Abstract
Woodchucks infected with woodchuck hepatitis virus (WHV) are an excellent model for studying acute, self-limited and chronic hepadnaviral infections. Defects in the immunological response leading to chronicity are still unknown. Specific T-helper cell responses to WHV core and surface antigens (WHcAg and WHsAg, respectively) are associated with acute resolving infection; however, they are undetectable in chronic infection. Up to now, cytotoxic T-lymphocyte (CTL) responses could not be determined in the woodchuck. In the present study, we detected virus-specific CTL responses by a CD107a degranulation assay. The splenocytes of woodchucks in the postacute phase of WHV infection (18 months postinfection) were isolated and stimulated with overlapping peptides covering the whole WHcAg. After 6 days, the cells were restimulated and stained for CD3 and CD107a. One peptide (c96-110) turned out to be accountable for T-cell expansion and CD107a staining. Later, we applied the optimized degranulation assay to study the kinetics of the T-cell response in acute WHV infection. We found a vigorous T-cell response against peptide c96-110 with peripheral blood cells beginning at the peak of viral load (week 5) and lasting up to 15 weeks postinfection. In contrast, there was no T-cell response against peptide c96-110 detectable in chronically WHV-infected animals. Thus, with this newly established flow cytometric degranulation assay, we detected for the first time virus-specific CTLs and determined one immunodominant epitope of WHcAg in the woodchuck.
Infection with hepatitis B virus (HBV) of adults often results in an acute, self-limited hepatitis, yet in about 5% of adult patients, chronic infection is established. The cellular immune response to HBV proteins seems to be crucial for viral clearance and is associated with liver cell damage causing acute, transient hepatitis. Previous studies revealed polyclonal and multispecific CD4+ T-helper lymphocyte responses and CD8+ cytotoxic T-lymphocyte (CTL) responses in patients with acute, self-limited hepatitis (6, 9, 26). In contrast, viral persistence and chronic hepatitis are associated with low or undetectable T-cell responses (17, 18). Nevertheless, details of the immunological events leading to viral elimination or viral persistence are still unknown.
Woodchucks infected with woodchuck hepatitis virus (WHV) are an excellent model for studying acute, self-limited and chronic hepadnaviral infections. Several fundamental findings on immune responses to hepadnaviral infection and the development of hepatocellular carcinoma have been established in this animal model (10, 23, 24; for a review, see reference 19). It was demonstrated that cellular immune responses to hepadnaviral proteins induce liver injury and are crucial for viral clearance. T-helper lymphocyte responses to WHV core, surface, and X antigens (WHcAg, WHsAg, and WhxAg, respectively) in the early phase of acute infection have been shown to correlate with resolution of the acute infection, whereas the absence of these responses correlates with chronic infection (11, 13). Utilizing a [2-3H]adenine-based proliferation assay, specific peptides of the WHcAg were identified as T-helper epitopes. In addition, immunization with a particular incomplete Freund's adjuvant-emulsified WHcAg-derived peptide (amino acids [aa] 97 to 110), which contains a major T-cell epitope, protects woodchucks from acute WHV infection (12). However, in all these studies, the contribution of WHV-specific cytotoxic T cells has not been investigated.
For the optimal use of this model to study, e.g., the early T-cell response after infection or DNA vaccination, the determination of CTLs is needed. Attempts to apply the standard chromium release assay to the woodchuck system by using recombinant vaccinia virus expressing WHcAg or WHsAg have not been successful yet (Pablo Sarobe, personal communication). Alternative CTL assays like the enzyme-linked immunospot assay or gamma interferon (IFN-γ) staining are not available due to the lack of appropriate antibodies in the woodchuck model. A novel approach for the detection of virus-specific CTLs is based on their granule-dependent effector function. The killing of target cells is mediated via two major pathways: granule-dependent (perforin/granzyme) and -independent (ligand-induced cell death, e.g., Fas-FasL) mechanisms (27). The granule-dependent pathway utilizes preformed lytic granules located within the cytoplasm of CD8+ T cells. These are surrounded by a lipid bilayer containing lysosome-associated membrane glycoproteins (LAMPs), including CD107a (LAMP-1). Rapidly after T-cell receptor stimulation, degranulation occurs, releasing the contents into the immunological synapse. Consequently, CD107a is exposed to the cell surface and can be stained by specific monoclonal antibodies, thereby providing a marker for degranulation (3).
The aim of this study was the characterization of WHV-specific CTLs during acute WHV infection. We established an assay for the detection of WHV-specific CTLs in woodchucks by measuring degranulation using a CD107a antibody. We isolated lymphocytes from the spleens of individuals in the postacute phase of infection and stimulated the cells in vitro with WHcAg-derived peptides. We identified a WHcAg-specific epitope by screening the WHcAg with overlapping peptides. Having established this technique, we determined the kinetics of virus-specific CTLs in the course of acute infection. WHV-specific CTLs were detected in peripheral blood mononuclear cells (PBMC) of acutely WHV-infected woodchucks beginning at week 5 postinfection.
MATERIALS AND METHODS
Woodchucks.
All animals were purchased from North Eastern Wildlife (Ithaca, NY) or raised in our local animal facilities. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (16) and were reviewed and approved by the local Animal Care and Use Committee (Animal Care Center, University of Duisburg-Essen, Essen, Germany, and the district government of Düsseldorf, Germany). Four groups of animals were included in the study.
(i) Naïve woodchucks.
Woodchucks 30341 and 30342 without any markers of previous WHV infection were used as control animals for CTL assays.
(ii) Woodchucks in the postacute phase of WHV infection.
Six woodchucks (15810, 17214, 21026, 21027, 21029, and 21032) were inoculated intravenously with 1 × 107 WHV genome equivalents. All animals resolved the infection and tested positive for anti-WHc and anti-WHs 18 months after inoculation (postacute phase of WHV infection). Spleen specimens were obtained either by biopsies or autopsies.
(iii) Acutely WHV-infected woodchucks.
Two woodchucks (22524 and 22531) were inoculated intravenously with 1 × 107 WHV genome equivalents and monitored weekly for WHV DNA by a dot blot technique. Blood for the isolation of PBMC was drawn weekly from anesthetized woodchucks via the vena saphena and collected in EDTA monovettes (Sarstedt, Nuembrecht, Germany).
(iv) Chronically WHV-infected woodchucks.
Three woodchucks (25702, 25706, and 26519) that had been infected with WHV during their first 6 months of life were included in this study. Blood was drawn from the woodchucks that were positive for WHV DNA and anti-WHc and negative for anti-WHs when they were 2 to 3 years old.
Spleen biopsy.
After the woodchuck was anesthetized with ketamine-xylazine, the abdomen was opened via a midline incision. The intestines were moved to the left side to allow for optimal exposure of the spleen. The splenic artery and vein of the upper pole were ligated prior to clamping and removing the superior third of the spleen. Bleeding from the remnant spleen was prevented using a mass ligation. After repositioning of the intestine, the abdominal wound was closed in two layers.
Peptides.
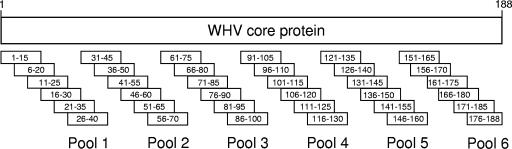
A set of 36 synthetic peptides covering the complete core antigen sequence of WHV 8 (188 aa) was purchased from EMC microcollections (Tübingen, Germany). The peptide library was constructed according to the strain that was used for inoculation, and therefore the sequences match exactly. The 15mer peptides overlap by 10 aa and were combined in six pools (Fig. 1). An unrelated cytomegalovirus (CMV)-derived peptide (YILEETSVM) served as a negative control.
FIG. 1.
Schematic illustration of 36 synthetic peptides covering the core protein of WHV 8. The 15-mer peptides were merged into six pools containing six peptides each.
Preparation and in vitro stimulation of lymphocytes from spleen and peripheral blood.
Spleens were homogenized thoroughly with a syringe plunger, and single-cell suspensions were prepared using a 70-μm nylon cell strainer (BD Biosciences, Heidelberg, Germany). Subsequently, cells were washed twice in phosphate-buffered saline (PBS; Gibco Invitrogen, Karlsruhe, Germany). Cell counting was performed manually using a hemocytometer and trypan blue exclusion microscopy.
Up to 5 × 106 splenocytes per well were plated in 24-well plates (Greiner Bio-One, Frickenhausen, Germany) in a total of 2 ml AIM-V medium (Gibco Invitrogen) supplemented with 10% fetal calf serum and 10 U/ml penicillin-streptomycin (PAA Laboratories, Pasching, Austria). For stimulation, peptide pools or individual peptides were added to a final concentration of 2 μg/ml per peptide. After 3 days, a 10-U/ml concentration of recombinant human interleukin 2 (Roche Diagnostics, Mannheim, Germany) was added.
In order to detect CTLs in the periphery, PBMC were separated by Ficoll density gradient centrifugation (Biocoll; Biochrom AG, Berlin, Germany). For stimulation, 1 × 106 cells per well were cultured for 2 days in 96-well flat-bottom plates (BD Labware, Franklin Lakes, NJ) in 200 μl of complete AIM-V medium in the presence of 2-μg/ml peptide pools or individual peptides.
CD107a degranulation assay.
Restimulation of spleen-derived lymphocytes was performed after 6 days of in vitro stimulation; PBMC were restimulated after 2 days. Thereafter, cells were transferred to 96-well round-bottom plates (Greiner Bio-One) and restimulated for 5 h with 200 μl complete AIM-V medium containing peptide pools or individual peptides in a final concentration of 2 μg/ml per peptide. Fluorescein isothiocyanate-conjugated anti-mouse CD107a antibody (BDPharmingen, Heidelberg, Germany) was added in a 1:100 dilution to the cells during the restimulation period to detect degranulation. After 5 h of restimulation, splenocytes and PBMC were washed once with PBS containing 0.1% bovine serum albumin (BSA; Calbiochem, CA). Subsequently, staining with peridinin chlorophyll a protein (PerCP)-conjugated anti-human CD4 antibody (clone L200; BDPharmingen, Heidelberg, Germany) was performed with PBS containing 0.1% BSA for 15 min at 4°C. After being washed, the cells were fixed and permeabilized using the Cytofix/Cytoperm intracellular staining kit (BD Biosciences, Heidelberg, Germany) according to the manufacturer's protocol. Thereafter, cells were stained with a primary rabbit anti-human CD3 antibody (DakoCytomation, Denmark) and a secondary phycoerythrin-labeled anti-rabbit immunoglobulin G antibody (Abcam, Cambridge, United Kingdom). Antibodies were diluted 1:80 and 1:150, respectively, in 1× BD Perm/Wash buffer including 5% BSA. Each intracellular staining was performed for 25 min at 4°C, followed by washing once.
Flow cytometry acquisition and analysis.
Data were acquired with a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany). In all cases, at least 100,000 events were collected for analysis. Data files were analyzed with FlowJo software (Tree Star, Ashland, Oregon).
Detection of WHV DNA and serology.
The spot blot technique was performed to detect WHV DNA in woodchuck sera as described previously (12). Additionally, WHV DNA was quantified by real-time PCR with a LightCycler DNA master SYBR green kit (Roche). The core-specific primers used for the PCR were wc1 (5′-TGG GGC CAT GGA CAT AGA TCC TTA-3′; nucleotides [nt] 2015 to 2038) and wc-149s (5′-AAG ATC TCT AAA TGA CTG TAT GTT CCG-3′; nt 2467 to 2451). LightCycler PCR was run at 95°C for 5 s, 53°C for 10 s, and 72°C for 10 s. A plasmid containing a full-length WHV genome served as the standard. The detection limit of this assay was 103 WHV genome equivalents per reaction.
Anti-WHcAg and anti-WHsAg were determined by enzyme-linked immunosorbent assay as described previously (20, 22).
Cloning of woodchuck CD107a DNA.
For the cloning of the woodchuck CD107a DNA, a woodchuck cDNA library derived from woodchuck PBMC RNA was used. Primers were designed based on available nucleotide sequences corresponding to human and mouse CD107a. Primer 1s (5′-ATT RCT TTT GGA AGA GGA YA-3′) and 5as (5′-TAT TTA RRA AAG TGS CAG C-3′) purchased from biomers.net (Ulm, Germany) were used in a one-round PCR under the following conditions: 94°C for 4 min, 3 cycles (94°C for 1 min, 60°C for 1 min, 72°C for 2 min), 3 cycles (94°C for 1 min, 58°C for 1 min, 72°C for 2 min), 27 cycles (94°C for 1 min, 56°C for 1 min, 72°C for 2 min), and 1 cycle 72°C for 7 min. Amplified DNA fragments were isolated from agarose gels by using the QIAquick gel extraction kit (QIAGEN, Hilden, Germany) and cloned into pCR2.1 TOPO vector using a TOPO TA cloning kit (Invitrogen, Karlsruhe, Germany). Recombinant clones were selected and sequenced in both directions using an ABI Prism 3100 sequencer and the BigDye primer cycle sequencing protocol (Applied Biosystems, Darmstadt, Germany).
Nucleotide sequence accession number.
The sequence of the fragment of woodchuck CD107a mRNA has been submitted to GenBank under accession number EF159727.
RESULTS
Cloning of woodchuck CD107a DNA.
Cloning of the woodchuck CD107a DNA was performed to assess the homology of woodchuck CD107a to human and murine CD107a and to evaluate the potential binding of commercial antibodies. The amplification of a putative woodchuck CD107a sequence using the woodchuck PBMC cDNA library as a target and the “consensus” primers for human and murine CD107a mRNA sequences repeatedly yielded a fragment with an approximate size of 1,500 bp (data not shown). Subsequent analysis revealed that a 970-bp fragment of amplified putative woodchuck CD107a nucleotide sequence demonstrated significant similarity with the sequences of CD107a mRNAs for several species reported to GenBank. Thus, the similarity of the woodchuck CD107a nucleotide sequence to the corresponding sequences from Mus musculus, Homo sapiens, and Macaca fascicularis was 75%, 81%, and 82%, respectively. At the amino acid level, the similarity levels reached 68%, 78%, and 79%, correspondingly. The extra 530 bp also share a homology of about 80% with CD107a sequences.
Due to the high similarity of the identified fragment of woodchuck CD107a to sequences from other species, the cross-reactivity of commercially available monoclonal antibodies was likely. Therefore, in pilot experiments, two antibodies to human CD107a and one antibody to mouse CD107a were tested with peptide-stimulated spleen cells of woodchucks. Only one of these displayed specific binding to woodchuck cells (data not shown) and, hence, was used in the following experiments. The epitope to which this anti-CD107a antibody binds is not known.
Characterization of WHV core-specific T cells from the spleens of animals with resolved infections.
Spleen cells are frequently used in the mouse system to demonstrate virus-specific CTLs in acute infection. Furthermore, in resolved infection, memory T cells are assumed to be present in the spleen. In our first set of experiments, we therefore used splenocytes from woodchucks with resolved infection to characterize WHV-specific CTLs. Cells were isolated from biopsy samples obtained by partial splenectomies of woodchucks in the postacute phase of infection. Regarding both animal health and spleen cell viability, two to three consecutive surgeries at intervals of 2 weeks could be carried out (data not shown). Splenocytes (5 × 106 per well) of these woodchucks were isolated 18 months postinfection and stimulated repeatedly in vitro with six pools containing six peptides each (15 aa) covering the complete WHcAg. For controls, cells either were left unstimulated, were stimulated with an unrelated CMV-derived peptide, or were stimulated with a peptide pool but not restimulated. Recombinant human interleukin 2 was added to the cells after 3 days to promote proliferation. After 6 days of stimulation, cells were restimulated for 5 h and stained for the degranulation marker CD107a. Since there is no antibody against woodchuck CD8 available, cells were also stained for T-cell markers CD3 and CD4. Analysis of data was performed by gating the main (lymphocyte) population in the forward scatter versus the side scatter plot. Subsequently, two-color plots depicting CD3 versus CD107a were generated. Gates were set according to a rat immunoglobulin G2a isotype control (data not shown).
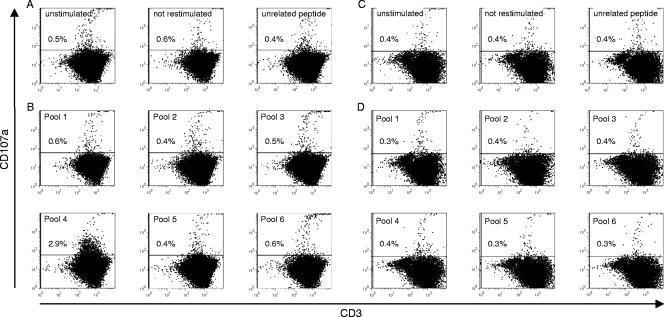
As Fig. 2A shows, there was a background of 0.4% to 0.6% CD107a+ T cells of all gated lymphocytes in unstimulated, not-restimulated, and control peptide-stimulated samples from woodchucks that had resolved infection. Stimulation with WHcAg-derived peptide pools 1, 2, 3, 5, and 6 also resulted in 0.4% to 0.6% CD107a+ T cells (Fig. 2B). However, cells that were stimulated with peptide pool 4 exhibited 2.9% CD107a staining (Fig. 2B), representing a nearly fivefold increase compared to the unstimulated control.
FIG. 2.
Representative dotplots of splenocytes from a woodchuck with resolved WHV infection (17214)(A and B) and from a naïve control animal (30341)(C and D). Lymphocytes were isolated and stimulated repeatedly in vitro for 6 days with WHcAg-derived peptide pools (B and D). As controls, cells were left unstimulated, not restimulated, or stimulated with an unrelated CMV peptide (A and C). Six animals that had resolved infection and two naïve control animals were analyzed for their responses to stimulation with peptide pools.
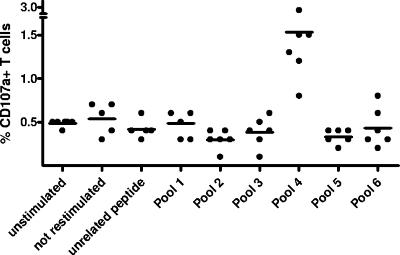
The splenocytes of six woodchucks that had resolved WHV infection were analyzed for degranulation after in vitro stimulation. All six animals showed a response to stimulation with pool 4 ranging from 0.8% to 2.9% of CD107a+ T cells, which is significantly higher than the background of 0.4% to 0.5% in unstimulated controls (Mann-Whitney U test, P < 0.01) (Fig. 3).
FIG. 3.
Degranulation responses of splenocytes from six woodchucks that had resolved WHV infection. Splenocytes were isolated, stimulated in vitro for 6 days as indicated, and subsequently subjected to the CD107a degranulation assay. The mean value for each group is indicated by a bar. The difference between the group receiving stimulation with peptide pool 4 and the unstimulated controls was analyzed by using the Mann-Whitney U test (P < .01).
The splenocytes of the two WHV-negative control animals showed no degranulation response (0.3% to 0.4%) after stimulation with WHcAg-derived peptide pool 4 (Fig. 2C and D).
Epitope mapping for WHV core-specific CTLs.
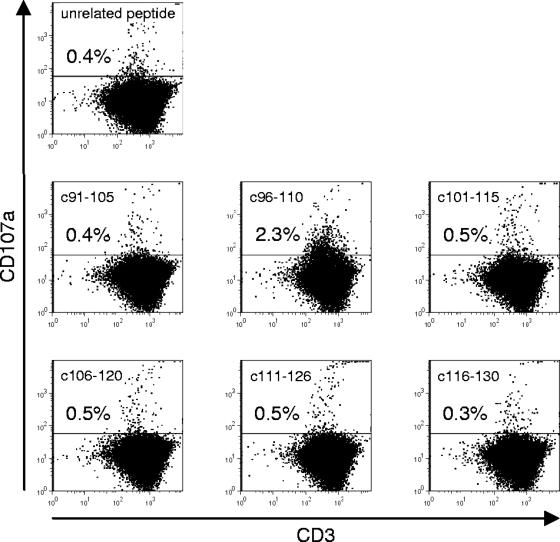
For the identification of major histocompatibility complex class I (MHC-I)-restricted epitopes within WHcAg, spleen-derived lymphocytes were stimulated with the six individual peptides of pool 4 (covering aa 91 to 130 of WHV core protein) for 6 days according to the protocol described above. Therefore, the splenocytes of animals 15810, 17214, and 21029 which were positive for pool 4 were isolated from biopsy samples obtained by partial splenectomies. As shown in Fig. 4, peptide c96-110 turned out to be accountable for the response resulting in CD107a staining (2.3%), whereas stimulation with the other peptides (c91-105 and c101-115 to c116-130 and the unrelated CMV peptide) induced no response (0.3% to 0.5%). The splenocytes of two out of three animals that were stimulated with the six single peptides of pool 4 exhibited responses to peptide c96-110, indicated by 2.3% and 4.6% CD107a+ T cells, respectively. The splenocytes of one animal did not respond to stimulation with peptide c96-110 (0.5%).
FIG. 4.
Representative dotplots of splenocytes from a woodchuck with resolved WHV infection (17214) stimulated with single peptides of pool 4 and an unrelated CMV peptide. In total, three woodchucks were analyzed for responses to stimulation with individual peptides.
Our first set of experiments indicates that we successfully established the CD107a degranulation assay to detect virus-specific CTLs in woodchucks in the postacute phase of infection. Due to the outbred background of woodchucks, stimulation with individual peptides had to be performed with cells of the same animals that had responded to stimulation with WHcAg-derived pool 4 to identify single epitopes. This was achieved by partial splenectomies. By stimulation with individual peptides of pool 4, we found a single CTL epitope (c96-110).
Detection of WHV core-specific CTLs during acute WHV infection.
From experiments with HBV-infected chimpanzees and studies with HBV-infected humans, it is known that virus-specific CTLs can be detected late after infection at the onset of viral clearance (8, 26, 28). Having established a detection system for WHV core-specific CTLs from spleen, we investigated the kinetics of CTL responses to the identified epitope during acute WHV infection. Therefore, peripheral blood was analyzed weekly for a period of 15 weeks postinfection. For the determination of virus-specific CTL responses in PBMC, however, improvement of the degranulation assay protocol was necessary, since in vitro cultivation for 6 days adversely affected PBMC viability and subsequent flow cytometric analysis. Optimization was achieved by reducing the in vitro stimulation period to 2 days. Direct ex vivo analyses of PBMC revealed no CD107a staining.
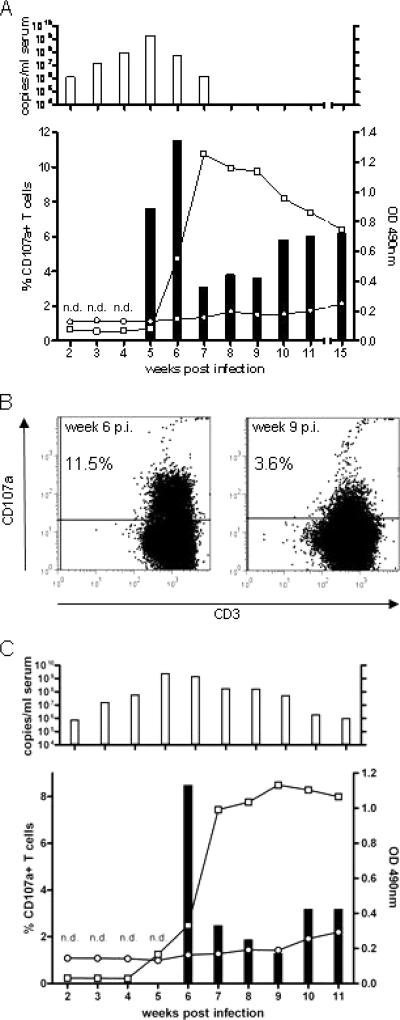
Two animals (22524 and 22531) were infected intravenously with WHV and monitored weekly for markers of infection. As shown for animal 22524 in Fig. 5A, WHV DNA was detected in the serum from week 2 to week 7 postinfection. Antibodies to WHV core protein were detectable in the serum for the first time at week 5 postinfection (Fig. 5A). We first determined a degranulation response after restimulation with pool 4 and the individual peptide c96-110 at week 5 postinfection. The strongest response to peptide stimulation could be seen at week 6 postinfection (11.5%) (Fig. 5A and B). This response declined to 3.1% in week 7 and reached a level of approximately 6% in the following weeks. T-cell responses were determined up to 15 weeks postinfection (Fig. 5A). Monitoring the infection course of the second WHV-infected woodchuck (22531) showed similar results (Fig. 5C).
FIG. 5.
(A) Humoral and cellular immune responses of woodchuck 22524 during acute WHV infection. T-cell responses were determined by using PBMC. (Upper panel) WHV DNA detected by quantitative PCR with a detection limit of 103 genome equivalents per reaction. (Lower panel) Bars, T-cell responses to stimulation with peptide pool 4; n.d., T-cell response not determined. (B) Exemplary dotplots from week 6 and week 9 postinfection (p.i.) showing various degranulation responses. (C) Course of infection of woodchuck 22531.
Absence of WHV core-specific CTLs during chronic WHV infection.
Viral persistence and the chronic outcome of infection are associated with the absence of CTL responses to hepadnaviral proteins in humans (17, 18). In woodchucks, the T-helper cell response is known to be absent in chronically WHV-infected animals (12). However, the influence of virus-specific CTLs has not been known until now. We therefore applied the optimized CD107a degranulation assay to the PBMCs of three chronically WHV-infected animals. These animals tested positive for WHV DNA and anti-WHc. As Fig. 6 shows, in these three animals, there was no difference in T-cell response after stimulation with peptide c96-110 (0.8% to 1.1%) compared to that in the unstimulated control (0.9%).
FIG. 6.
Absence of degranulation response of PBMC from chronically WHV-infected animals (25702, 25706, 26519) after stimulation with peptide c96-10 (bottom row) compared to the unstimulated control (middle row). PBMC of woodchuck 22524 that had resolved infection served as a positive control (top row).
DISCUSSION
Virus-specific T cells exert different functions to eliminate the infecting virus. The mechanisms are secretion of IFN-γ to downregulate viral replication or killing of infected cells by cytotoxic molecules. In recent years, several methods have been established to characterize these virus-specific CD8+ T cells and their functions. Some assays are functional tests (IFN-γ release, cytotoxic activity); others use tetramers of MHC-I molecules to count the total numbers of epitope-specific cells regardless of function. Recently, tests which directly measure the effector function of virus-specific CTLs have been established (3). Upon recognition of the target cell via the T-cell receptor complex, degranulation occurs, i.e., the release of apoptosis-inducing proteins like granzymes and perforin into the immunological synapse. Consequently, the transmembrane protein CD107a is exposed to the cell surface. In many studies, flow cytometric analysis of this molecule has been described as a suitable method for the detection of CTLs (3, 21, 29). We applied this novel approach based on the cytolytic effector function to the woodchuck model for hepadnaviral infection. We demonstrated that stimulated woodchuck cells can be stained with an anti-mouse CD107a antibody. This staining was possible due to the cross-reactivity of an anti-mouse CD107a antibody to woodchuck CD107a. The cloning and sequencing of a major part of woodchuck CD107a revealed high protein sequence homologies to murine, human, and nonhuman primate CD107a of approximately 80%, which may explain the cross-reactive binding of the anti-mouse CD107a antibody. The present flow cytometric experiments for the characterization of CTLs are the first to be performed in the woodchuck model. In order to determine virus-specific CTLs, an in vitro restimulation protocol with subsequent staining was established. Therefore, lymphocytes of the spleens of animals that had resolved the infection were subjected to analyses. We demonstrated the expansion and degranulation of CD3+ T cells after stimulation with peptide pools and single peptides. The obtained percentages of CD107a-positive T cells are in line with findings from studies with other systems (3, 5). For a more detailed characterization of T cells, staining with antibodies to CD3 and CD4 was performed, since an antibody to woodchuck CD8 is not yet available. Three-color flow cytometry allowed us to define degranulating CD3+ T lymphocytes as CD4−, which suggests that the analyzed cells are CD8+ T lymphocytes.
After screening for a CTL epitope with spleen-derived lymphocytes, optimization of the established degranulation assay protocol for application to the PBMC of acutely WHV-infected woodchucks was achieved. Reducing the in vitro stimulation period from 6 to 2 days turned out to be optimal for the detection of virus-specific CTLs in peripheral blood. Thus, this newly established flow cytometric degranulation assay is a feasible tool to determine CTL responses to viral antigens in WHV-infected woodchucks.
With this newly established assay, we identified one CTL epitope (c96-110) within the WHcAg. The minimum optimal amino acid sequence within c96-110 has to be determined in follow-up studies. The existence of a single MHC-I-restricted epitope in the nucleocapsid corresponds with previous findings of human MHC-I-restricted epitopes of HBV core protein. The unique HLA-A2-restricted HBV core peptide is located at aa 18 to 27 (1; for a review, see reference 4). Furthermore, HLA-A31- and HLA-Aw68-restricted CTLs recognize only HBV core peptide 141-151 (14; for a review, see reference 4). Most of the animals that we analyzed recognize the identified epitope despite their outbred background, implying that these animals had the same MHC-I background. This is suggested by the fact that four out of six individuals in the postacute phase of infection were closely related to each other (one parent and three offspring). The presenting MHC-I molecule, however, needs to be defined. The MHC-I genes of woodchucks, termed Mamo I genes, are similar in their molecular structure to those of humans and other mammals. Until now, 20 different alleles of Mamo I genes have been identified (30).
It is generally thought that the control of hepadnaviral infection and the elimination of the virus are mediated by the class I-restricted CTL response. On the one hand, several studies have shown that the peripheral blood CTL response to HBV is polyclonal and multispecific in patients with acute viral hepatitis who have successfully cleared the virus (2, 9, 18, 25). We therefore investigated the kinetics of CTL appearance in peripheral blood during the acute and postacute phases of WHV infection. The results indicate that the peak T-cell response occurs as late as 6 weeks after infection and coincides with viral clearance. On the other hand, patients that are persistently infected with HBV mount a weak and oligoclonal CTL response to viral antigens. Our studies with chronically WHV-infected animals confirm these findings, as we determined the absence of CTL responses to peptide c96-110.
The elimination of HBV occurs in two steps: initially, cytokines like IFN-γ are released to downregulate viral replication, and then infected cells are eliminated as a result of the cytotoxic activity of CTLs. The cytokine-mediated inhibitory effect of CTLs on viral replication was shown in transgenic mice (7). In the woodchuck model, the influence of noncytolytic mechanisms on viral clearance has not been determined until now due to the lack of appropriate tools. The cytolytic step of viral clearance was demonstrated in the mouse model: hepatitis B surface antigen-specific murine CTL clones cause a necroinflammatory liver disease when they are injected into hepatitis B surface antigen-positive transgenic mice (15). Since the cytolytic potential of virus-specific T cells is displayed in our assay, we conclude that WHV-specific CTLs contribute importantly to the control of viral infection and that cytolytic events are required for complete elimination of the virus.
In conclusion, we demonstrated for the first time the detection of WHV-specific CTL responses in WHV-infected woodchucks and identified a CTL epitope within the WHcAg. Our results show that the newly established degranulation assay protocol is applicable to both spleen-derived lymphocytes and PBMC. In future experiments, we will determine further epitopes within other viral proteins, particularly within the WHsAg. The ability to determine virus-specific CTL responses in woodchucks is also helpful for studying very early events in hepadnaviral infection. In HBV-infected humans, the analyses of T-cell responses in the early phase are limited because of the often unknown point of exposure. Additionally, we will use this method to monitor immune therapy studies in chronically infected woodchucks, including therapeutic vaccination protocols during antiviral treatment.
Acknowledgments
We gratefully acknowledge the advice of Antonio Bertoletti and Adam Gehring (Centre for Molecular Medicine, A*Star, Singapore). We thank Reiner Schulte (German Primate Center, Göttingen, Germany) for the identification of cross-reactive anti-CD4 antibody.
This work was supported by a grant to I.F. and M.R. from the Deutsche Forschungsgemeinschaft (GK 1045/1).
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Bertoletti, A., F. V. Chisari, A. Penna, S. Guilhot, L. Galati, G. Missale, P. Fowler, H.-J. Schlicht, A. Vitiello, R. C. Chesnut, F. Fiaccadori, and C. Ferrario. 1993. Definition of a minimal optimal cytotoxic T-cell epitope within the hepatitis B virus nucleocapsid protein. J. Virol. 67:2376-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertoletti, A., C. Ferrari, F. Fiaccadori, A. Penna, R. Margolskee, H. J. Schlicht, P. Fowler, S. Guilhot, and F. V. Chisari. 1991. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc. Natl. Acad. Sci. USA 88:10445-10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 4.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29-60. [DOI] [PubMed] [Google Scholar]
- 5.Elrefaei, M., B. Barugahare, F. Ssali, P. Mugyenyi, and H. Cao. 2006. HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J. Immunol. 176:1274-1280. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari, C., A. Penna, A. Bertoletti, A. Valli, A. D. Antoni, T. Giuberti, A. Cavalli, M. A. Petit, and F. Fiaccadori. 1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 145:3442-3449. [PubMed] [Google Scholar]
- 7.Guidotti, L. G., K. Ando, M. V. Hobbs, T. Ishikawa, L. Runkel, R. D. Schreiber, and F. V. Chisari. 1994. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl. Acad. Sci. USA 91:3764-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 9.Maini, M. K., C. Boni, G. S. Ogg, A. S. King, S. Reignat, C. K. Lee, J. R. Larrubia, G. J. Webster, A. J. McMichael, C. Ferrari, R. Williams, D. Vergani, and A. Bertoletti. 1999. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology 117:1386-1396. [DOI] [PubMed] [Google Scholar]
- 10.Mason, W. S., A. R. Jilbert, and J. Summers. 2005. Clonal expansion of hepatocytes during chronic woodchuck hepatitis virus infection. Proc. Natl. Acad. Sci. USA 102:1139-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menne, S., J. Maschke, M. Lu, H. Grosse-Wilde, and M. Roggendorf. 1998. T-cell response to woodchuck hepatitis virus (WHV) antigens during acute self-limited WHV infection and convalescence and after viral challenge. J. Virol. 72:6083-6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menne, S., J. Maschke, T. K. Tolle, M. Lu, and M. Roggendorf. 1997. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J. Virol. 71:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menne, S., C. A. Roneker, M. Roggendorf, J. L. Gerin, P. J. Cote, and B. C. Tennant. 2002. Deficiencies in the acute-phase cell-mediated immune response to viral antigens are associated with development of chronic woodchuck hepatitis virus infection following neonatal inoculation. J. Virol. 76:1769-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Missale, G., A. Redeker, J. Person, P. Fowler, S. Guilhot, H. J. Schlicht, C. Ferrari, and F. V. Chisari. 1993. HLA-A31- and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J. Exp. Med. 177:751-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriyama, T., S. Guilhot, K. Klopchin, B. Moss, C. A. Pinkert, R. D. Palmiter, R. L. Brinster, O. Kanagawa, and F. V. Chisari. 1990. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science 248:361-364. [DOI] [PubMed] [Google Scholar]
- 16.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 17.Rehermann, B., K.-M. Chang, J. McHutchison, R. Kokka, M. Houghton, C. M. Rice, and F. V. Chisari. 1996. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J. Virol. 70:7092-7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehermann, B., P. Fowler, J. Sidney, J. Person, A. Redeker, M. Brown, B. Moss, A. Sette, and F. V. Chisari. 1995. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J. Exp. Med. 181:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roggendorf, M. 2005. The woodchuck: a model for immunopathogenesis and therapy of hepadnaviral infection. Monogr. Virol. 25:1-24. [Google Scholar]
- 20.Roos, S., K. Fuchs, and M. Roggendorf. 1989. Protection of woodchucks from infection with woodchuck hepatitis virus by immunization with recombinant core protein. J. Gen. Virol. 70:2087-2095. [DOI] [PubMed] [Google Scholar]
- 21.Rubio, V., T. B. Stuge, N. Singh, M. R. Betts, J. S. Weber, M. Roederer, and P. P. Lee. 2003. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat. Med. 9:1377-1382. [DOI] [PubMed] [Google Scholar]
- 22.Schodel, F., G. Neckermann, D. Peterson, K. Fuchs, S. Fuller, H. Will, and M. Roggendorf. 1993. Immunization with recombinant woodchuck hepatitis virus nucleocapsid antigen or hepatitis B virus nucleocapsid antigen protects woodchucks from woodchuck hepatitis virus infection. Vaccine 11:624-628. [DOI] [PubMed] [Google Scholar]
- 23.Summers, J., A. R. Jilbert, W. Yang, C. E. Aldrich, J. Saputelli, S. Litwin, E. Toll, and W. S. Mason. 2003. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc. Natl. Acad. Sci. USA 100:11652-11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summers, J., J. M. Smolec, and R. Snyder. 1978. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc. Natl. Acad. Sci. USA 75:4533-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thimme, R., K.-M. Chang, J. Pemberton, A. Sette, and F. V. Chisari. 2001. Degenerate immunogenicity of an HLA-A2-restricted hepatitis B virus nucleocapsid cytotoxic T-lymphocyte epitope that is also presented by HLA-B51. J. Virol. 75:3984-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thimme, R., S. Wieland, C. Steiger, J. Ghrayeb, K. A. Reimann, R. H. Purcell, and F. V. Chisari. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77:68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trapani, J. A., and M. J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2:735-747. [DOI] [PubMed] [Google Scholar]
- 28.Webster, G. J., S. Reignat, M. K. Maini, S. A. Whalley, G. S. Ogg, A. King, D. Brown, P. L. Amlot, R. Williams, D. Vergani, G. M. Dusheiko, and A. Bertoletti. 2000. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 32:1117-1124. [DOI] [PubMed] [Google Scholar]
- 29.Zelinskyy, G., S. J. Robertson, S. Schimmer, R. J. Messer, K. J. Hasenkrug, and U. Dittmer. 2005. CD8+ T-cell dysfunction due to cytolytic granule deficiency in persistent Friend retrovirus infection. J. Virol. 79:10619-10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou, J. H., S. Ferencik, V. Rebmann, D. L. Yang, M. Lu, M. Roggendorf, and H. Grosse-Wilde. 2003. Molecular genetic and biochemical analysis of woodchuck (Marmota monax) MHC class I polymorphism. Tissue Antigens 61:240-248. [DOI] [PubMed] [Google Scholar]