Abstract
Matrix metalloproteinases (MMPs) play important roles in cancer invasion, angiogenesis, and inflammatory infiltration. Kaposi's sarcoma is a highly disseminated angiogenic tumor of proliferative endothelial cells linked to infection by Kaposi's sarcoma-associated herpesvirus (KSHV). In this study, we showed that KSHV infection increased the invasiveness of primary human umbilical vein endothelial cells (HUVEC) in a Matrigel-based cell invasion assay. KSHV-induced cell invasion was abolished by an inhibitor of MMPs, BB-94, and occurred in both autocrine- and paracrine-dependent fashions. Analysis by zymography and Western blotting showed that KSHV-infected HUVEC cultures had increased secretion of MMP-1, -2, and -9. KSHV increased the secretion of MMP-2 within 1 h following infection without upregulating its mRNA expression level. In contrast, the secretion of MMP-1 and -9 was not increased until 6 h after KSHV infection and was correlated with the upregulation of their mRNA expression levels. Promoter analysis by reporter assays and electrophoretic mobility shift assays identified an AP-1 cis-element as the dominant KSHV-responsive site in the MMP-1 promoter. Together, these results suggest that KSHV infection modulates the production of multiple MMPs to increase cell invasiveness and thus contributes to the pathogenesis of KSHV-induced malignancies.
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8, is an oncogenic gammaherpesvirus identified in an AIDS patient with Kaposi's sarcoma (KS) (10). Extensive epidemiological studies have linked KSHV infection to all four clinical forms of KS, including classical KS, transplantation or immunosuppressive KS, African or endemic KS, and AIDS-related or epidemic KS (14). KSHV has also been associated with several other lymphoproliferative diseases, including primary effusion lymphoma and multicentric Cattleman's disease (14).
Kaposi's sarcoma is an angiogenic vascular tumor of endothelial spindle cells (14). Skin lesions are a common manifestation of KS; however, KS is also a highly disseminated tumor often involved with visceral organs. The KS tumor is characterized by vast aberrant proliferation of small vessels that lack a basement membrane, displaying leaky behavior, with microhemorrhages and hemosiderin deposition (11). Besides endothelial spindle cells, a KS tumor also consists of fibroblasts, smooth muscle cells, and infiltrating inflammatory cells. In KS lesions, the majority of the spindle tumor cells are latently infected by KSHV; however, a small number of them also undergo spontaneous lytic replication (19). Lytic replication generates infectious virions for spreading to other cells and at the same time produces virus-encoded cytokines as well as induces cellular cytokines through viral lytic proteins or de novo viral infection, all of which could contribute to KS pathogenesis by promoting KS angiogenesis, inflammatory infiltration, and tumor dissemination through autocrine and paracrine mechanisms (19).
Matrix metalloproteinases (MMPs) are a family of related enzymes that share some common functional and structural features (44). MMPs are expressed in a wide variety of cancers and are involved in cancer invasion, metastasis, and angiogenesis (7, 13). MMPs regulate cellular functions and microenvironments by cleaving extracellular matrix proteins, an activity that depends on metal ions (44). MMPs are first synthesized as inactive proenzymes or zymogens. Their latency is maintained by an unpaired cysteine sulfhydryl group near the C-terminal end of the propeptide domain. This sulfhydryl acts as a ligand for the zinc ion at the active site. The activation of MMPs requires the cysteine-to-zinc switch through normal proteolytic removal of the propeptide domain or ectopic perturbation of the cysteine-zinc interaction, a process that replaces the thiol group with a water molecule, allowing it to attack the peptide bonds of the targets (44). A total of 22 members of human MMPs have been identified so far. Among them, MMP-1, a collagenase, and MMP-2 and -9, two gelatinases, are critical players in cancer angiogenesis and metastasis (7, 13).
A number of MMPs, including MMP-1, -2, -3, -9, and -19, have been detected in KS tumor cells, implicating their roles in the pathogenesis of these tumors (6, 23, 29, 42). Indeed, an inhibitor of MMPs, COL-3, had an overall 44% response rate in AIDS-KS patients. Patients who responded to the treatment had reduced levels of MMP-2 and vascular endothelial growth factor in their sera (12). In addition, a recent study showed that KSHV ORF-K1 induced the expression of MMP-9 (46). In spite of these studies, how KSHV infection regulates the expression and secretion of MMPs remains unclear.
In this study, we have found that KSHV infection promotes MMP-dependent cell invasion of primary human umbilical vein endothelial cells (HUVEC). We have shown that KSHV infection induces the secretion and activation of MMP-1 (collagenase 1), MMP-2 (gelatinase A), and MMP-9 (gelatinase B). Furthermore, we have found that KSHV induction of MMP-1 occurs at the transcriptional level and is mediated by the AP-1 pathway. These results illustrate an important role of MMPs in KSHV-induced cell invasion and the pathogenesis of KSHV-related malignancies.
MATERIALS AND METHODS
Cell culture and virus infection.
HUVEC and the growth medium EGM2 were purchased from Clonetics (Walkersville, MD). Concentrated virus stocks were prepared from a recombinant KSHV BAC36 as previously described (16, 51). Fresh virus preparations with titers of about 106 green fluorescent protein (GFP)-positive cells/ml (GFU) were used in the experiments. KSHV infection of HUVEC was carried out as previously described (16, 51). For all experiments, cells were infected at a multiplicity of infection of 2, i.e., 2 GFU per cell, to give a 50% infection rate at 2 days postinfection (dpi), unless specified otherwise.
Extracellular matrix invasion assay.
Cell invasiveness was measured using a modified Boyden chamber method (41). Briefly, transwell insert chambers (Becton Dickinson, Franklin Lakes, NJ) with 8-μm-pore filters were coated with 100 μl of Matrigel at 200 μg/ml diluted in phosphate-buffered saline (Becton Dickinson). Mock- or KSHV-infected HUVEC were seeded at 105 cells/ml in the upper chambers with 500 μl of the basic EBM-2 supplemented with 0.2% fetal bovine serum. A 750-μl aliquot of complete endothelial cell growth medium (EGM-2 with growth factors) was placed in the lower wells serving as a source of chemoattractants. The cells were incubated for 24 h except if indicated otherwise. Cells that were able to degrade the Matrigel layer and migrated to the lower surface of the filter were fixed with 70% ethanol, stained with hematoxylin and eosin, and counted under a light microscope. For each experiment, three to five wells were used for each treatment. Five random fields were counted for each well, and the average cell number per field in a well was calculated. To evaluate the role of MMPs in KSHV-mediated cell invasion, a broad-spectrum synthetic inhibitor of MMPs, BB-94 (British Biotechnology, Oxford, United Kingdom), was added to both the insert and lower chamber of the transwell system at a final concentration of 10 μg/ml during the assay. To evaluate paracrine-mediated cell invasion, normal HUVEC were examined in the invasion assay in the presence of supernatants from mock- or KSHV-infected HUVEC cultures. The supernatants were collected at 24 hpi and subjected to high-speed centrifugation at 20,000 × g for 2 h to eliminate any cellular debris and virions before the invasion assay. The absence of any infectious virions in the supernatants was confirmed by virus titration as previously described (16, 51).
Substrate-specific zymography for MMPs.
The secretion and enzymatic activity of MMPs were examined by specific zymograms using collagen (MMP-1) or gelatin (MMP-2 and MMP-9) as substrate. Briefly, supernatants from mock- or KSHV-infected HUVEC were mixed with a sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer without 2-mercaptoethanol as previously described (17) and resolved in a 10% polyacrylamide gel containing 1 mg/ml of collagen type I (Sigma, St. Louis, MO) or gelatin (Sigma). After electrophoresis, the gel was incubated with a renaturing solution containing 50 mM Tris-HCl at pH 8.0 and 2.5% Triton X-100 and then activated in a buffer containing 0.5 mM CaCl2 and 1 mM ZnCl2 overnight at 37°C. The gel was then stained with 1% Coomassie brilliant blue R-250 and destained in a destaining buffer containing 5% acetic acid and 10% methanol. The specific bands of MMPs were visualized and quantified using a Kodak Image Station 2000R system (Eastman Kodak Company, Rochester, NY).
All the MMPs were identified based on their molecular weights resolved by zymography. MMP-1 was resolved in proenzyme, intermediate, and mature forms, termed pro-MMP-1, inter-MMP-1, and active MMP-1, which had molecular masses of 52, 48, and 41 kDa, respectively (see Fig. 3A, below). MMP-2 was also resolved in three forms, termed pro-MMP-2, inter-MMP-2, and active MMP-2, which had molecular masses of 72, 64, and 62 kDa, respectively (see Fig. 3B, below). The three forms of MMP-9, termed pro-MMP-9, inter-MMP-9, and active MMP-9, had molecular masses of 92, 89, and 86 kDa, respectively (see Fig. 3C, below). The different forms of MMPs at times did not resolve in distinct bands in zymography, as shown for MMP-1 (see Fig. 3A, panel b). The identification of the MMPs was also confirmed by Western blot assay, though this assay generally had lower sensitivity than the zymography. The Western blot assay also had lower resolution than the zymography and was generally incapable of distinguishing the different forms MMPs because of the diffusion of the bands during the transfer of the proteins from gels to the membranes (see Fig. 3, below).
FIG. 3.
KSHV infection increases secretion of MMP-1, -2, and -9 by HUVEC cultures. Data shown are representative illustrations of zymograms (MMP-1 [A, panels a and b], MMP-2 [B, panel a] and MMP-9 [C, panel a]), Western blots (A, panel c, and B and C, panel b), and quantifications (D, E, and F) of different forms of MMP-1, -2, and -9 at different time points following KSHV infection. The numbers at the top of the bars are the fold increases of KSHV-infected cultures over mock-infected cultures at the same time points (D, E, and F). The zymograms were carried out with either collagen (MMP-1) or gelatin (MMP-2 and MMP-9) as substrate. The MMPs were identified based on their molecular weights and Western blots with specific antibodies. All three MMPs had three forms, which were resolved as three bands in zymograms. Because of the closeness of the molecular weights of the different MMP isoforms, they were often not resolved as three distinct bands (A, panel b). Western blots usually failed to resolve the three isoforms of the MMPs because of the diffusion of the proteins during transfer from gels to the membranes.
Western blot analysis.
To detect MMPs, protein preparations from supernatants of mock- and KSHV-infected HUVEC cultures were separated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels under reducing conditions and transferred to nitrocellulose membranes as previously described (17). The membrane was first incubated with a primary antibody to a specific MMP and then with a respective secondary antibody conjugated to horseradish peroxidase (HRP). Specific signals were revealed with chemiluminescence substrates and recorded on films or with the Kodak Image Station 2000R system described above. For the detection of MMP-1, a mouse anti-human MMP-1 primary monoclonal antibody (Calbiochem, Oakland, CA) and a goat anti-mouse immunoglobulin G (IgG)-HRP conjugate (Calbiochem) were used. For the detection of MMP-2, a rabbit anti-human MMP-2 primary polyclonal antibody (Sigma) and a goat anti-rabbit IgG-HRP conjugate were used (Bio-Rad Laboratories, Hercules, CA). For the detection of MMP-9, a mouse anti-MMP-9 primary monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and the same goat anti-mouse IgG-HRP conjugate were used (Bio-Rad).
RT-qPCR.
Reverse transcription-quantitative real-time PCR (RT-qPCR) was performed as previously described (49). Briefly, total RNA from KSHV-infected HUVEC was prepared with TRI reagent as recommended by the manufacturer (Sigma). The RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI) and reverse transcribed to obtain the first-strand cDNA using the Superscript III first-strand synthesis system (Invitrogen, Carlsbad, CA). A control without reverse transcriptase was conducted in parallel. qPCR was carried out in a DNA engine Opticon 2 continuous fluorescence detector (Bio-Rad) as previously described (49). The primers for MMP-1 were 5′AATGTGCTACACGGATACCC3′ (MMP-1-foward) and 5′CTTTGTGGCCAATTCCAGGA3′ (MMP-1-reverse), which amplify a product of 213 bp. The primers for MMP-2 were 5′GCTCATCGCAGATGCCTGGAATGC3′ (MMP-2-foward) and 5′GGAGGAGTACAGTCAGCATCTATTC3′ (MMP-2-reverse), which amplify a product of 374 bp. The primers for MMP-9 were 5′CGGAGTGGCAGGGGGAAGATGCTG3′ (MMP-9-foward) and 5′GCAGGATGTCATAGGTCACGTAGC3′ (MMP-9-reverse), which amplify a product of 255 bp. Glyceraldehyde-3-phoshate dehydrogenase (GAPDH) was used for normalization. The GAPDH primers were 5′ACAGTCAGCCGCATCTTCTT3′ (forward) and 5′ACGACCAAATCCGTTGACTC3′ (reverse).
Reporter constructs and reporter assay.
The human MMP-1 promoter-luciferase reporter plasmids were kindly provided by H. Esumi (National Cancer Center Research Institute East) (24). These included the full-length MMP-1 promoter reporter p1773 containing the −1773 to +49 MMP-1 promoter sequence and sequential truncated MMP-1 promoter constructs p1129 (−1129 to +49), p623 (−623 to +49), p137 (−137 to +49), and p14 (−14 to +49). Site-directed mutagenesis was carried out using the p137 reporter construct as a substrate by mutating either the AP-1 or Ets1 cis-element, or both of them, to generate the corresponding mutants p137mutAP1, p137mutEts1, and p137mutAP1&Ets1, respectively. Other plasmids used for the study were dominant negative constructs (DN) of c-Fos, termed A-Fos (a gift from Charles Vinson at the National Institutes of Health), and c-Jun, termed DN-c-Jun (a gift from Bradford W. Ozanne, Beatson Institute). The Ets1 DN mutant (DN-Ets1) lacking a transcription activation domain at amino acid 306 to 441 was obtained by PCR cloning of the fragment into the pCMV-myc vector (BD Biosciences, San Jose, CA) using primers 5′CTATGTGCGGGACCGTGCTGACC3′ and 5′TCACTCGTCGGCATCTGGCTTGAC3′ as described previously (48).
Transfection was carried out using the Targefect-HUVEC reagent according to the instructions of the manufacturer (Targeting Systems, Santee, CA). In all the experiments, we estimated the transfection efficiency to be 30 to 40% using the GFP expression vector pEGFP (Clontech Laboratories, Inc., Mountain View, CA) as a control. Transfection efficiency was normalized by cotransfection with pSV-β-galactosidase (Promega, Madison, WI). The luciferase activities were detected using a luciferase assay kit (Promega) and a Veritus microplate luminometer (Turner Biosystems, Sunnyvale, CA) as previously described (47).
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts were prepared from mock- or KSHV-infected HUVEC as previously described (47). Based on the results of promoter deletion and mutagenesis analysis, a wild-type probe (5′TAATCAAGAGGATGTTATAAAGCATGAGTCAGAC3′) from the MMP-1 promoter region −64 to −97 containing a putative AP-1 site and a putative Ets1 site was designed. Corresponding mutant probes with the AP-1 site (5′TAATCAAGAGGATGTTATAAAGCAACTATCAGAC3′), Ets1 site (5′TAATCAAGAAAATGTTATAAAGCATGAGTCAGAC3′), or both sites (5′TAATCAAGAAAATGTTATAAAGCAACTATCAGAC3′) mutated were also used in the experiments. Antibodies to either c-Fos or Ets1 (Santa Cruz) were used for the supershift experiments.
RESULTS
KSHV infection enhances HUVEC invasion of the extracellular matrix.
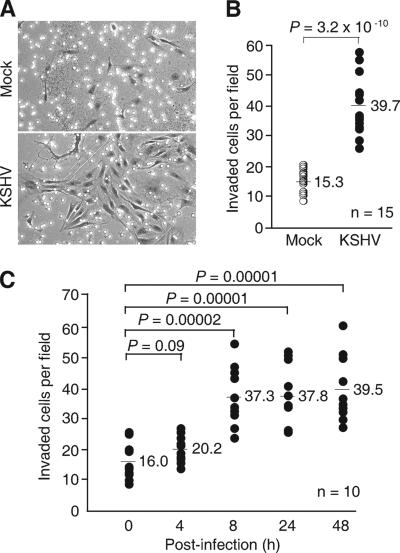
To determine the effect of KSHV infection on cell invasion, we infected HUVEC with a recombinant KSHV BAC36 at 2 GFU, which gave approximately 50% primary infection efficiency as monitored by GFP expression at 2 dpi (16). At 24 hpi, we assayed the ability of the cells in invading the Matrigel barriers with properties similar to the basement membrane. Without chemoattractant gradients, the intrinsic invasiveness of normal HUVEC through a Matrigel barrier was usually undetectable (data not shown). However, in the presence of chemoattractant gradients, some normal HUVEC succeeded in invading the Matrigel barrier (Fig. 1A). KSHV infection increased 2.6-fold of the cells traveling through the Matrigel barrier (Fig. 1A and B; 39.7 cells/field versus 15.3 cells/field; P = 3.2 × 10−10), indicating that the invasiveness of the HUVEC was enhanced following KSHV infection.
FIG. 1.
KSHV infection increases HUVEC invasion of the extracellular matrix. (A and B) Mock- or KSHV-infected HUVEC were assayed for their ability to invade a Matrigel layer at 24 hpi. A representative illustration (A) and quantification (B) of mock- or KSHV-infected HUVEC that had invaded the Matrigel layer are shown. (C) HUVEC invasiveness assayed at different time post-KSHV infection. All the invasion assays were carried out for 24 h as described in Materials and Methods. Mock- or KSHV-infected HUVEC that had invaded the Matrigel layers were quantified in three independent experiments, each with three to five repeats (A and B). The total number of repeats (n) for each condition is shown. Each dot represents the average number of cells per microscopic field in one test well in the invasion assay. The bars and their associated numbers are the average numbers of invaded cells per field from all the wells under different test conditions. P values shown are from a two-tailed Fisher's exact test.
Next, we determined the time course of KSHV promotion of cell invasiveness. HUVEC cultures were either mock infected or infected with KSHV for 0 to 48 h and assayed for their invasiveness. As expected, the invasiveness of the mock-infected cells remained unchanged over time during the test period (data not shown). KSHV infection enhanced cell invasion as early as 8 hpi (37.3 cells/field versus 16.0 cells/field; P = 0.00002) and persisted up to 48 hpi (Fig. 1C). Nevertheless, the cell invasiveness of KSHV-infected cultures remained at the same elevated levels after 8 hpi (37.3, 37.8, and 39.5 cells/field at 8, 24, and 48 hpi, respectively). At 4 hpi, the cell invasiveness was also increased compared to cells at 0 hpi but was not statistically significant (20.2 cells/field versus 16.0 cells/field; P = 0.09).
KSHV-enhanced cell invasion is MMP dependent and is effectuated by both autocrine and paracrine mechanisms.
MMPs are frequently involved in cell invasion (44). To determine whether KSHV promotion of cell invasion was involved with MMPs, we carried out the cell invasion assay in the presence of a broad-spectrum inhibitor of MMPs, BB-94. As shown in Fig. 2A, KSHV infection increased HUVEC cell invasiveness by 1.8-fold (32.6 cells/field versus 18.2 cells/field; P = 0.0005). BB-94 abolished KSHV promotion of cell invasion (20.6 cells/field versus 32.2 cells/field; P = 0.005), indicating that MMPs were important effectors in the KSHV-mediated cell invasion.
FIG. 2.
KSHV-enhanced HUVEC invasiveness is MMP dependent and is mediated by a paracrine mechanism. (A) KSHV-induced cell invasion was MMP dependent. A specific inhibitor of MMPs, BB-94, abolished KSHV-increased cell invasiveness. (B) KSHV infection increased paracrine-dependent cell invasion. Virion-free supernatants from mock- or KSHV-infected HUVEC cultures (MediumMock and MediumKSHV, respectively) were assayed for their abilities to promote cell invasion of normal HUVEC with or without the presence of the inhibitor of MMPs, BB-94. The invasion assays were carried out as described for Fig. 1. HUVEC that had invaded the Matrigel layer were quantified in two independent experiments, each with three to four repeats. The total number of repeats (n) for each condition is shown in the figures. Each dot represents the average number of cells per microscopic field in one test well in the invasion assay. The bars and their associated numbers are the average numbers of invaded cells per field from all the wells under different test conditions. P values shown are from a two-tailed Fisher's exact test.
If MMPs are involved in KSHV promotion of cell invasion, they should act not only on the KSHV-infected cells but also on the KSHV-negative cells in the same population through secretion into the medium. Indeed, an equal number of GFP-positive and GFP-negative cells were observed in the invading cell population (data not shown). To further demonstrate that KSHV infection could promote cell invasion in a paracrine fashion, we examined the invasiveness of normal HUVEC in the presence of virion-free supernatants from mock- or KSHV-infected cultures. As shown in Fig. 2B, supernatants from mock-infected HUVEC cultures had a marginal effect on cell invasiveness (13.1 cells/field versus 12.6 cells/field; P = 0.8). In contrast, supernatants from KSHV-infected HUVEC cultures increased the invasiveness of normal HUVEC by 1.6-fold (19.9 cells/field versus 12.6 cells/field; P = 0.01). Addition of the inhibitor of MMPs, BB-94, abolished the enhanced cell invasiveness exerted by the supernatants of KSHV-infected HUVEC cultures (19.9 cells/field versus 9.4 cells/field; P = 0.0009). Together, these results suggest that KHSV infection could promote MMP-dependent cell invasion through both autocrine and paracrine mechanisms.
KSHV infection increased the secretion of MMP-1, -2, and -9.
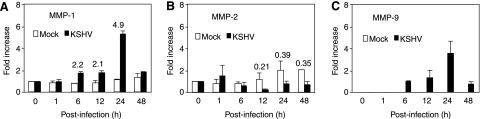
During cell invasion, cells secrete MMPs, which can then exert their effects by digesting the extracellular matrix proteins to promote cell invasion (44). Since the above results demonstrated an MMP-dependent increase in cell invasiveness following KSHV infection, we examined the effect of KSHV infection on the secretion of MMPs at different time points postinfection. Normal HUVEC secreted low levels of pro-MMP-1, inter-MMP-1, and active MMP-1, all of which increased slightly in mock-infected cultures at 24 and 36 hpi (Fig. 3A and D). KSHV infection increased the secretion of all three forms of MMP-1 as early as 6 hpi. At 24 hpi, KSHV-infected HUVEC cultures secreted 46.1-, 36.3-, and 6.0-fold more pro-MMP-1, inter-MMP-1, and active MMP-1, respectively, than the mock-infected culture did (Fig. 3A and D). At 36 hpi, KSHV-infected HUVEC cultures secreted 39.9-, 41.8-, and 6.6-fold more of the three respective MMP-1 forms than the mock-infected culture did (Fig. 3A and D).
We also detected three forms of MMP-2, termed pro-MMP-2, inter-MMP-2, and active MMP-2, respectively. Mock-infected HUVEC cultures secreted pro-MMP-2 as early as 1 hpi. KSHV infection increased the secretion of pro-MMP-2 by 3-fold at 1 hpi and by 1.9-fold at 6 hpi (Fig. 3B and E, panel a). Both mock- and KSHV-infected cultures continued to secrete high levels of pro-MMP-2 for up to 36 hpi. However, less pro-MMP-2 was detected in the KSHV-infected HUVEC cultures than in the mock-infected cultures after 24 hpi (Fig. 3B and E, panels a). Nevertheless, we detected higher levels of inter-MMP-2 and active MMP-2 in the KSHV-infected cultures than in the mock-infected cultures after 24 hpi (Fig. 3B, panel a, and E, panels b and c). At 24 hpi, KSHV-infected HUVEC cultures secreted 1.5- and 2.1-fold more inter-MMP-2 and active MMP-2, respectively, than the mock-infected cultures did (Fig. 3B and E, panels b and c). At 36 hpi, KSHV-infected HUVEC cultures secreted 1.1- and 2.0-fold more inter-MMP-2 and active MMP-2 than the mock-infected culture did (Fig. 3B and E, panels b and c).
Similar to MMP-1, normal HUVEC secreted low or undetectable levels of all three forms of MMP-9, termed pro-MMP-9, inter-MMP-9, and active MMP-9, which were increased in mock-infected cultures at 24 and 36 hpi (Fig. 3C and F). KSHV infection increased the secretion of all three forms of MMP-9 as early as 6 hpi. At 24 hpi, KSHV-infected HUVEC cultures secreted 24.6- and 2.5-fold more pro-MMP-9 and active MMP-9, respectively, than the mock-infected cultures did (Fig. 3C and F, panels a and c). At 36 hpi, KSHV-infected HUVEC cultures secreted 45.0- and 21.8-fold more pro-MMP-9 and active MMP-9, respectively, than the mock-infected cultures did (Fig. 3C and F, panels a and c). Interestingly, the levels of inter-MMP-9 remained at similar levels in both mock- and KSHV-infected cultures at 24 and 36 hpi (Fig. 3C and F, panel b).
KSHV infection upregulates the expression of MMP-1 and -9 transcripts.
The increased secretion of MMP-1 by KSHV infection was not detected until 6 hpi, suggesting that KSHV could mediate the expression of MMP-1 transcript. Thus, we examined the expression of MMP-1 transcript by RT-qPCR. In agreement with the results of weak secretion in the mock-infected cultures (Fig. 3A and D), MMP-1 transcript was also detected at low levels in these cultures (Fig. 4A). KSHV infection upregulated the expression of MMP-1 transcript at 6 hpi and persisted for up to 24 hpi (Fig. 4A), at which time the expression of MMP-1 in the KSHV-infected cultures was 4.9-fold higher than that of mock-infected cultures (Fig. 4A).
FIG. 4.
Detection of mRNA expression of MMP-1, -2, and -9 in mock- and KSHV-infected HUVEC at different times postinfection by RT-qPCR. Total cellular RNA isolated at different time points following mock or KSHV infection was examined by RT-qPCR with respective specific primers for MMP-1 (A), -2 (B), and -9 (C). RT-qPCR for GAPDH was carried out to calibrate the amount of RNA input. The numbers at the tops of the bars are fold increases of KSHV-infected cultures over mock-infected cultures at the same time point. (A) MMP-1 transcript was expressed at low levels in mock-infected cells but was upregulated following KSHV infection at 6 hpi, which was sustained for up to 24 hpi. (B) MMP-2 transcript was strongly expressed in mock-infected cells. KSHV infection did not alter the expression of MMP-2 transcript before 6 hpi but downregulated its expression at and after 12 hpi. (C) MMP-9 transcript was not detectable in mock-infected cells but was significantly upregulated following KSHV infection at 6 hpi, which was sustained for up to 48 hpi. The fold increase was calculated either with the transcript expression levels at 0 hpi set as 1 (A and B) or with the first detectable time point set as 1 (C).
In contrast to MMP-1, MMP-2 had strong expression in normal HUVEC cultures, and KSHV infection increased the secretion of MMP-2 as early as 1 hpi, suggesting that KSHV infection was unlikely to increase MMP-2 secretion by regulating the expression of its transcript. Previous studies have shown that MMP-2 is constitutively expressed in endothelial cells, and different isoforms of MMP-2 are stored in the storage vesicles that can be stimulated for release by extracellular factors (32, 40, 44). Indeed, MMP-2 transcript was strongly expressed in mock-infected HUVEC cultures and largely unaffected by KSHV infection before 6 hpi (Fig. 4B). While the expression of the MMP-2 transcript was increased 1.2-, 2.1-, and 2.2-fold at 12, 24, and 48 hpi in mock-infected cultures compared to that at 0 hpi, it was decreased 4.8-, 2.6-, and 2.8-fold in KSHV-infected cultures compared to the respective mock-infected cultures (Fig. 4B). These results were in agreement with the lower secreted levels of pro-MMP-2 detected at 24 and 36 hpi (Fig. 3B and E, panel a).
The pattern of KSHV induction of MMP-9 secretion was similar to that of MMP-1, suggesting that KSHV could also mediate the expression of MMP-9 transcript. The expression of MMP-9 transcript was undetectable in mock-infected HUVEC cultures, indicating its low expression levels in normal HUVEC cultures. KSHV infection indeed upregulated the expression of MMP-9 transcript. We detected the transcript at 6 hpi, which peaked at 24 hpi and was sustained for up to 48 hpi (Fig. 4C).
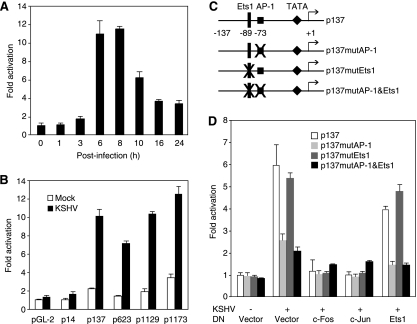
KSHV infection activates the MMP-1 promoter via the AP-1 cis-element.
Among the three MMPs examined in this study, MMP-1 secretion was robustly induced by KSHV infection (Fig. 3A and D). The expression of MMP-1 transcript was also upregulated following KSHV infection (Fig. 4A). Thus, KSHV infection could directly regulate MMP-1 expression and secretion at the transcriptional level. We further examined the effect of KSHV infection on the MMP-1 promoter activity in a reporter assay. A full-length MMP-1 promoter reporter p1173 containing the upstream promoter region −1773 to +49 was transfected into HUVEC. At different times posttransfection, cells were infected with KSHV, harvested, and assayed for reporter activities. As shown in Fig. 5A, KSHV infection indeed activated the MMP-1 reporter, with the highest level reaching 11- to 12-fold at 6 and 8 hpi. To map the MMP-1 promoter region that was activated by KSHV infection, we performed reporter assays with a series of deletion reporter constructs of the MMP-1 promoter. As shown in Fig. 5B, the dominant KSHV-responsive region was located between −14 and −137 in the MMP-1 promoter. Analysis of this DNA fragment identified a putative AP-1 binding site and a putative Ets1 binding site (Fig. 5C). To determine whether the putative AP-1 and Ets1 binding sites were responsive to KSHV infection, we carried out site-directed mutagenesis using the p137 reporter construct as a template and generated corresponding reporter constructs p137mutAP-1, p137mutEts1, and p137mutAP-1&Ets1, with the AP-1 site, Ets1 site, or both sites ablated, respectively (Fig. 5C). As shown in Fig. 5D, mutation of either the AP-1 site or both the AP-1 and Ets1 sites reduced KSHV activation of the MMP-1 promoter reporter by 70%. In contrast, mutation of the Ets1 site alone had a marginal effect on KSHV activation of the MMP-1 promoter reporter. To confirm the involvement of the AP-1 site in the mediation of KSHV activation of the MMP-1 promoter, we overexpressed DN of either c-Fos or c-Jun in the reporter assay. As expected, DN of either c-Fos or c-Jun abolished KSHV activation of the MMP-1 promoter reporter. In contrast, DN of Ets1 only slightly reduced KSHV activation of the MMP-1 promoter reporter. These results illustrated the direct involvement of AP-1 in KSHV activation of the MMP-1 promoter.
FIG. 5.
KSHV infection activates the MMP-1 promoter via the AP-1 cis-element. (A) Kinetics of activation of the full-length MMP-1 promoter reporter by KSHV infection. HUVEC were transfected with a full-length MMP-1 promoter reporter construct p1173 and assayed for luciferase activity at 36 h posttransfection. Prior to harvest, the cells were infected with KSHV for 0, 1, 3, 6, 8, 10, 16, and 24 h. (B) Deletion analysis of the MMP-1 promoter to identify the region that was responsive to KSHV infection in HUVEC. HUVEC were transfected with different MMP-1 deletion promoter reporter constructs and assayed for luciferase activity at 36 h posttransfection. Prior to harvesting, the cells were infected with KSHV for 6 h. (C) Illustration of the region (−14 to −137) in the MMP-1 promoter that was responsive to KSHV infection and generation of mutant reporter constructs p137mutAP-1, p137mutEts1, and p137mutAP-1&Ets1 with the AP-1 site, Ets1 site, or both AP-1 and Ets1 sites ablated. (D) Mutagenesis analysis of the MMP-1 promoter to confirm the AP-1 site as the major KSHV-responsive cis-element. HUVEC cotransfected with wild-type MMP-1 promoter reporter construct p137 and its mutants, together with c-Fos, c-Jun, or Ets1 expression vector, or control vector, were assayed for luciferase activity at 36 h posttransfection. Prior to harvesting, the cells were mock infected or infected with KSHV for 6 h.
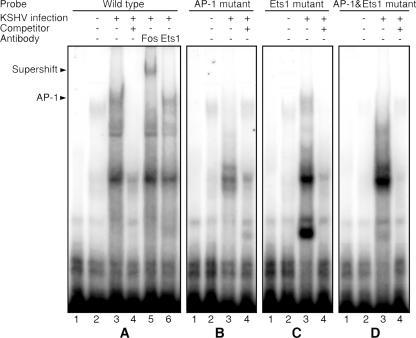
KSHV infection induces specific binding of AP-1 to the MMP-1 promoter.
To rule out any nonspecific binding of the AP-1 site by other proteins that could also result in the increase of the reporter activity, we examined the direct binding of AP-1 to the MMP-1 promoter upon KSHV infection by EMSA. An oligonucleotide encompassing both the AP-1 and Ets1 sites (−97 to −64) was used as a probe. Mock-infected HUVEC only had faint protein-DNA complex bands (Fig. 6A). In contrast, KSHV-infected cells formed several strong protein-DNA complex bands. Addition of unlabeled cold probes abolished several bands at the top part of the gel, indicating that these protein-DNA interactions were likely specific (Fig. 6A). To identify the protein-DNA complexes, we added a specific antibody to either c-Fos or Ets1 to the reaction, since the probe contained the putative binding sites for these two transcriptional factors (Fig. 6A). The c-Fos antibody caused a supershift of the top major band, indicating that this band was an AP-1-DNA complex. The c-Fos antibody had no effect on the other bands, including a minor band that was located immediately below the AP-1-DNA complex band. In contrast, the Ets1 antibody had no effect on any of the shifted bands, indicating that Ets1 was not involved in the formation of any of the protein-DNA complexes in the KSHV-infected cells (Fig. 6A). Mutation of the AP-1 site or both AP-1 and Ets1 sites in the probe abolished the AP-1-DNA complex, while mutation of the Ets1 site alone had no effect on any of the protein-DNA complexes (Fig. 6B to D). Together, these results indicated that the AP-1 site but not the Ets1 site was the dominant site in the MMP-1 promoter that responded to KSHV infection, thus further confirming an essential role of the AP-1 pathway in KSHV activation of the MMP-1 promoter. It needs to be emphasized that the detection of several other bands in addition to the identified AP-1 band in the EMSA indicated that other unidentified factors could also be involved in KSHV induction of MMP-1.
FIG. 6.
EMSA results confirmed the AP-1 site as the major KSHV-responsive cis-element in the MMP-1 promoter. (A) KSHV infection induced specific binding of the AP-1 complex to the MMP-1 promoter. Nuclear extracts from either mock-infected HUVEC (lane 2) or HUVEC infected with KSHV for 6 h (lanes 3 to 6) were subjected to EMSA using a 32P-labeled oligonucleotide probe designed from the region mapped to be responsive to KSHV infection (−64 to −97). The labeled probe alone is shown in lane 1. In the competition assay, a 100× excess amount of unlabeled probe was added to the reaction mixture (lane 4). Addition of 1 μg of antibody to c-Fos (lane 5) but not Ets1 (lane 6) supershifted the AP-1-DNA complex band. (B to D) Mutagenesis analysis of the MMP-1 promoter to identify the major AP-1-DNA complex that was responsive to KSHV infection. Nuclear extracts from either mock-infected HUVEC (lane 2) or HUVEC infected with KSHV for 6 h (lanes 3 and 4) were subjected to EMSA using 32P-labeled mutant probes with the putative AP-1 site (B), Ets1 site (C), or both AP-1 and Ets1 sites (D) mutated. The labeled probes alone are shown in lane 1. In the competition assay, a 100× excess amount of unlabeled probe was added to the reaction mixtures (lanes 4).
DISCUSSION
In this study, we have shown that KSHV infection enhances HUVEC invasion in an in vitro Matrigel-based cell invasion assay. In searching for factors that promoted the observed cell invasion, we found that KSHV infection increased the secretion of several proteases, including MMP-1, -2, and -9, in the HUVEC cultures. In agreement with these observations, the increased cell invasiveness was abolished when the proteolytic activities of the MMPs were inhibited by a specific inhibitor of MMPs, BB-94. Furthermore, we found that the increased secretion of MMPs resulted in the promotion of both autocrine and paracrine cell invasion. Together, these results illustrated an essential role of the MMPs in KSHV-mediated cell invasion.
A number of viruses also induce the secretion of MMPs. Epstein-Barr virus upregulates the expression of MMP-1 and -9 via latent membrane protein 1, while hepatitis B virus enhances cell invasion by promoting the expression of MMP-9 (26, 50). Interestingly, human immunodeficiency virus type 1 Tat protein and basic fibroblast growth factor, both of which are implicated in the promotion of AIDS-KS progression, synergistically activate MMP-2 by inducing the membrane-type MMP1 (MT-MMP-1) and the membrane-bound tissue inhibitor of metalloproteinase 2 (TIMP2) (42).
Proteases are important factors in the evolution of cancer (33). MMPs regulate cell signaling and homeostasis of the extracellular environment through specific and efficient proteolysis of their substrates (33). Besides regulation of cell-cell and cell-matrix interactions by disrupting their contacts, proteolysis of extracellular matrix substrates generates new molecules with properties that are distinct from their precursor proteins, thus resulting in the alterations of cellular ligand-receptor interactions, such as those of chemokine-chemokine receptors and the Fas-Fas ligand (15, 21, 22, 31, 35, 36, 38). Thus, MMPs contribute to different malignant phenotypes of cancer, including cell invasion, metastasis, angiogenesis, epithelial-mesenchymal transition, genomic instability, vascular permeability, and inflammatory infiltration (5, 28, 33, 37). Among the MMPs that are induced by KSHV, both MMP-2 and MMP-9 belong to gelatinases whose substrates are type IV and V collagens, laminin, fibronectin, and gelatin (50). On the other hand, MMP-1 is a collagenase and the principal endothelium-derived proteinase whose substrate includes types I, II, III, and V of native fibrillar collagens (43). MMP-1 plays an important role in the remodeling of collagenous connective tissues. It can be speculated that these KSHV-induced MMPs contribute to different facets of KS pathogenesis. In agreement with these observations, it has been shown that KS tumors express a number of MMPs, including MMP-1, -2, -3, -9, and -19 (6, 23, 29, 42). Treatment of AIDS-KS patients with an inhibitor of MMPs, COL-3, resulted in favorable outcomes in 44% of patients (12).
The secretive patterns of MMPs induced by KSHV infection varied with the individual MMPs. Although strong activity of MMP-2 was detected in normal HUVEC cultures, KSHV infection further increased its secretion at the early stage of infection. Pro-MMP-2 was induced as early as 1 hpi and persisted up to 6 hpi. The increased secretion of inter-MMP-2 followed that of pro-MMP-2 at 6 and 24 hpi, while the increased secretion of active MMP-2 was observed at 24 and 36 hpi. Nevertheless, the increased secretion of all three forms of MMP-2 by KSHV was limited, with 3.0-, 1.8-, and 2.1-fold increases for pro-MMP-2, inter-MMP-2, and active MMP-2, respectively, at their peaks of induction (Fig. 3E). Interestingly, the expression of MMP-2 mRNA was largely unchanged before 6 hpi but was decreased 4.8-, 2.6-, and 2.9-fold at 12, 24, and 48 hpi, respectively, in KSHV-infected cultures compared to the respective mock-infected cultures (Fig. 4B). While the mechanism of KSHV-mediated reduction of MMP-2 transcript expression remains to be further elucidated, these results suggest that, similar to Epstein-Barr virus, MMP-2 is unlikely to be a major determinant in KSHV-induced cell invasion.
A number of studies have shown that MMP-2 is constitutively expressed in endothelial cells and that its activation is a result of cooperative effects among MT1-MMP, TIMP2, and MMP-2 itself (44). Different isoforms of MMP-2 are also located in the storage vesicles in human endothelial cells and regulated through a specific secretive pathway (32, 40). Upon stimulation by extracellular factors, such as binding of cytokines to their receptors, the MMP-2 isoforms are released to the extracellular environment. Based on the pattern of MMP-2 secretion, it appeared that KSHV also induced MMP-2 through the secretive pathway. The interaction between KSHV ligand(s) and cellular receptor(s) at the early stage of infection could trigger a prompt yet transient shedding of MMP-2-containing vesicles, which could then be activated by the membrane-anchored MMP-2 activation complex.
In contrast to the strong secretion of MMP-2, the production of MMP-9 by normal HUVEC was barely detectable by zymography. KSHV infection increased the secretion of MMP-9 at 6 hpi and persisted for up to 36 hpi. In agreement with these results, the expression of MMP-9 mRNA was also upregulated by KSHV infection at 6 hpi and persisted for up to 48 hpi, suggesting transcriptional regulation of MMP-9 expression by KSHV infection. In fact, previous studies have shown the expression of MMP-9 is under the control of a 2.2-kb upstream regulatory region harboring binding sites for AP-1, Ets2, NF-κB, and Sp1, some of which are activated during KSHV infection (19, 47, 48). In endothelial cells, the expression of MMP-9 is regulated by multiple signaling pathways, such as the phosphatidylinositol 3-kinase pathway (25, 39). Further studies are required to define the mechanism by which KSHV regulates the expression and secretion of MMP-9.
The pattern of KSHV-induced secretion of MMP-1 was similar to that of MMP-9, which started at 6 hpi and persisted up to 36 hpi. The expression of MMP-1 mRNA was also upregulated by KSHV infection at 6 hpi and persisted up to 24 hpi. These results indicated that the expression of MMP-1 could be regulated by KSHV at the transcriptional level. Indeed, we found that the MMP-1 promoter was responsive to KSHV infection. Further promoter mapping defined the AP-1 cis-element as the predominant site that mediated KSHV activation of the MMP-1 promoter. Consistent with these results, our previous studies have shown that KSHV infection of HUVEC activates AP-1 through multiple mitogen-activated protein kinase (MAPK) pathways (34, 47). Thus, it is likely that MAPK pathways also mediate KSHV induction of MMP-1. Several KSHV proteins mediate the activation of AP-1 and MAPK pathways. Two KSHV gene products, LANA (LNA, ORF73) and vFLIP (ORF-K13), directly activate AP-1 (2, 3). vFLIP and five other KSHV gene products, vGPCR (ORF74), vPK (ORF36), LAMP (ORF-K15), kaposin B (ORF-K12), and ORF49, and also the binding of KSHV glycoproteins to cellular receptors, activate MAPK pathways (1, 3, 4, 8, 18, 20, 27, 47), all of which could contribute to KSHV-induced secretion of MMP-1.
Besides MMPs, we and others have also shown that de novo KSHV infection induces angiogenic factors and inflammatory cytokines (9, 30, 45, 47, 48). Although the majority of tumor cells are latently infected by KSHV in KS tumors, a small number of them also undergo spontaneous lytic replication, generating infectious virions for spreading to other cells (19). More importantly, this process produces virus-encoded cytokines and also induces cellular cytokines through viral lytic proteins or de novo viral infection. Our results have shown that de novo viral infection also induces MMPs that could contribute to cell invasion and other pathological features related to KS pathogenesis.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (CA096512, DE017333, and CA124332), an American Cancer Society Research Scholar grant (RSG-04-195), and a Type B Outstanding Abroad Young Scientist Award from the National Science Foundation of China (30328001) to S.-J. Gao.
We thank Bradford W. Ozanne (Beatson Institute, Glasgow, United Kingdom), Charles Vinson (National Institute of Health, Maryland), and H. Esumi (National Cancer Center Research Institute East, Chiba, Japan) for kindly providing reagents. We thank members of the Gao laboratory for technical assistance and helpful comments.
Footnotes
Published ahead of print on 18 April 2007.
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 2.An, J., A. K. Lichtenstein, G. Brent, and M. B. Rettig. 2002. The Kaposi's sarcoma-associated herpesvirus (KSHV) induces cellular interleukin 6 expression: role of the KSHV latency-associated nuclear antigen and the AP1 response element. Blood 99:649-654. [DOI] [PubMed] [Google Scholar]
- 3.An, J., Y. Sun, R. Sun, and M. B. Rettig. 2003. Kaposi's sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-κB and JNK/AP1 pathways. Oncogene 22:3371-3385. [DOI] [PubMed] [Google Scholar]
- 4.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 5.Balbin, M., A. Fueyo, A. M. Tester, A. M. Pendas, A. S. Pitiot, A. Astudillo, C. M. Overall, S. D. Shapiro, and C. Lopez-Otin. 2003. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 35:252-257. [DOI] [PubMed] [Google Scholar]
- 6.Blankaert, D., T. Simonart, J. P. Van Vooren, D. Parent, C. Liesnard, C. M. Farber, T. Marique, and J. Werenne. 1998. Constitutive release of metalloproteinase-9 (92-kd type IV collagenase) by Kaposi's sarcoma cells. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:203-209. [DOI] [PubMed] [Google Scholar]
- 7.Bogenrieder, T., and M. Herlyn. 2003. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene 22:6524-6536. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann, M. M., M. Glenn, L. Rainbow, A. Kieser, C. Henke-Gendo, and T. F. Schulz. 2003. Activation of mitogen-activated protein kinase and NF-κB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 77:9346-9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll, P. A., E. Brazeau, and M. Lagunoff. 2004. Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology 328:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 11.Cheung, T. W. 2004. AIDS-related cancer in the era of highly active antiretroviral therapy (HAART): a model of the interplay of the immune system, virus, and cancer. On the offensive: the Trojan Horse is being destroyed. Part A: Kaposi's sarcoma. Cancer Investig. 22:774-786. [DOI] [PubMed] [Google Scholar]
- 12.Cianfrocca, M., T. P. Cooley, J. Y. Lee, M. A. Rudek, D. T. Scadden, L. Ratner, J. M. Pluda, W. D. Figg, S. E. Krown, and B. J. Dezube. 2002. Matrix metalloproteinase inhibitor COL-3 in the treatment of AIDS-related Kaposi's sarcoma: a phase I AIDS malignancy consortium study. J. Clin. Oncol. 20:153-159. [DOI] [PubMed] [Google Scholar]
- 13.Deryugina, E. I., and J. P. Quigley. 2006. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 25:9-34. [DOI] [PubMed] [Google Scholar]
- 14.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67:175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fingleton, B., T. Vargo-Gogola, H. C. Crawford, and L. M. Matrisian. 2001. Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia 3:459-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao, S. J., J. H. Deng, and F. C. Zhou. 2003. Productive lytic replication of a recombinant Kaposi's sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 77:9738-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, S. J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233-241. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez, C. M., E. L. Wong, B. S. Bowser, G. K. Hong, S. Kenney, and B. Damania. 2006. Identification and characterization of the Orf49 protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 80:3062-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene, W., K. Kuhne, F. C. Ye, J. G. Chen, F. C. Zhou, X. F. Lei, and S.-J. Gao. 2007. Molecular biology of KSHV in relation to AIDS-associated oncogenesis, p. 69-127. In C. Meyers (ed.), AIDS-associated viral oncogenesis. Springer Science Business Media, New York, NY. [DOI] [PMC free article] [PubMed]
- 20.Hamza, M. S., R. A. Reyes, Y. Izumiya, R. Wisdom, H. J. Kung, and P. A. Luciw. 2004. ORF36 protein kinase of Kaposi's sarcoma herpesvirus activates the c-Jun N-terminal kinase signaling pathway. J. Biol. Chem. 279:38325-38330. [DOI] [PubMed] [Google Scholar]
- 21.Handsley, M. M., and D. R. Edwards. 2005. Metalloproteinases and their inhibitors in tumor angiogenesis. Int. J. Cancer 115:849-860. [DOI] [PubMed] [Google Scholar]
- 22.Ho, A. T., E. B. Voura, P. D. Soloway, K. L. Watson, and R. Khokha. 2001. MMP inhibitors augment fibroblast adhesion through stabilization of focal adhesion contacts and up-regulation of cadherin function. J. Biol. Chem. 276:40215-40224. [DOI] [PubMed] [Google Scholar]
- 23.Impola, U., M. A. Cuccuru, M. V. Masala, L. Jeskanen, F. Cottoni, and U. Saarialho-Kere. 2003. Preliminary communication: matrix metalloproteinases in Kaposi's sarcoma. Br. J. Dermatol. 149:905-907. [DOI] [PubMed] [Google Scholar]
- 24.Ishii, Y., T. Ogura, M. Tatemichi, H. Fujisawa, F. Otsuka, and H. Esumi. 2003. Induction of matrix metalloproteinase gene transcription by nitric oxide and mechanisms of MMP-1 gene induction in human melanoma cell lines. Int. J. Cancer 103:161-168. [DOI] [PubMed] [Google Scholar]
- 25.Ko, H. M., J. H. Kang, J. H. Choi, S. J. Park, S. Bai, and S. Y. Im. 2005. Platelet-activating factor induces matrix metalloproteinase-9 expression through Ca2+- or PI3K-dependent signaling pathway in a human vascular endothelial cell line. FEBS Lett. 579:6451-6458. [DOI] [PubMed] [Google Scholar]
- 26.Lu, J., H. H. Chua, S. Y. Chen, J. Y. Chen, and C. H. Tsai. 2003. Regulation of matrix metalloproteinase-1 by Epstein-Barr virus proteins. Cancer Res. 63:256-262. [PubMed] [Google Scholar]
- 27.McCormick, C., and D. Ganem. 2005. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307:739-741. [DOI] [PubMed] [Google Scholar]
- 28.McQuibban, G. A., J. H. Gong, E. M. Tam, C. A. McCulloch, I. Clark-Lewis, and C. M. Overall. 2000. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 289:1202-1206. [DOI] [PubMed] [Google Scholar]
- 29.Meade-Tollin, L. C., D. Way, and M. H. Witte. 1999. Expression of multiple matrix metalloproteinases and urokinase type plasminogen activator in cultured Kaposi sarcoma cells. Acta Histochem. 101:305-316. [DOI] [PubMed] [Google Scholar]
- 30.Naranatt, P. P., H. H. Krishnan, S. R. Svojanovsky, C. Bloomer, S. Mathur, and B. Chandran. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 64:72-84. [DOI] [PubMed] [Google Scholar]
- 31.Nawrocki-Raby, B., C. Gilles, M. Polette, C. Martinella-Catusse, N. Bonnet, E. Puchelle, J. M. Foidart, F. Van Roy, and P. Birembaut. 2003. E-cadherin mediates MMP down-regulation in highly invasive bronchial tumor cells. Am. J. Pathol. 163:653-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen, M., J. Arkell, and C. J. Jackson. 1998. Active and tissue inhibitor of matrix metalloproteinase-free gelatinase B accumulates within human microvascular endothelial vesicles. J. Biol. Chem. 273:5400-5404. [DOI] [PubMed] [Google Scholar]
- 33.Overall, C. M., and O. Kleifeld. 2006. Tumour microenvironment. Opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 6:227-239. [DOI] [PubMed] [Google Scholar]
- 34.Pan, H., J. Xie, F. Ye, and S. J. Gao. 2006. Modulation of Kaposi's sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J. Virol. 80:5371-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parks, W. C., C. L. Wilson, and Y. S. Lopez-Boado. 2004. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 4:617-629. [DOI] [PubMed] [Google Scholar]
- 36.Pirila, E., A. Sharabi, T. Salo, V. Quaranta, H. Tu, R. Heljasvaara, N. Koshikawa, T. Sorsa, and P. Maisi. 2003. Matrix metalloproteinases process the laminin-5 gamma 2-chain and regulate epithelial cell migration. Biochem. Biophys. Res. Commun. 303:1012-1017. [DOI] [PubMed] [Google Scholar]
- 37.Radisky, D. C., D. D. Levy, L. E. Littlepage, H. Liu, C. M. Nelson, J. E. Fata, D. Leake, E. L. Godden, D. G. Albertson, M. A. Nieto, Z. Werb, and M. J. Bissell. 2005. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436:123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schenk, S., and V. Quaranta. 2003. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 13:366-375. [DOI] [PubMed] [Google Scholar]
- 39.Steinle, J. J., C. J. Meininger, R. Forough, G. Wu, M. H. Wu, and H. J. Granger. 2002. Eph B4 receptor signaling mediates endothelial cell migration and proliferation via the phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 277:43830-43835. [DOI] [PubMed] [Google Scholar]
- 40.Taraboletti, G., S. D'Ascenzo, P. Borsotti, R. Giavazzi, A. Pavan, and V. Dolo. 2002. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 160:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson, E. W., S. Nakamura, T. B. Shima, A. Melchiori, G. R. Martin, S. Z. Salahuddin, R. C. Gallo, and A. Albini. 1991. Supernatants of acquired immunodeficiency syndrome-related Kaposi's sarcoma cells induce endothelial cell chemotaxis and invasiveness. Cancer Res. 51:2670-2676. [PubMed] [Google Scholar]
- 42.Toschi, E., G. Barillari, C. Sgadari, I. Bacigalupo, A. Cereseto, D. Carlei, C. Palladino, C. Zietz, P. Leone, M. Sturzl, S. Butto, A. Cafaro, P. Monini, and B. Ensoli. 2001. Activation of matrix-metalloproteinase-2 and membrane-type-1-matrix-metalloproteinase in endothelial cells and induction of vascular permeability in vivo by human immunodeficiency virus-1 Tat protein and basic fibroblast growth factor. Mol. Biol. Cell 12:2934-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vihinen, P., and V. M. Kahari. 2002. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int. J. Cancer 99:157-166. [DOI] [PubMed] [Google Scholar]
- 44.Visse, R., and H. Nagase. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92:827-839. [DOI] [PubMed] [Google Scholar]
- 45.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36:687-693. [DOI] [PubMed] [Google Scholar]
- 46.Wang, L., N. Wakisaka, C. C. Tomlinson, S. M. DeWire, S. Krall, J. S. Pagano, and B. Damania. 2004. The Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 64:2774-2781. [DOI] [PubMed] [Google Scholar]
- 47.Xie, J., H. Pan, S. Yoo, and S. J. Gao. 2005. Kaposi's sarcoma-associated herpesvirus induction of AP-1 and interleukin 6 during primary infection mediated by multiple mitogen-activated protein kinase pathways. J. Virol. 79:15027-15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye, F. C., D. J. Blackbourn, M. Mengel, J. P. Xie, L. W. Qian, W. Greene, I. T. Yeh, D. Graham, and S. J. Gao. 2007. Kaposi's sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J. Virol. 81:3980-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoo, S. M., F. C. Zhou, F. C. Ye, H. Y. Pan, and S. J. Gao. 2005. Early and sustained expression of latent and host modulating genes in coordinated transcriptional program of KSHV productive primary infection of human primary endothelial cells. Virology 343:47-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshizaki, T., H. Sato, M. Furukawa, and J. S. Pagano. 1998. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc. Natl. Acad. Sci. USA 95:3621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]