rhabdomyosarcoma (RD-18S), and green monkey kidney cell lines were prepared on the wells of a 96-well microplate (18, 21). In January 2004, we substituted the Vero E6 cell line, which was provided by the National Institute of Infectious Diseases, Tokyo, Japan, for the Vero cell line. The growth medium for the Vero E6 cell line consisted of Eagle's minimum essential medium (MEM) with 10% fetal bovine serum (FBS) and antibiotics (streptomycin [0.1 μg/ml] and penicillin G [100 units/ml]). The maintenance medium for the Vero E6 cell line consisted of MEM with crystallized trypsin (T-8003; Sigma, St. Louis, MO) (2 μg/ml), 5% MEM vitamin solution (100× concentrate; Sanko Junyaku, Tokyo, Japan), 0.2% glucose, and antibiotics. After the plates were washed with phosphate-buffered saline without calcium and magnesium (PBS), 100 μl of the maintenance medium was added to each well of the plates. Then, each specimen was centrifuged at 3,000 rpm for 15 min and 75 μl of the supernatant was inoculated onto two wells of each cell line. The inoculated plates were incubated at 33°C in a CO2 incubator. We observed the plates two or three times per week for CPEs for 14 days for all cell lines except the Vero E6 cell line, which was observed for approximately 1 month, without passage or medium change. When a suspected hMPV CPE was observed, viral identification was carried out by RT-PCR and sequence analysis.
Infectivity assay of hMPVs.
We prepared confluent monolayers of Vero, Vero E6, and LLC-MK2 cell lines in 96-well microplates, using MEM containing 10% FBS. Cells were washed twice with PBS and inoculated with 50 μl of serial 10-fold dilutions, ranging from 100 to 10−2, of hMPV strains 1508-Yamagata-05 and 1918-Yamagata-05, which were isolated in this study and had been passaged twice in Vero E6 cells, and Sendai-1311-04 and Sendai-155-D06, which were isolated at the Virus Research Center, Sendai Medical Center, Sendai, Japan, and had been passaged 10 times in LLC-MK2 cells. We used four replicate wells per dilution of each strain. After centrifugation for 30 min at 2,000 rpm, the virus inocula were incubated for 30 min at 33°C in a CO2 incubator and aspirated, and then 0.1 ml of MEM without trypsin was added as the maintenance medium to each well. At 24 h postinoculation, the medium was removed and the cells were rinsed with PBS twice and then fixed with 70% acetone in PBS for 10 min at room temperature. Fixed cultures were immunostained for the expression of hMPV antigen by incubation for 45 min at 37°C with guinea pig anti-hMPV polyclonal antibodies, which were prepared at the Department of Infectious Diseases, Yamagata University School of Medicine, followed by incubation for 45 min with peroxidase-labeled anti-guinea pig immunoglobulin G antibodies (AP108P; CHEMICON International, Inc.) at room temperature. After each reaction, the cells were washed several times in PBS containing 5% skim milk. In the final step, the peroxidase reaction was allowed to proceed for 20 min using 0.03% H2O2 and 0.2 mg/ml of 3-3′-diaminobenzidine tetrahydrochloride (Dojindo, Kumamoto, Japan). The cells were then rinsed with distilled water, and the stained cells were counted microscopically. We determined the numbers of hMPV-infected cells per well for four replicate wells for each virus dilution and calculated the mean number.
RT-PCR, sequence analysis, and phylogenetic analysis.
RNA extraction, RT-PCR, and sequence analysis were carried out as described previously (17, 19). Briefly, viral RNA was extracted from 100 μl of the viral culture fluid by using ISOGEN-LS (Nippon Gene, Tokyo, Japan). The viral RNA was then transcribed into cDNA with Moloney murine leukemia virus reverse transcriptase (Nippon Gene, Tokyo, Japan) and a random primer (Takara Bio Inc., Otsu, Japan). Using cDNA, a part of the fusion region was amplified by PCR with 40 cycles at 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min and a final extension at 72°C for 10 min. The PCR products were purified with a QIAquick PCR purification kit (QIAGEN, Hilden, Germany) and then sequenced using a BigDye Terminator V1.1 cycle sequencing kit on an ABI Prism 310 (Applied Biosystems, Foster City, CA) automatic sequencer. For PCR and sequencing analysis, primers MPVF1f, MPVF1r, BF101, and BF104 were used (25, 32). Sequence data were analyzed with CLUSTAL W version 1.83, and a phylogenetic tree was constructed via the neighbor-joining method (28), using the same software.
Nucleotide sequence accession numbers.
Sequence data were added under accession numbers AB251496 through AB251574 at GenBank.
RESULTS
Isolation and CPEs for hMPV in the Vero E6 cell line in Yamagata.
All hMPVs in this study were isolated using the Vero E6 cell line. We succeeded in isolating a total of 79 hMPV strains from 77 children and 2 adults, with a total isolation rate of 1.9% (79/4,112).
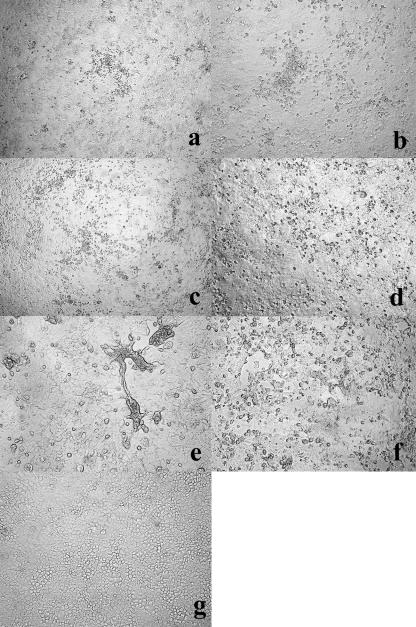
The CPEs for hMPV in the primary cultures were accompanied by granular, small, roundup, and refringent cells, but there was no clear syncytium formation (Fig. 1a to d). Most early CPEs were recognized as focal or scattered granular patterns, as shown in Fig. 1a and b, whose images were taken on day 17 after specimen inoculation. Thereafter, the areas of granular, refringent cells spread slowly, as shown in Fig. 1c and d, whose images were taken 29 days after specimen inoculation, with eventual destruction of the cells in some cases. We found the CPE for hMPV in a primary culture within 6 to 7 days after specimen inoculation for 6 strains, within 8 to 14 days for 46 strains, within 15 to 21 days for 23 strains, and after more than 21 days for 4 strains. On the other hand, the CPEs observed after a passage of recovered viral fluid showed large and/or small syncytia within 4 days after inoculation (Fig. 1e and f). Findings of initial CPEs without syncytial formation in the primary culture and with syncytial formation after the passage indicated that the CPEs for hMPVs in the Vero E6 cell line were quite variable. The most remarkable characteristic of the Vero E6 cell line was the stability of the monolayer, which enabled us to observe the hMPV CPE several weeks or even 1 month after cell preparation and specimen inoculation (Fig. 1c, d, and g), whereas regular Vero cells, which had been used until December 2003, degenerated within 14 days. Adenoviruses, enteroviruses, and herpes simplex viruses could also be replicated using the Vero E6 cell line. Since these viruses grow faster than hMPV, we could not isolate hMPVs in cases where these viruses were recovered.
FIG. 1.
Microscopic studies of hMPV-infected (a to f) and uninfected (g) Vero E6 cell lines. The various CPEs were induced by the hMPV strains. (a and b) Vero E6 cell primary culture 17 days after inoculation with 1159-Yamagata-05 and 1645-Yamagata-05, respectively. Early CPEs for hMPV were recognized as granular and roundup cell formation, and there was no clear syncytium formation. (c and d) Vero E6 cell primary culture 29 days after inoculation with 970-Yamagata-05 and 1159-Yamagata-05, respectively. The granular formation has spread and progressed, though the Vero E6 monolayer is still stable. (e) Vero E6 cell culture 4 days after inoculation with viral fluid from 1159-Yamagata-05 after two passages. Infected cells showed marked syncytium formation progressing to detachment from the cell monolayer. (f) Vero E6 cell culture 4 days after inoculation with viral fluid from 871-Yamagata-05 after two passages. The CPE consisted of roundup cells and small rather than large syncytia. (g) Uninfected Vero E6 cells 22 days after preparation. Original magnifications, ×100 (a and c) and ×200 (b and d to g).
Infectivity assay of hMPVs in Vero, Vero E6, and LLC-MK2 cells.
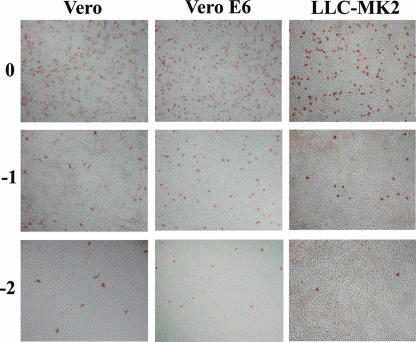
We compared the sensitivities of Vero E6 cells to hMPVs with those of Vero and LLC-MK2 cells. We inoculated the hMPV strains, fixed the cells 24 h after infection, and counted the number of immunostained cells. The two hMPV strains previously passaged twice in Vero E6 cells (1508-Ymagata-05 and 1918-Yamagata-05) infected more than 20 times more cells in Vero E6 cells than in LLC-MK2 cells; however, there were no significant differences in infectivity between Vero E6 and Vero cells. For example, at a dilution of 10−2, the mean numbers of infected cells for four replicate wells infected with the 1918-Yamagata -05 strain were 63.25 in Vero cells, 74.25 in Vero E6 cells, and 2.5 in LLC-MK2 cells. We also performed the infectivity assay for two strains (Sendai-1311-04 and Sendai-155-D06) previously passaged 10 times in LLC-MK2 cells. In replicate experiments performed on different days, LLC-MK2-passaged viruses infected 5 to 7.5 times more cells in Vero and Vero E6 cells than in LLC-MK2 cells. Examples of stained images of the three cell lines inoculated with the strain Sendai-1311-04 are shown in Fig. 2. The mean numbers of infected cells for four wells at the dilution of 10−2 were 121 in Vero cells, 132 in Vero E6 cells, and 26.5 in LLC-MK2 cells.
FIG. 2.
Microscopic views of Vero, Vero E6, and LLC-MK2 cells infected with hMPV strain Sendai-1311-04, which was originally isolated using LLC-MK2 cells and had been passaged 10 times in the same cell line. We inoculated the cultures in parallel with 100, 10−1, and 10−2 dilutions of the virus stock, fixed the culture at 24 h postinfection, and immunostained them for the viral antigen.
Sequence and phylogenetic analysis.
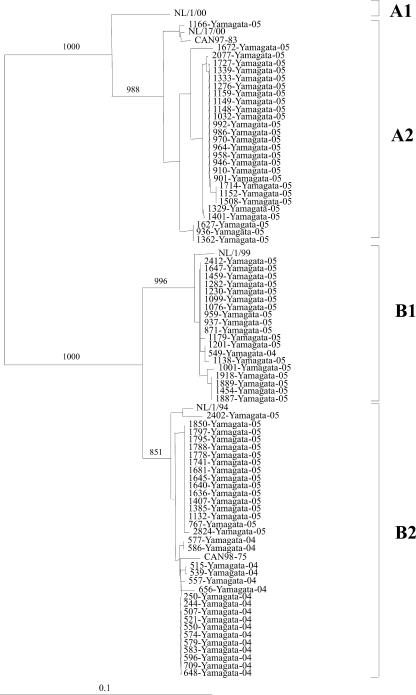
As shown in Fig. 3, phylogenetic analysis revealed that 17 out of the 18 strains isolated in 2004 clustered in the B2 subgenogroup and only 1 strain in the B1 subgenogroup. In 2005, there was a mixture of three subgenogroups: 27 subgenogroup A2 strains (44.3%), 18 subgenogroup B1 strains (29.5%), and 16 subgenogroup B2 strains (26.2%). The nucleotide identities among the strains within subgenogroups A2, B1, and B2 from Yamagata were between 97 and 100%, 99 and 100%, and 98 and 100%, respectively. The nucleotide differences between the results for 2004 and 2005 were only 0 to 1% for subgenogroup B1 strains and 0 to 2% for subgenogroup B2 strains.
FIG. 3.
Phylogenetic tree for the partial (441-bp) sequence of the fusion regions of hMPV strains isolated in Yamagata, Japan, between 2004 and 2005 as well as for the reference strains. The branch lengths are proportional to the numbers of nucleotide differences. The numbers above the branches are the bootstrap probabilities (%). The marker denotes the measurement of relative phylogenetic distance. The reference strains (CAN98-75, CAN97-83, NL/1/00, NL/17/00, NL/1/99, and NL/1/94) were based on references 25 and 32.
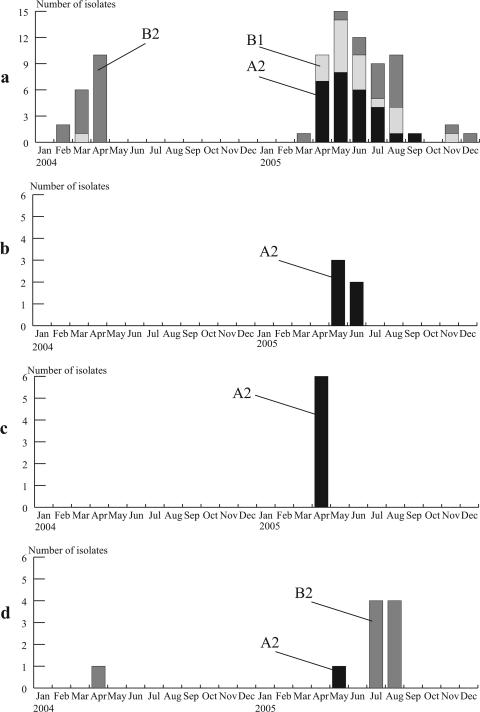
The monthly distribution of subgenogroups is shown in Fig. 4a. Outbreaks of hMPV subgenogroup B2 strains occurred between February and April 2004, peaking in April, whereas subgenogroup A2 and B1 strains had peaks between April and June 2005, when 21 and 13 isolates, respectively, were recovered. Ten out of 16 subgenogroup B2 viruses were isolated between July and August 2005.
FIG. 4.
Monthly distribution and subgenogroups of hMPV strains isolated in Yamagata, Japan, in 2004 and 2005. (a) Subgenogroups A2, B1, and B2 were grouped according to the phylogenetic analysis whose results are shown in Fig. 3. Monthly distributions are also shown for one primary school (b) and two nurseries (c and d) at which we isolated more than five hMPV strains.
Outbreaks at primary schools and nurseries.
During the study period, there were two nurseries and one primary school at which we isolated more than five hMPV strains. Between 2 May and 13 June 2005, we isolated five subgenogroup A2 strains, which had 99 to 100% nucleotide identity with each other, at one primary school (Fig. 4b). At one nursery, we isolated six subgenogroup A2 strains, which had 100% identical nucleotide sequences, between 16 and 28 April 2005 (Fig. 4c). At another nursery, we isolated one subgenogroup B2 strain in April 2004, one subgenogroup A2 strain in May 2005, and eight subgenogroup B2 strains between 19 July and 10 August 2005 (Fig. 4d). The nucleotide identity among the eight subgenogroup B2 isolates in 2005 was 100%, and there was 99% identity between these isolates and the one isolate from 2004. Interestingly, we isolated a subgenogroup A2 strain in May 2005 and a subgenogroup B2 strain in August 2005 from the same 2-year-old girl.
DISCUSSION
First, we report the effectiveness of the Vero E6 cell line for the isolation of hMPV. The appearance of a recognizable hMPV CPE can take 2 or more weeks with the LLC-MK2 cell line (3, 12, 23, 35). In our experience with the Vero E6 cell line, we could identify the CPE for only six strains within 6 to 7 days after specimen inoculation whereas 2 to 4 weeks was needed to detect the CPE for the other strains. For this reason, we could not shorten the time needed to detect CPE using the Vero E6 cell line compared with that for the LLC-MK2 cell line. However, blind passage was often necessary to isolate hMPVs using the LLC-MK2 cell line after approximately 2 weeks of primary incubation (35). The merit of the Vero E6 cell line is that it forms a monolayer that can be maintained in a stable condition for a long period and enable us to observe hMPV CPE for approximately 1 month without passage or medium change. Furthermore, the infectivity assay in this study indicated that the infection efficiency of hMPV in Vero E6 cells was better than that in LLC-MK2 cells. Thus, the cell line stability and the better infectivity efficiency suggested that the Vero E6 cell line is effective for the isolation of the slow-growing hMPV.
Our infectivity assay suggested that Vero cells are generally sensitive to hMPVs. van den Hoogen et al. found that Vero cell clone 118 permitted infection with viruses from all four lineages and CPEs were easily observed, whereas the CPE of prototype strain NL/1/00, which belongs to the A lineage, was more clearly observed than that of prototype strain NL/1/99, which belongs to the B lineage, on the tMK cell line (33). The findings that the Vero E6 cell line and Vero cell clone 118 possess characteristics that make them advantageous for the isolation of hMPV and for the observation of its CPE suggest that some clones of the Vero cell line might be especially suitable for the isolation of hMPVs. We have not confirmed that hMPVs belonging to the A1 lineage can be replicated in the Vero E6 cell line. Since all four subgenogroups do not always appear within a 2-year period (1), we should continue to investigate whether we are able to isolate subgenogroup A1 viruses using Vero E6 cells.
hMPV has its main clinical impact in the winter months in countries with moderate climates (2, 3, 11, 30, 31, 36). However, a few reports have mentioned the detection of hMPV in summer months (7, 22, 34, 35). Although our limited results showed that hMPV infections occurred with a peak in April 2004 and in May 2005, we also isolated hMPVs over the summer, from June to September, in 2005. In particular, we isolated eight strains at one nursery between July and August in 2005 and five strains at one primary school between May and June 2005 (Fig. 4b and d). Therefore, the results for our virus isolation support the notion that hMPV infections occur throughout the year (35, 36).
Cocirculation of hMPV genogroups and subgenogroups has been previously reported (1, 11, 16, 36). Ludewick et al. reported that a shift in the predominant group from subgenogroup B2 to A1 was observed between 2000 and 2002 (16). Williams et al. reported that the cocirculation of multiple hMPV subgenogroups had continued for 20 years, with subgenogroups substituting from year to year (36). We also found the cocirculation of two or three subgenogroups in the Yamagata area in 2004 and 2005, as shown in Fig. 4a. Interestingly, the hMPV subgenogroup isolated from each of the three clusters at two nurseries and one primary school was specific to that place, though two or three subgenogroups had been cocirculating in Yamagata during the respective periods. These results suggest that close contact among children is an important factor in the transmission of hMPVs. Furthermore, observations from one nursery (Fig. 4a and d) suggested that hMPVs circulating within a community can enter facilities such as primary schools and nurseries.
In 1987, on the 25th anniversary of the isolation of Vero cells from African green monkey kidney tissue, Earley and Johnson suggested that Vero cells had been one of the most powerful basic resources in the entire field of virology and should continue to be a major cell substrate for virologists in years to come (9). In this paper, we report that one clone of Vero, the Vero E6 cell line, is sensitive not only to measles virus, Ebola virus, Cremian-Congo hemorrhagic fever virus, and SARS coronavirus, as has been already reported (20, 26, 27, 29), but also to hMPVs. Since only a few hMPVs have been isolated using the tissue culture method, we hope that the Vero E6 cell line might contribute to further research on hMPVs, especially toward clarification of the epidemiology and etiology of this virus.
Acknowledgments
We thank the doctors, nurses, and other members of the Yamagata Prefecture for their assistance and collaboration in the surveillance of viral infectious diseases and B. Simizu for providing us with reference 9.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Agapov, E., K. C. Sumino, M. Gaudreault-Keener, G. A. Storch, and M. J. Holtzman. 2006. Genetic variability of human metapneumovirus infection: evidence of a shift in viral genotype without a change in illness. J. Infect. Dis. 193:396-403. [DOI] [PubMed] [Google Scholar]
- 2.Bastien, N., D. Ward, P. van Caeseele, K. Brandt, S. H. S. Lee, G. McNabb, B. Klisko, E. Chan, and Y. Li. 2003. Human metapneumovirus infection in the Canadian population. J. Clin. Microbiol. 41:4642-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin, G., Y. Abed, G. Pelletier, L. Ruel, D. Moisan, S. Côté, T. C. T. Peret, D. D. Erdman, and L. J. Anderson. 2002. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 186:1330-1334. [DOI] [PubMed] [Google Scholar]
- 4.Boivin, G., G. De Serres, S. Côté, R. Gilca, Y. Abed, L. Rochette, M. G. Bergeron, and P. Déry. 2003. Human metapneumovirus infections in hospitalized children. Emerg. Infect. Dis. 9:634-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, P. K. S., J. S. Tam, C. Lam, E. Chan, A. Wu, C. Li, T. A. Buckley, K. Ng, G. M. Joynt, F. W. T. Cheng, K. To, N. Lee, D. S. C. Hui, J. L. K. Cheung, I. Chu, E. Liu, S. S. C. Chung, and J. J. Y. Sung. 2003. Human metapneumovirus detection in patients with severe acute respiratory syndrome. Emerg. Infect. Dis. 9:1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, P. K. S., K. To, A. Wu, G. M. K. Tse, K. Chan, S. Lui, J. J. Y. Sung, J. S. Tam, and B. Tomlinson. 2004. Human metapneumovirus-associated atypical pneumonia and SARS. Emerg. Infect. Dis. 10:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chano, F., C. Rousseau, C. Laferrière, M. Couillard, and H. Charest. 2005. Epidemiological survey of human metapneumovirus infection in a large pediatric tertiary care center. J. Clin. Microbiol. 43:5520-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Døllner, H., K. Risnes, A. Radtke, and S. A. Nordbø. 2004. Outbreak of human metapneumovirus infection in Norwegian children. Pediatr. Infect. Dis. J. 23:436-440. [DOI] [PubMed] [Google Scholar]
- 9.Earley, E. M., and K. M. Johnson. 1988. The lineage of the Vero, Vero 76 and its clone C1008 in the United States, p. 26-29. In B. Simizu and T. Terasima (ed.), Vero cells—origin, properties, and biomedical applications. Department of Microbiology, School of Medicine, Chiba University, Chiba, Japan.
- 10.Ebihara, T., R. Endo, H. Kikuta, N. Ishiguro, H. Ishiko, M. Hara, Y. Takahashi, and K. Kobayashi. 2004. Human metapneumovirus infection in Japanese children. J. Clin. Microbiol. 42:126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esper, F., R. A. Martinello, D. Boucher, C. Weibel, D. Ferguson, M. L. Landry, and J. S. Kahn. 2004. A 1-year experience with human metapneumovirus in children aged <5 years. J. Infect. Dis. 189:1388-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falsey, A. R., D. Erdman, L. J. Anderson, and E. E. Walsh. 2003. Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 187:785-790. [DOI] [PubMed] [Google Scholar]
- 13.Hamelin, M., Y. Abed, and G. Boivin. 2004. Human metapneumovirus: a new player among respiratory viruses. Clin. Infect. Dis. 38:983-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ijpma, F. F. A., D. Beekhuis, M. F. Cotton, C. H. Pieper, J. L. L. Kimpen, B. G. van den Hoogen, G. J. J. van Doornum, and D. M. E. Osterhaus. 2004. Human metapneumovirus infection in hospital referred South African children. J. Med. Virol. 73:486-493. [DOI] [PubMed] [Google Scholar]
- 15.Landry, M. L., D. Ferguson, S. Cohen, T. C. T. Peret, and D. D. Erdman. 2005. Detection of human metapneumovirus in clinical samples by immunofluorescence staining of shell vial centrifugation cultures prepared from three different cell lines. J. Clin. Microbiol. 43:1950-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludewick, H. P., Y. Abed, N. van Niekerk, G. Boivin, K. P. Klugman, and S. A. Madhi. 2005. Human metapneumovirus genetic variability, South Africa. Emerg. Infect. Dis. 11:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzaki, Y., K. Sugawara, E. Takashita, Y. Muraki, S. Hongo, N. Katsushima, K. Mizuta, and H. Nishimura. 2004. Genetic diversity of influenza B virus: the frequent reassortment and cocirculation of the genetically distinct reassortant viruses in a community. J. Med. Virol. 74:132-140. [DOI] [PubMed] [Google Scholar]
- 18.Mizuta, K., C. Abiko, H. Goto, T. Murata, and S. Murayama. 2003. Enterovirus isolation from children with acute respiratory infections and presumptive identification by a modified microplate method. Int. J. Infect. Dis. 7:138-142. [DOI] [PubMed] [Google Scholar]
- 19.Mizuta, K., N. Katsushima, S. Ito, K. Sanjoh, T. Murata, C. Abiko, and S. Murayama. 2003. A rare appearance of influenza A(H1N2) as a reassortant in a community such as Yamagata where A(H1N1) and A(H3N2) co-circulate. Microbiol. Immunol. 47:359-361. [DOI] [PubMed] [Google Scholar]
- 20.Ng, M., S. Tan, E. See, E. Ooi, and A. Ling. 2003. Proliferative growth of SARS coronavirus in Vero E6 cells. J. Gen. Virol. 84:3291-3303. [DOI] [PubMed] [Google Scholar]
- 21.Numazaki, Y., T. Oshima, A. Ohmi, A. Tanaka, Y. Oizumi, S. Komatsu, T. Takagi, M. Karahashi, and N. Ishida. 1987. A microplate method for isolation of viruses from infants and children with acute respiratory infections. Microbiol. Immunol. 31:1085-1095. [DOI] [PubMed] [Google Scholar]
- 22.Osterhaus, A., and R. Fouchier. 2003. Human metapneumovirus in the community. Lancet 361:890-891. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier, G., P. Déry, Y. Abed, and G. Boivin. 2002. Respiratory tract reinfections by the new human metapneumovirus in an immunocompromised child. Emerg. Infect. Dis. 8:976-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Percivalle, E., A. Sarasini, L. Visai, M. G. Revello, and G. Gerna. 2005. Rapid detection of human metapneumovirus strains in nasopharyngeal aspirates and shell vial cultures by monoclonal antibodies. J. Clin. Microbiol. 43:3443-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peret, T. C. T., G. Boivin, Y. Li, M. Couillard, C. Humphrey, A. D. M. E. Osterhaus, D. D. Erdman, and L. J. Anderson. 2002. Characterization of human metapneumoviruses isolated from patients in North America. J. Infect. Dis. 185:2660-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rota, J. S., K. B. Hummel, P. A. Rota, and W. J. Bellini. 1992. Genetic variability of the glycoprotein genes of current wild-type measles isolates. Virology 188:135-142. [DOI] [PubMed] [Google Scholar]
- 27.Saijo, M., T. Qing, M. Niikura, A. Maeda, T. Ikegami, C. Prehaud, I. Kurane, and S. Morikawa. 2002. Recombinant nucleoprotein-based enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Crimean-Congo hemorrhagic fever virus. J. Clin. Microbiol. 40:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez, A., and M. P. Kiley. 1987. Identification and analysis of Ebola virus messenger RNA. Virology 157:414-420. [DOI] [PubMed] [Google Scholar]
- 30.Stockton, J., I. Stephenson, D. Fleming, and M. Zambon. 2002. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg. Infect. Dis. 8:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. M. Fouchier, and A. D. M. E. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Hoogen, B. G., S. Herfst, L. Sprong, P. A. Cane, E. Forleo-Neto, R. L. de Swart, A. D. M. E. Osterhaus, and R. A. M. Fouchier. 2004. Antigenic and genetic variability of human metapneumoviruses. Emerg. Infect. Dis. 10:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Hoogen, B. G., A. D. M. E. Osterhaus, and R. A. M. Fouchier. 2004. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr. Infect. Dis. J. 23:S25-S32. [DOI] [PubMed] [Google Scholar]
- 34.van den Hoogen, B. G., G. J. J. van Doornum, J. C. Fockens, J. J. Cornelissen, W. E. P. Beyer, R. de Groot, A. D. M. E. Osterhaus, and R. A. M. Fouchier. 2003. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J. Infect. Dis. 188:1571-1577. [DOI] [PubMed] [Google Scholar]
- 35.Williams, J. V., P. A. Harris, S. J. Tollefson, L. L. Halburnt-Rush, J. M. Pingsterhaus, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2004. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 350:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams, J. V., C. K. Wang, C. Yang, S. J. Tollefson, F. S. House, J. M. Heck, M. Chu, J. B. Brown, L. D. Lintao, J. D. Quinto, D. Chu, R. R. Spaete, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2006. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J. Infect. Dis. 193:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]