Abstract
In response to various stress stimuli, heat shock genes are induced to express heat shock proteins (Hsps). Previous studies have revealed that expression of heat shock genes is regulated both at transcriptional and posttranscriptional level, and the rapid transcriptional induction of heat shock genes involves activation of the specific transcription factor, heat shock factor 1 (HSF1). Furthermore, the transcriptional induction can vary in intensity and kinetics in a signal- and cell-type-dependent manner. In this study, we demonstrate that mechanical loading in the form of hydrostatic pressure increases heat shock gene expression in human chondrocyte-like cells. The response to continuous high hydrostatic pressure was characterized by elevated mRNA and protein levels of Hsp70, without activation of HSF1 and transcriptional induction of hsp70 gene. The increased expression of Hsp70 was mediated through stabilization of hsp70 mRNA molecules. Interestingly, in contrast to static pressurization, cyclic hydrostatic loading did not result in the induction of heat shock genes. Our findings show that hsp70 gene expression is regulated posttranscriptionally without transcriptional induction in chondrocyte-like cells upon exposure to high continuous hydrostatic pressure. We suggest that the posttranscriptional regulation in the form of hsp70 mRNA stabilization provides an additional mode of heat shock gene regulation that is likely to be of significant importance in certain forms of stress.
Keywords: heat shock transcription factor, heat shock element, cartilage, chondrocyte
Common to all organisms exposed to environmental stresses, both chemical and physical, is the induction of an evolutionarily highly conserved class of proteins known as heat shock proteins (Hsps) or stress proteins. Hsps play a protective role as molecular chaperones in cells by facilitating the folding, intracellular transport, assembly, and disassembly of other proteins. The induction of Hsps is regulated at both transcriptional and posttranscriptional levels (for review, see refs. 1 and 2). Essential for transcriptional regulation is the activity of the stress-responsive heat shock transcription factors (HSFs), the molecules that are present in unstressed cells in an inactive state and become activated in response to stress stimuli (for review, see ref. 3). Originally, a single HSF was identified in yeast and fruit fly, but subsequently multiple HSFs have been cloned in vertebrates. To date, three distinct HSFs in humans and chickens and two in mouse are known (4–8). However, only one of these factors, HSF1, is activated in response to classical stress stimuli. Upon activation, HSF1 is converted from a monomer to a trimer; the complex is then hyperphosphorylated and translocated to the nucleus, where it binds to the promoter of heat shock genes and initiates transcription (for review, see ref. 3).
Heat shock response involves multiple regulatory processes and the induction of target gene expression varies in intensity and kinetics in a modulator- and cell-type-dependent manner. In cells exposed to heat, heavy metals, or arachidonate, stress-induced trimerization, hyperphosphorylation, and acquisition of DNA-binding activity of HSF1 are accompanied with transcriptional induction of the hsp70 gene (9–11). However, the anti-inflammatory drugs indomethacin and sodium salicylate activate HSF1 to bind DNA but fail to induce transcription of heat shock genes, and in neither case does HSF1 undergo hyperphosphorylation (12–15). These studies indicate that inducible phosphorylation is not a prerequisite for regulation of HSF1 DNA-binding activity, but hyperphosphorylation is required to fully activate heat shock element (HSE)-driven transcription (11, 15). Furthermore, the complex regulation of heat shock response is reflected by studies showing that HSF1 is not necessarily involved in the transcriptional induction of heat shock genes. For example, the induction of hsp70 transcription by adenovirus E1a or serum stimulation can be mediated through the basal transcriptional complex, containing the CCAAT, GC, and TATA elements (16, 17). In addition to the transcriptional control, a posttranscriptional mechanism by hsp70 mRNA stabilization has been reported upon heat shock (18).
Articular cartilage is a tissue that protects the bone ends from excessive wear, and it has to withstand very high compressive loads. The compressive forces within joint articular cartilage can rise in normal conditions to 20 MPa on standing (19). As in all connective tissues, there is a relationship between mechanical factors and tissue behavior in articular cartilage. At present, it is widely accepted that joint loading is an essential requirement for the maintenance of normal composition of articular cartilage (20–23). Moderate exercise appears to support maintenance of the biological properties of cartilage (24, 25), whereas strenuous exercise may eventually induce adverse reactions in the joint (26). Although the pathogenesis of osteoarthrosis is still unknown, local biomechanical forces exposed to articular cartilage potentially play a crucial role in the initiation of osteoarthrosis.
Experiments carried out under in vitro conditions using mechanical loading of cartilage explants indicate that both the frequency and the amplitude of the force applied on the cartilage affect the synthesis of cartilage-specific proteoglycans (27–31). High continuous hydrostatic pressure (HP) inhibits proteoglycan synthesis and secretion, reduces the steady-state level of aggrecan mRNA, alters the shape of the Golgi apparatus, and inhibits the stress fiber organization of microfilaments (32–35). These findings prompted us to investigate whether such conditions would be adverse and induce stress response in the cells that synthesize cartilage-specific macromolecules. The expression of hsp70 genes, encoding classical Hsps, was studied after exposure of simian virus 40 (SV40)-immortalized human chondrocytes (36) to various levels of HP. Although the expression of Hsp70 was increased at both mRNA and protein levels, neither an acquired DNA binding of HSF1 nor an additional transcription of hsp70 could be detected during static exposure to HP. However, the accumulation of Hsp70 protein was coincident with stabilization of hsp70 mRNA molecules. Thus, we report an increase in the steady-state level of hsp70 mRNA and accumulation of Hsp70 protein without transcriptional induction of the corresponding gene.
MATERIALS AND METHODS
Cell Culture and Exposure to HP.
SV40-immortalized T/C28a4 human chondrocytes, established after immortalization of juvenile costal chondrocytes with SV40 tumor antigen (36), were cultured in a humidified 5% CO2/95% air atmosphere at 37°C in DMEM with 10% fetal calf serum, penicillin (50 units/ml), streptomycin (50 units/ml), and 3 mM glutamine. Cells were grown to a density of 7.2–8.0 × 104 cells per cm2 on 60-mm plates. Before exposure to HP or elevated temperature, medium was changed and 15 mM Hepes (pH 7.3) was added. For heat shock, the plates were sealed with Parafilm and immersed in a water bath at 42°C or 43°C. To study the mRNA stability, actinomycin D (2.5 mg/ml) was dissolved in methanol and applied to cultures at final concentration of 5 μM. To expose the cells to HP, the culture dishes were filled with the medium described above and sealed with a covering plastic membrane. The apparatus for hydrostatic pressurization of the cells has been described in detail (31). The pressure levels of the test chamber were selected to be 4 MPa and 30 MPa. Static and cyclic modes of pressure loading were used. In the cyclic mode, the frequency of the pressure pulses was 0.5 Hz (1-s load/1-s rest).
Western Blot and Sedimentation Analysis.
For Western blot analysis, whole cell extracts were prepared as described (37). The protein extracts (15 μg per lane) were electrophoresed on SDS/10% polyacrylamide gels and transferred to nitrocellulose membrane. Monoclonal antibodies (StressGen) recognizing the inducible form of Hsp70 (SPA-810) and Hsc70 (SPA-815) and peroxidase-conjugated secondary antibodies (Dako and Amersham) were used for the Western blots. The membranes were developed with an enhanced chemiluminescence method (Amersham). Polyclonal anti-HSF1 antiserum was used in the analysis of HSF1 hyperphosphorylation as described (11). Centrifugation of the whole cell extracts (500 μg of protein) in a 15–50% glycerol density gradient (38) was used to separate the oligomeric form of HSF1 from the monomeric form. The positions of proteins were visualized by Western blot analysis with the polyclonal anti-HSF1 antiserum. The protein standards (cytochrome c, 1.9 S; BSA, 3.2 S; carbonic anhydrase, 4.3 S; alcohol dehydrogenase, 7.4 S) were used for determining sedimentation positions.
Nuclear Run-On Assay.
Transcription run-on analysis was performed with an equal number of isolated nuclei in the presence of 100 μCi of [α-32P]dUTP (1 Ci = 37 GBq) as described (39). Radiolabeled RNA was isolated and hybridized to nitrocellulose-immobilized plasmids specific for human hsp70 (40) and rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (41). pBluescript plasmid (Stratagene) was used as a vector control. The hybridization and washing conditions were as described (42). Quantitation of the dots was performed with a PhosphorImager (Molecular Dynamics).
Gel Mobility Shift Assay.
Electrophoretic mobility shift analysis was performed after mixing 15 μg of whole cell extract and a γ-32P-labeled synthetic double-stranded oligonucleotide corresponding to the two overlapping HSEs located between positions −115 and −81 of the human hsp70 gene promoter (37). Protein–DNA complexes were resolved on a 4% nondenaturing polyacrylamide gel. Gels were dried and the radioactivity on the gel was detected by autoradiography. The specificity of the binding was ascertained by adding a 100-fold molar excess of unlabeled oligonucleotide to the samples.
Northern Blot Analysis.
Total cellular RNA was isolated by using TRIzol reagent (GIBCO/BRL). Total RNA (10 μg or 20 μg) was separated on a 1% agarose/formaldehyde gel, transferred to nylon membrane (Hybond-N, Amersham), and hybridized with [α-32P]dCTP-labeled plasmids specific for human hsp70 (40), rat GAPDH (41), and human 28S rRNA (43). Autoradiography signals were quantified by using a PhosphorImager (Molecular Dynamics), and the values obtained were normalized either against GAPDH or 28S rRNA.
RESULTS
Accumulation and Increased Synthesis of Hsp70 Protein During 30-MPa Static HP Treatment.
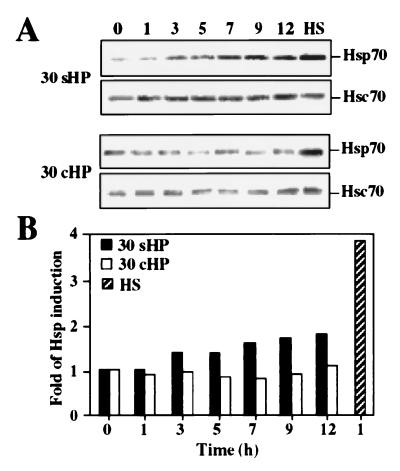
To investigate whether mechanical loading causes stress response, as characterized by increased amounts of Hsps, the SV40-immortalized human chondrocytes T/C-28a4 were exposed to 30-MPa and 4-MPa HP at static and cyclic pressure for up to 12 h. Whole cell extracts were prepared and analyzed by Western blot. Heat shock at 43°C for 5 h was used as the positive control for stress response. The amount of Hsp70 started to increase after 3 h of static treatment at 30-MPa HP (30sHP), and an approximately 2-fold accumulation of Hsp70 was detected after 12 h of exposure (Fig. 1). In contrast, no increase in Hsp70 levels was obtained by cyclic 30-MPa HP (30cHP; 0.5 Hz). As expected, the levels of Hsc70 were not affected by any of the exposures. In comparison, an approximately 4-fold increase in Hsp70 level was observed in response to elevated temperature (Fig. 1). Upon exposure to 4-MPa static or cyclic HP, Hsp70 and Hsc70 levels were not changed (data not shown). The total protein synthesis was determined when cells were exposed to 30-MPa and 4-MPa HP both at static and cyclic modes for 1–12 h and labeled with 50 μCi of [35S]methionine. In accordance with the Western blot data shown in Fig. 1, Hsp70 synthesis was elevated during static treatment at 30 MPa, but neither 30 MPa cyclic nor 4 MPa static or cyclic loading altered the synthesis of Hsp70 (data not shown). In heat-shocked cells, Hsp70 synthesis was markedly increased, whereas synthesis of the constitutively expressed Hsc70 remained constant in both heat-shocked and pressurized cells (data not shown).
Figure 1.
(A) Western blot analysis with antibodies against Hsp70 and Hsc70. Samples were from whole cell extracts of nonstressed control cells (0 h), cells exposed to 30-MPa static (30 sHP) or cyclic (30 cHP) HP for 1–12 h, and cells exposed to heat shock (lane HS) at 43°C for 5 h. Experiments were repeated three times. (B) Analysis of Hsp70 protein accumulation was performed by chemiluminescence reaction, and protein levels were quantitated densitometrically.
HP Did Not Induce Transcription of hsp70 Gene or HSF1 DNA-Binding Activity.
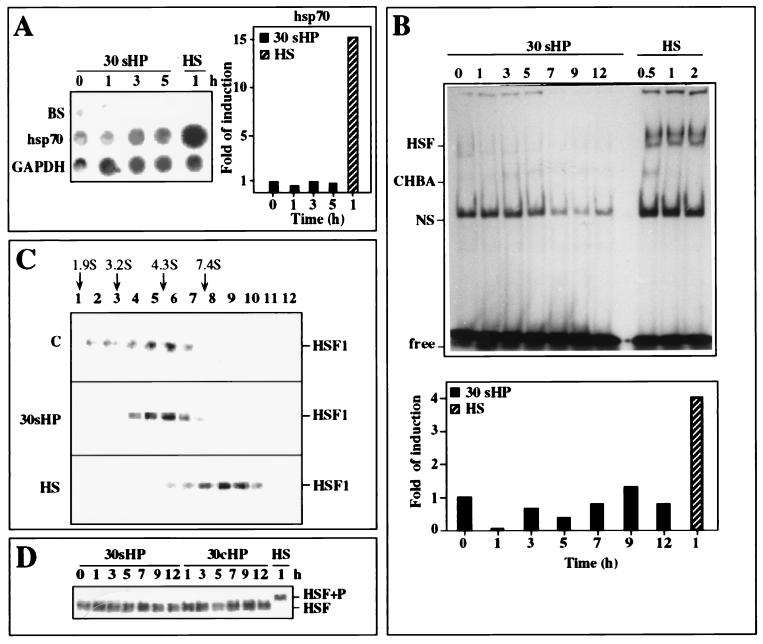
To elucidate the regulatory mechanism by which HP induced the Hsp response in chondrocytes, transcription of hsp70 gene was analyzed by nuclear run-on assay. The cells were exposed to either 30-MPa static pressure for various time periods from 1 to 5 h or to a 42°C heat shock for 1 h. As shown in Fig. 2A, hsp70 transcription was not affected by HP, whereas an approximately 15-fold induction was observed in heat-shocked cells.
Figure 2.
Analysis of hsp70 transcription rate and HSF1 activation. (A) Transcription rates of hsp70 and GAPDH genes were analyzed by nuclear run-on assay. Equal number of nuclei were isolated from nonstressed control cells (0 h) and from cells exposed to 30-MPa HP (30 sHP) for 1–5 h or to heat shock (HS) at 42°C for 1 h. Band BS indicates the Bluescript plasmid that was used as a vector control, and GAPDH was used as a loading control. Quantitative analysis of the hsp70 transcription rate, relative to GAPDH, was performed by using a PhosphorImager (Molecular Dynamics). (B) Gel mobility shift assay of HSF1-binding activity to a 35-bp 32P-labeled oligonucleotide containing the HSE was performed with whole cell extracts isolated from nonstressed control cells (0 h) or cells exposed to 30-MPa static HP (30 sHP) for 1–12 h or to heat shock (lanes HS) at 43°C for 0.5, 1, and 2 h. Quantitative analysis of HSF1 DNA-binding activity, relative to levels of nonspecific HSE-binding activity (NS) was performed by using a PhosphorImager. CHBA, constitutive binding activity to HSE; Free, unbound labeled HSE oligonucleotide. (C) HSF1 in the whole cell extracts (500 μg of protein) was analyzed by 15–50% glycerol gradient sedimentation method, and the positions of HSF1 proteins were visualized by Western blot analysis. Cells were either untreated (blot C) or exposed to 30-MPa static HP (blot 30 sHP) for 1 h or to heat shock (blot HS) at 43°C for 1 h. The sedimentation positions of protein standards are indicated (cytochrome c, 1.9 S; BSA, 3.2 S; carbonic anhydrase, 4.3 S; alcohol dehydrogenase, 7.4 S). (D) HSF1 hyperphosphorylation analysis with antibody against HSF1 by Western blotting from control nonstressed cells (0 h) or cells exposed to 30-MPa static (30 sHP) or 30-MPa cyclic HP (30 cHP) for 1–12 h or to heat shock (lane HS) at 43°C for 1 h. HSF1+P indicates the position of the HSF1 after hyperphosphorylation.
To confirm that the HP-mediated Hsp response was not transcriptionally regulated, HSF1 DNA-binding activity was analyzed by electrophoretic mobility shift assay with a synthetic oligonucleotide containing the proximal HSE of the human hsp70 promoter. The chondrocytes were exposed to 30-MPa static pressure for various time periods extending to 12 h or to a 43°C heat shock for 0.5, 1, and 2 h. In untreated chondrocytes, a low basal level of HSE-binding activity could be observed, which was not increased by static 30-MPa HP. However, HSE-binding activity was clearly stimulated in heat-shocked cells (Fig. 2B). Addition of a 100-fold competitive unlabeled oligonucleotide to the samples prevented the DNA-binding activity, showing the specificity of the assay (data not shown).
Due to the low but clearly detectable basal HSE-binding activity in untreated cells (Fig. 2B), we analyzed the oligomeric state of HSF1 in nonstressed control cells and in cells exposed either to 30-MPa continuous HP for 1 h or to a 43°C heat shock for 1 h. For sedimentation analysis, whole cell extracts were fractionated by centrifuging aliquots through 15–50% glycerol gradients, and the location of HSF1 was determined by Western blot analysis. In the samples isolated from untreated cells and those exposed to static HP, HSF1 was present mainly in fractions 5 and 6, whereas in heat-shocked cells, a clear shift of HSF1 position into fractions 9 and 10 could be detected (Fig. 2C). On the basis of earlier results (38, 44), a shift in sedimentation indicates trimerization of HSF1 upon activation by heat-stress, whereas in pressurized cells HSF1 remained in a monomeric form.
Finally, the phosphorylation state of HSF1, based on altered migration of hyperphosphorylated HSF1 on SDS/PAGE, was studied. As shown in Fig. 2D, we could not observe any changes in HSF1 migration after 30-MPa static or cyclic HP exposure extended to 12 h (Fig. 2D), leading to a conclusion that, in contrast to heat-shocked chondrocytes, no induction of HSF1 DNA-binding activity or hsp70 transcription occurred during HP treatment. Hence, the mechanisms regulating the cellular stress response upon exposure to high continuous HP and elevated temperature are likely to be distinct.
Steady-State Levels of hsp70 mRNA Increased During 30-MPa Static HP Treatment.
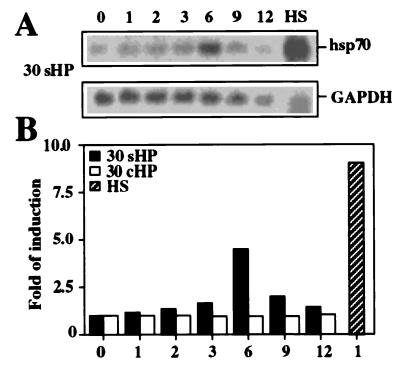
Because the accumulation of Hsp70 was not due to transcriptional activation, the steady-state levels of the corresponding mRNA were determined by Northern blot analysis. Similar to the analyses shown above, the cells were nonstressed or exposed to 30-MPa static and cyclic HP for up to 12 h or to a 43°C heat shock for 1 h. During continuous HP, the steady-state level of hsp70 mRNA increased up to 6 h, after which an attenuation was observed (Fig. 3A). The amount of hsp70 mRNA increased slightly less than 5-fold (Fig. 3B), which is rather well comparable with the increase in Hsp70 protein levels (Fig. 1). In accordance with our protein data (Fig. 1), the 30-MPa cyclic HP treatment did not affect the steady-state level of hsp70 mRNA (Fig. 3B). Furthermore, a 4-MPa continuous or cyclic HP did not change hsp70 mRNA levels (data not shown). Approximately 10-fold induction of the steady-state level of hsp70 mRNA was observed in response to elevated temperature (Fig. 3B) that corresponds to the nuclear run-on data shown in Fig. 2A. Thus, in the beginning of static pressurization at 30 MPa, the translation of hsp70 mRNA into Hsp70 protein quite closely followed the amount of hsp70 mRNA present in the cells. However, the accumulation of Hsp70 protein continued for at least up to 12 h despite the decrease in the steady-state mRNA level observed after 6 h of the HP treatment.
Figure 3.
Analysis of the steady-state level of hsp70 mRNA by Northern blot hybridization. (A) Samples were from control nonstressed cells (0 h) or cells exposed to 30-MPa static HP (30 sHP) up to 12 h or to heat shock (lane HS) at 43°C for 1 h. The RNA samples were hybridized with the 32P-labeled cDNA probes for hsp70 and GAPDH. The experiments were repeated three times. (B) Quantitative analysis of hsp70 mRNA levels, relative to GAPDH, was performed by using a PhosphorImager.
Stabilization of hsp70 mRNA in HP-Mediated Heat Shock Response.
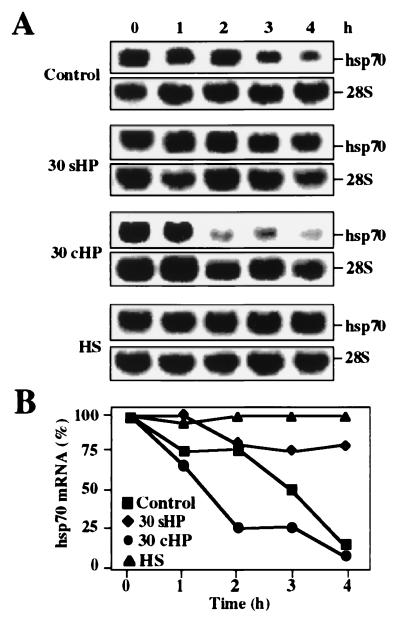
Because hsp70 mRNA levels increased relatively slowly and without transcriptional induction during 30-MPa static HP treatment, we investigated whether the stability of hsp70 mRNA was altered. For this purpose, the cells were treated with actinomycin D after the hydrostatic loading to block the novel synthesis of mRNA molecules. The RNA samples were collected at 1-h intervals after addition of actinomycin D for up to 4 h, and the Northern blots were probed for hsp70 mRNA and 28 rRNA (Fig. 4A). Quantitative densitometry of the bands indicated that hsp70 mRNA remained at considerably higher level in the cells exposed to static 30-MPa HP than in the untreated control cells (Fig. 4B). However, in cells exposed to 30-MPa cyclic HP, hsp70 mRNA decayed even faster than in control cells. In heat-shocked cells, the steady-state level of hsp70 mRNA hardly changed in the presence of actinomycin D (Fig. 4), indicating effective stabilization in agreement with previous studies (18).
Figure 4.
Analysis of hsp70 mRNA stability by Northern blot hybridization. (A) Control nontreated cells (blot C), cells exposed to 30-MPa static (30 sHP) or cyclic HP (30 cHP) for 3 h, and to heat shock (HS) at 43°C for 1 h were incubated in the presence of 5 μM actinomycin D for 0–4 h. The RNA samples were hybridized with hsp70 and 28S probe. The 28S rRNA served as a normalization control. (B) Quantitative analysis of hsp70 mRNA levels, relative to 28S rRNA, was performed by using a PhosphorImager.
DISCUSSION
In this study, we examined the effect of mechanical loading on heat shock genes in chondrocyte-like cells. We subjected cell cultures to 4- and 30-MPa HP both in static and cyclic modes for various times. Strikingly, a specific heat shock response was initiated by 30-MPa static HP. The stress response resulted in the accumulation of Hsp70 at both mRNA and protein levels, which was due to stabilization of hsp70 mRNA. HSF1 activation or transcriptional induction could not be detected when cells were exposed to different modes of HP. Interestingly, hsp70 gene expression was not observed in cyclically loaded cells. This indicates a discoordinate stress stimuli between static and cyclic HP in the regulation of hsp70 genes.
Previous studies have shown that heat shock genes are regulated at both transcriptional and posttranscriptional levels with the highly conserved pattern of regulatory events that are independent of cell type or differentiation (1). Upon stress, transcriptional induction of hsp70 gene is mainly accompanied with induced HSF1 trimerization, acquisition of DNA-binding activity, and hyperphosphorylation (9–11, 15). In addition, transcription of heat shock genes can be mediated through a basal transcriptional complex in certain promoting circumstances (16, 17), and regulation of hsp70 mRNA translation at the level of elongation has been reported (44). Our observation that the Hsp response is associated with hsp70 mRNA stabilization rather than HSF1 activation or transcriptional induction in the pressurized cells reveals a mechanism in the complex pattern involved in the regulation of heat shock gene expression.
We show herein that 30-MPa continuous HP caused Hsp response due to hsp70 mRNA stabilization at early time points. However, after a peak value at 6 h the mRNA level decreased. We have previously shown that, under the high static loading conditions used in this experiment, the total protein synthesis is remarkably decreased (34). Interestingly, a previous study showing a coincident block in total protein synthesis and stabilization of hsp70 mRNA by heat shock (18) suggests that regulation of mRNA turnover might be important in the control of protein synthesis under cellular stress. Although the mechanisms underlying mRNA stabilization are not well known today, at least certain conserved nucleotide sequences present in the mRNAs are known to affect their turnover rates (45, 46). Whether the stabilization of hsp70 mRNA in the beginning of continuous 30-MPa pressurization involves an increased expression of some specific RNA-binding proteins remains open. However, there are known examples that show binding of a translated protein to its corresponding mRNA (47, 48) or binding of a hormone-inducible protein to another hormone-stabilized mRNA species (49), thereby affecting the message stability. In a similar way, in the pressurized chondrocytes, the accumulation of Hsp70 protein may lead to interactions with hsp70 mRNA that regulate message stability.
Our results indicate that 30-MPa static HP increased the levels of hsp70 mRNA and Hsp70 protein to a lesser extent than heat shock. Furthermore, a relatively slow Hsp70 response, with no sign of transcriptional activation, could be seen in pressurized cells compared with heat-shocked cultures. These findings reveal a moderate stress response in the cells exposed to HP. HSF1 activation and transcriptional induction appeared not to be absolutely required for stress response in these loading conditions that lead to a posttranscriptional regulation of hsp70 gene expression through mRNA stabilization.
Earlier reports of the effects of HP have shown that continuous high HP leads to a reversible depolymerization of microtubules in vitro (50), a change from the normal stacked appearance of the Golgi apparatus to a tightly packed perinuclear clump (33), and an inhibition of total proteoglycan and protein synthesis in bovine chondrocytes (32, 34). In line with our results, a 50-MPa HP treatment was recently shown to increase hsp70 mRNA levels in chondrocyte-like HCS-2/8 cells (51). Our study provides a mechanism that explains the induction. Hsps are known to act as molecular chaperones that participate in the biogenesis of proteins including their synthesis, folding, assembly, disassembly, and translocation (1, 52–55). We suggest that high static HP may initiate the synthesis of Hsp70 proteins to prevent misfolding of the cellular proteins, and they might aid in the protection of new matrix protein synthesis. Intermittent hydrostatic pressure appears to give the cells an opportunity to recover from the strenuous conditions, because cyclic loading did not have as adverse an impact on cytoskeleton as continuous loading (35), and no stress response in any cyclic loading condition was observed in this study.
It has been well documented that the Hsp response can vary in a cell-type-specific manner (56, 57). Chondrocytes are highly differentiated cells that produce and maintain extracellular matrix including cartilage-specific proteoglycans and collagens. The immortalized chondrocytes used in this study express type II collagen and aggrecan in defined culture conditions (36) and have served as a reproducible model that exhibits cellular responses similar to primary human chondrocyte cultures (58). Articular cartilage has to withstand very high compressive loads. Within joint, the compressive forces on articular cartilage can rise from 0.1–0.2 MPa when unloaded to 20 MPa on standing, and cycle between 4 and 5 MPa when walking (19). Whether HP or other mechanical loading stimuli would cause a different heat shock response in cells that normally do not experience a continuous HP gradient or whether HP treatment affects differentiation in a cell-type-specific manner remains to be examined. However, our data show that Hsp response may arise in excessive loading conditions, possibly signaling an important pathway occurring as part of the pathological response in cartilage diseases.
Acknowledgments
This work was supported by the Academy of Finland, the Sigrid Juselius Foundation, and the Finnish Cultural Foundation and its North Savo Fund.
ABBREVIATIONS
- Hsp
heat shock protein
- HSF
heat shock factor
- HSE
heat shock element
- HP
hydrostatic pressure
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- SV40
simian virus 40
References
- 1.Lindquist S, Craig E A. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto R I. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- 3.Wu C. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 4.Rabindran S K, Giorgi G, Clos J, Wu C. Proc Natl Acad Sci USA. 1991;88:6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarge K D, Zimarino V, Holm K, Wu C, Morimoto R I. Genes Dev. 1991;5:1902–1911. doi: 10.1101/gad.5.10.1902. [DOI] [PubMed] [Google Scholar]
- 6.Schuetz T J, Gallo G J, Sheldon L, Tempst P, Kingston R E. Proc Natl Acad Sci USA. 1991;88:6911–6915. doi: 10.1073/pnas.88.16.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakai A, Morimoto R I. Mol Cell Biol. 1993;13:1983–1997. doi: 10.1128/mcb.13.4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakai A, Tanabe M, Kawazoe W, Inazawa J, Morimoto R I, Nagata K. Mol Cell Biol. 1997;17:469–481. doi: 10.1128/mcb.17.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baler R, Dahl G, Voellmy R. Mol Cell Biol. 1993;13:2486–2496. doi: 10.1128/mcb.13.4.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurivich D A, Sistonen L, Sarge K D, Morimoto R I. Proc Natl Acad Sci USA. 1994;91:2280–2284. doi: 10.1073/pnas.91.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarge K D, Murphy S P, Morimoto R I. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurivich D A, Sistonen L, Kroes R A, Morimoto R I. Science. 1992;255:1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- 13.Jurivich D A, Pachetti C, Qiu L, Welk J F. J Biol Chem. 1995;270:24489–24495. doi: 10.1074/jbc.270.41.24489. [DOI] [PubMed] [Google Scholar]
- 14.Lee B S, Chen J, Angelidis C, Jurivich D A, Morimoto R I. Proc Natl Acad Sci USA. 1995;92:7207–7211. doi: 10.1073/pnas.92.16.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotto J J, Kline M, Morimoto R I. J Biol Chem. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- 16.Wu B J, Morimoto R I. Proc Natl Acad Sci USA. 1985;82:6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams G T, McClanahan T K, Morimoto R I. Mol Cell Biol. 1989;9:2574–2587. doi: 10.1128/mcb.9.6.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theodorakis N G, Morimoto R I. Mol Cell Biol. 1987;7:4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muir H. BioEssays. 1995;17:1039–1048. doi: 10.1002/bies.950171208. [DOI] [PubMed] [Google Scholar]
- 20.Palmoski M, Perricone E, Brandt K D. Arthritis Rheum. 1979;22:508–517. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- 21.Palmoski M J, Colyer R A, Brandt K D. Arthritis Rheum. 1980;23:325–334. doi: 10.1002/art.1780230310. [DOI] [PubMed] [Google Scholar]
- 22.Kiviranta I, Jurvelin J, Tammi M, Säämänen A-M, Helminen H J. Arthritis Rheum. 1987;30:801–808. doi: 10.1002/art.1780300710. [DOI] [PubMed] [Google Scholar]
- 23.Tammi M, Paukkonen K, Kiviranta I, Jurvelin J, Säämänen A-M, Helminen H J. In: Joint Loading—Biology and Health of Articular Structures. Helminen H J, Kiviranta I, Tammi M, Säämänen A-M, Paukkonen K, Jurvelin J, editors. Bristol, U.K.: Wright; 1987. pp. 64–88. [Google Scholar]
- 24.Säämänen A-M, Tammi M, Kiviranta I, Jurvelin J, Helminen H J. Arthritis Rheum. 1989;32:1282–1292. doi: 10.1002/anr.1780321014. [DOI] [PubMed] [Google Scholar]
- 25.Jurvelin J, Kiviranta I, Säämänen A-M, Tammi M, Helminen H J. J Biomech. 1990;23:1239–1246. doi: 10.1016/0021-9290(90)90381-c. [DOI] [PubMed] [Google Scholar]
- 26.Arokoski J, Kiviranta I, Jurvelin J, Tammi M, Helminen H J. Arthritis Rheum. 1993;36:1451–1459. doi: 10.1002/art.1780361018. [DOI] [PubMed] [Google Scholar]
- 27.Gray M L, Pizzanelli A M, Grodzinsky A J, Lee R C. J Orthop Res. 1988;6:777–792. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 28.Sah R L, Kim Y-J, Doong J, Grodzinsky A J, Plaas A H K, Sandy J D. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 29.Larsson T, Aspden R M, HeinegŚrd D. Matrix. 1991;11:388–394. doi: 10.1016/s0934-8832(11)80193-9. [DOI] [PubMed] [Google Scholar]
- 30.Parkkinen J J, Lammi M J, Helminen H J, Tammi M. J Orthop Res. 1992;10:610–620. doi: 10.1002/jor.1100100503. [DOI] [PubMed] [Google Scholar]
- 31.Parkkinen J J, Ikonen J, Lammi M J, Laakkonen J, Tammi M, Helminen H J. Arch Biochem Biophys. 1993;300:458–465. doi: 10.1006/abbi.1993.1062. [DOI] [PubMed] [Google Scholar]
- 32.Hall A C, Urban J P G, Gehl K A. J Orthop Res. 1991;9:1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- 33.Parkkinen J, Lammi M J, Pelttari A, Helminen H J, Tammi M, Virtanen I. Ann Rheum Dis. 1993;52:192–198. doi: 10.1136/ard.52.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lammi M J, Inkinen R I, Parkkinen J J, Jortikka M, Häkkinen T P, Nelimarkka L O, Järveläinen H T, Tammi M. Biochem J. 1994;304:723–730. doi: 10.1042/bj3040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parkkinen J J, Lammi M J, Inkinen R, Jortikka M, Tammi M, Virtanen I, Helminen H J. J Orthop Res. 1995;13:495–502. doi: 10.1002/jor.1100130404. [DOI] [PubMed] [Google Scholar]
- 36.Goldring M B, Birkhead J R, Suen L-F, Yamin R, Mizuno S, Glowacki J, Arbiser J L, Apperley J F. J Clin Invest. 1994;94:2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosser D D, Theodorakis N G, Morimoto R I. Mol Cell Biol. 1988;8:4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sistonen L, Sarge K D, Morimoto R I. Mol Cell Biol. 1994;14:2087–2099. doi: 10.1128/mcb.14.3.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banerji S, Theodorakis N G, Morimoto R I. Mol Cell Biol. 1984;4:2437–2448. doi: 10.1128/mcb.4.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu B, Hunt C, Morimoto R I. Mol Cell Biol. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard J M. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sistonen L, Sarge K D, Phillips B, Abravaya K, Morimoto R I. Mol Cell Biol. 1992;12:4104–4111. doi: 10.1128/mcb.12.9.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iruela-Arispe L, Hasselaar P, Sage H. Lab Invest. 1991;64:174–186. [PubMed] [Google Scholar]
- 44.Theodorakis N G, Banerji S S, Morimoto R I. J Biol Chem. 1988;263:14579–14585. [PubMed] [Google Scholar]
- 45.Hel Z, Skamene E, Radzioch D. Mol Cell Biol. 1996;16:5579–5590. doi: 10.1128/mcb.16.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amara F M, Entwistle J, Kuschak T I, Turley E A, Wright J A. J Biol Chem. 1996;271:15279–15284. doi: 10.1074/jbc.271.25.15279. [DOI] [PubMed] [Google Scholar]
- 47.Chu E, Takimoto C H, Voeller D, Grem J L, Allegra C J. Biochemistry. 1993;32:4756–4760. doi: 10.1021/bi00069a009. [DOI] [PubMed] [Google Scholar]
- 48.Voeller D M, Changchien L M, Maley G F, Maley F, Takechi T, Turner R E, Montford W R, Allegra C J, Chu E. Nucleic Acids Res. 1995;23:869–875. doi: 10.1093/nar/23.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dodson R E, Shapiro D J. Mol Cell Biol. 1994;14:3130–3138. doi: 10.1128/mcb.14.5.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salmon E D. Science. 1975;189:884–886. doi: 10.1126/science.1171523. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi K, Kubo T, Kobayashi K, Imanishi J, Takigawa M, Arai Y, Hirasawa Y. J Orthop Res. 1997;15:150–158. doi: 10.1002/jor.1100150122. [DOI] [PubMed] [Google Scholar]
- 52.Gething M J, Sambrook J. Nature (London) 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 53.Georgopoulos C, Welch W J. Annu Rev Cell Biol. 1993;9:601–635. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 54.Hendrick J P, Hartl F U. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 55.Morimoto R I, Tissieres A, Georgopoulos C. The Biology of Heat Shock Proteins and Molecular Chaperones. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 1–593. [Google Scholar]
- 56.Blake M J, Gershon D, Fargnoli J, Holbrook N J. J Biol Chem. 1990;265:15275–15279. [PubMed] [Google Scholar]
- 57.Mathur S K, Sistonen L, Brown I R, Murphy S P, Sarge K D, Morimoto R I. Proc Natl Acad Sci USA. 1994;91:8695–8699. doi: 10.1073/pnas.91.18.8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loeser R F, Varnum B C, Carlson C S, Goldring M B, Liu E T, Sadiev S, Kute T E, Wallin R. Arthritis Rheum. 1997;40:1455–1465. doi: 10.1002/art.1780400814. [DOI] [PubMed] [Google Scholar]