Abstract
In a longitudinal cohort diarrhea study, a girl living in Lima, Peru, and her brother and dog were diagnosed with Cryptosporidium canis infections during the same period. Both children had transient diarrhea, but the dog was asymptomatic. This is the first report of possible transmission of cryptosporidiosis between humans and dogs.
The role of companion animals in the transmission of human cryptosporidiosis is not clear. It is been suggested for some time that dogs can be a significant source of human cryptosporidiosis (1, 5, 16). This suggestion, however, has been largely based on the observation of direct transmission of Cryptosporidium parvum from calves to humans and the erroneous suggestion that C. parvum is responsible for cryptosporidiosis in all mammals. Case-control studies conducted in the United States have suggested only a weak association between the occurrence of cryptosporidiosis in human immunodeficiency virus-positive persons and contact with dogs (6). Weak associations between the occurrence of pediatric cryptosporidiosis and contact with dogs or cats have also been observed in Guinea-Bissau and Indonesia (8, 11). In contrast, contact with dogs and cats is not a risk factor for cryptosporidiosis in England (7) and is actually a protective factor in Australia (15).
Recent molecular epidemiological studies have shown that dogs and cats are infected almost exclusively with Cryptosporidium canis and Cryptosporidium felis, respectively, whereas humans are infected mostly with Cryptosporidium hominis and C. parvum (19). Thus, the role of companion animals in the transmission of human cryptosporidiosis may be limited. Even though a small number of humans are infected with C. canis and C. felis, recent findings of concurrent C. hominis infection in C. canis-infected persons suggest that many of the C. canis infections in humans may be due to anthroponotic rather than zoonotic transmission (2).
In this study, we report the first possible transmission of cryptosporidiosis between humans and a dog living in the same household in Lima, Peru.
Study design.
The findings reported here were from a longitudinal prospective pediatric cohort study of enteric parasites conducted in Pampas de San Juan, Peru, from March 2002 to March 2006. This study was approved by the institutional review boards of the participating research institutions. As part of the study procedures, we visited children enrolled in the study (a total of 660 children under 8 years old during the entire study period) daily to check for the occurrence of diarrhea and other clinical symptoms and collected weekly stool specimens from all participants for microscopic detection of ova and parasites, Cryptosporidium, Giardia, Cyclospora, and microsporidia. Once a child became positive for Cryptosporidium, Cyclospora, or microsporidia, all siblings and animals in the household were sampled and daily stool specimens were collected from the infected child until the resolution of the infection (when specimens were consecutively negative for the pathogen of interest for 7 days).
Laboratory analyses.
Cryptosporidium oocysts were microscopically detected using a modified acid-fast stain. Positive stool specimens were preserved in 2.5% potassium dichromate and stored at 4°C for genotyping. DNA was extracted from the washed stools using the FastDNA for soil kit (Qbiogene, Irvine CA), and the Cryptosporidium spp. present were genotyped using a small-subunit (SSU) rRNA-based PCR-restriction fragment length polymorphism (RFLP) analysis (18). The identification of C. canis in this study was confirmed by DNA sequence analysis of the PCR products. DNA sequencing was performed using a BigDye terminator cycle sequencing ready reaction kit and an ABI 3100 automated sequencer (Applied Biosystems, Foster City, CA) after PCR products were purified with Microcon centrifugal filter devices (Millipore, Bedford, MA).
Index case.
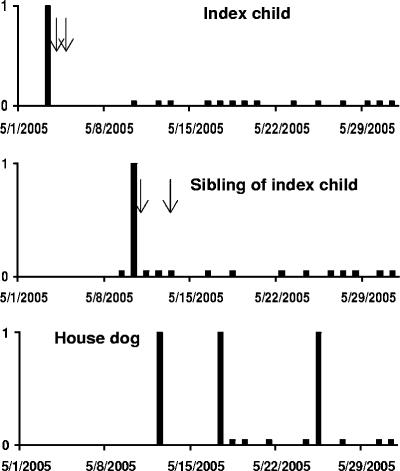
On 3 May 2005, we identified a Cryptosporidium infection in a 32-month-old girl in a household; the girl had transient diarrhea on two subsequent days (4 and 5 May 2005). The examination of additional specimens collected from the girl for 15 days yielded no further positive stools (Fig. 1). The girl had previously had another episode of cryptosporidiosis on 15 March 2004, and she had a subsequent episode of cryptosporidiosis on 26 September 2005. The first episode of infection lasted for 1 day and was associated with no clinical symptoms. The third episode of cryptosporidiosis lasted for at least 10 days and was also not associated with any clinical symptoms.
FIG. 1.
Timeline of Cryptosporidium canis infection in the household. Cryptosporidium canis infection was first detected in the index child (top panel) on 3 May 2005, then in a sibling on 10 May 2005 (middle panel), and subsequently in the household dog on 12, 17, and 25 May 2005 (lower panel). High bars indicate C. canis-positive stools, and low bars indicate C. canis-negative stools. Arrows indicate the occurrence of diarrhea. The index child also had two other episodes of cryptosporidiosis, on 15 March 2004 and 26 September 2005.
Household infections.
After the girl was diagnosed with the second episode of cryptosporidiosis (index case) on 3 May 2005, we collected 15 stool specimens between 9 May and 1 June 2005 from a sibling (a 6.5-year-old boy) living in the household. Only one stool specimen, collected on 10 May, was positive for Cryptosporidium. The sibling had diarrhea on 10 May and 13 May (Fig. 1). Four animals living in the household, including a chick, a hen, a rabbit, and a dog, were also sampled. However, only the dog was positive for Cryptosporidium on the day of initial sampling, 12 May 2005. Two of the 17 subsequent fecal specimens collected from the dog from 17 May to 16 June 2005 were also Cryptosporidium positive, one on 17 May and one on 25 May. The infected dog remained healthy during the infection episode (Fig. 1).
Cryptosporidium genotype in the household.
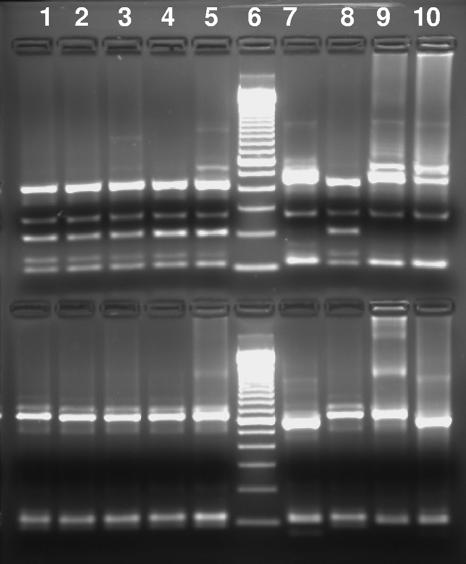
The organism in the only Cryptosporidium-positive stool specimen collected from the index child on 3 May 2005 during the second episode of cryptosporidiosis was genotyped as C. canis. Likewise, the organism in the stool specimen collected on 10 May from the sibling was also genotyped as C. canis. The organisms in all three fecal specimens collected from the dog in the household during the period (on 12, 17, and 25 May 2005) were genotyped as C. canis (Fig. 2). All SSU rRNA gene sequences obtained from specimens from the index child, the sibling, and the dog were identical to each other and to the reference C. canis sequence (AF112576) deposited in the GenBank database.
FIG. 2.
PCR-RFLP analysis of the SSU rRNA gene of Cryptosporidium spp. in stool specimens collected from the same household. Lane 1, specimen from the index child on 3 May 2005; lane 2, specimen from the sibling on 10 May 2005; lanes 3 to 5, specimens from the household dog on 12, 17, and 25 May 2005; lane 6, 100-bp molecular size ladder; lane 7, specimen from the index child collected during the third episode of cryptosporidiosis (on 26 September 2005); lane 8, positive control for C. canis; lane 9, positive control for C. parvum; lane 10, positive control for C. hominis. Notice that the upper SspI band in C. canis is smaller than those in C. parvum and C. hominis. The upper area shows SspI RFLP products, whereas the lower area shows VspI RFLP products. The fragment of ∼200 bp in the SspI lanes of C. canis was present in PCR products of C. canis but absent in C. hominis or C. parvum PCR products. It is also not seen in VspI RFLP products.
During the first episode of infection, no stool specimens for Cryptosporidium genotyping were available from the index child. The organisms in the only two Cryptosporidium-positive stool specimens collected during the third episode of cryptosporidiosis from the index child on 26 September and 6 October 2005 were genotyped as C. hominis (Fig. 2).
Public health significance.
This is the first report of possible transmission of C. canis among members living in the same household. Previously, all reported cases of direct zoonotic transmission of cryptosporidiosis were caused by C. parvum. These cases were a result of individuals either caring for Cryptosporidium-infected calves or contacting infected calves or lambs during farm visits (3, 4, 9, 10, 13, 14, 17). In this study, the index child, her sibling, and the household dog were all infected with C. canis, a cryptosporidium commonly found in dogs but only occasionally found in children (12, 18). Intrafamilial transmission of C. canis probably occurred in the household because C. canis infection in the study community is rare (18) and we did not see an increased occurrence of C. canis in children in other households during the period, which would reveal the occurrence of a possible waterborne outbreak of C. canis infection in the community. Because the sampling of the sibling and dog occurred after the initial cryptosporidiosis diagnosis in the index child due to the nature of the study design, it was impossible to decide the direction of Cryptosporidium transmission in the cluster cases. It can be argued that the rare occurrence of C. canis in humans and the common occurrence of C. canis in dogs suggest that the dog was more likely the source of infection in the two children. However, as mentioned previously, person-to-person transmission of C. canis in humans was shown to occur in AIDS patients in the study area (2). Thus, we cannot eliminate the possibility that the children had infected each other and actually transmitted the C. canis to the dog. Nevertheless, results of the study clearly demonstrate that C. canis is a Cryptosporidium sp. of public health significance, and under favorable conditions, the transmission of C. canis from dogs to humans may occur.
Acknowledgments
This work was supported in part by grants from NIH NIAID and Peru TMRC, R21 AI 059661 and 5P01AI051976, respectively, and by USDA CSREES grant 2001-51110-11340.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 18 April 2007.
REFERENCES
- 1.Bauer, D. 1994. The capacity of dogs to serve as reservoirs for gastrointestinal disease in children. Ir. Med. J. 87:184-185. [PubMed] [Google Scholar]
- 2.Cama, V., R. H. Gilman, A. Vivar, E. Ticona, Y. Ortega, C. Bern, and L. Xiao. 2006. Mixed Cryptosporidium infections and HIV. Emerg. Infect. Dis. 12:1025-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbett-Feeney, G. 1987. Cryptosporidium among children with acute diarrhoea in the west of Ireland. J. Infect. 14:79-84. [DOI] [PubMed] [Google Scholar]
- 4.Current, W. L., N. C. Reese, J. V. Ernst, W. S. Bailey, M. B. Heyman, and W. M. Weinstein. 1983. Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies of an outbreak and experimental transmission. N. Engl. J. Med. 308:1252-1257. [DOI] [PubMed] [Google Scholar]
- 5.Enriquez, C., N. Nwachuku, and C. P. Gerba. 2001. Direct exposure to animal enteric pathogens. Rev. Environ. Health 16:117-131. [DOI] [PubMed] [Google Scholar]
- 6.Glaser, C. A., S. Safrin, A. Reingold, and T. B. Newman. 1998. Association between Cryptosporidium infection and animal exposure in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:79-82. [DOI] [PubMed] [Google Scholar]
- 7.Goh, S., M. Reacher, D. P. Casemore, N. Q. Verlander, R. Chalmers, M. Knowles, J. Williams, K. Osborn, and S. Richards. 2004. Sporadic cryptosporidiosis, North Cumbria, England, 1996-2000. Emerg. Infect. Dis. 10:1007-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsumata, T., D. Hosea, E. B. Wasito, S. Kohno, K. Hara, P. Soeparto, and I. G. Ranuh. 1998. Cryptosporidiosis in Indonesia: a hospital-based study and a community-based survey. Am. J. Trop. Med. Hyg. 59:628-632. [DOI] [PubMed] [Google Scholar]
- 9.Levine, J. F., M. G. Levy, R. L. Walker, and S. Crittenden. 1988. Cryptosporidiosis in veterinary students. J. Am. Vet. Med. Assoc. 193:1413-1414. [PubMed] [Google Scholar]
- 10.Miron, D., J. Kenes, and R. Dagan. 1991. Calves as a source of an outbreak of cryptosporidiosis among young children in an agricultural closed community. Pediatr. Infect. Dis. J. 10:438-441. [DOI] [PubMed] [Google Scholar]
- 11.Molbak, K., P. Aaby, N. Hojlyng, and A. P. da Silva. 1994. Risk factors for Cryptosporidium diarrhea in early childhood: a case-control study from Guinea-Bissau, West Africa. Am. J. Epidemiol. 139:734-740. [DOI] [PubMed] [Google Scholar]
- 12.Morgan, U. M., L. Xiao, P. Monis, A. Fall, P. J. Irwin, R. Fayer, K. M. Denholm, J. Limor, A. Lal, and R. C. Thompson. 2000. Cryptosporidium spp. in domestic dogs: the “dog” genotype. Appl. Environ. Microbiol. 66:2220-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pohjola, S., H. Oksanen, L. Jokipii, and A. M. Jokipii. 1986. Outbreak of cryptosporidiosis among veterinary students. Scand. J. Infect. Dis. 18:173-178. [DOI] [PubMed] [Google Scholar]
- 14.Reif, J. S., L. Wimmer, J. A. Smith, D. A. Dargatz, and J. M. Cheney. 1989. Human cryptosporidiosis associated with an epizootic in calves. Am. J. Public Health 79:1528-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson, B., M. I. Sinclair, A. B. Forbes, M. Veitch, M. Kirk, D. Cunliffe, J. Willis, and C. K. Fairley. 2002. Case-control studies of sporadic cryptosporidiosis in Melbourne and Adelaide, Australia. Epidemiol. Infect. 128:419-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson, R. A., and R. N. Pugh. 2002. Dogs, zoonoses and immunosuppression. J. R. Soc. Health 122:95-98. [DOI] [PubMed] [Google Scholar]
- 17.Smith, K. E., S. A. Stenzel, J. B. Bender, E. Wagstrom, D. Soderlund, F. T. Leano, C. M. Taylor, P. A. Belle-Isle, and R. Danila. 2004. Outbreaks of enteric infections caused by multiple pathogens associated with calves at a farm day camp. Pediatr. Infect. Dis. J. 23:1098-1104. [PubMed] [Google Scholar]
- 18.Xiao, L., C. Bern, J. Limor, I. Sulaiman, J. Roberts, W. Checkley, L. Cabrera, R. H. Gilman, and A. A. Lal. 2001. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 183:492-497. [DOI] [PubMed] [Google Scholar]
- 19.Xiao, L., and U. M. Ryan. 2004. Cryptosporidiosis: an update in molecular epidemiology. Curr. Opin. Infect. Dis. 17:483-490. [DOI] [PubMed] [Google Scholar]