Abstract
The RNA helicases RIG-I and MDA5 detect virus infection of dendritic cells (DCs) leading to cytokine induction. Maximal sensitivity for virus detection by these helicases is obtained after their upregulation, which is thought to occur primarily through type I interferon (IFN) signaling. Here we demonstrate that in response to paramyxovirus infection, RIG-I upregulation requires type I IFN whereas MDA5 expression is increased by Sendai virus infection independently of signaling mediated by type I IFN, STAT1, tumor necrosis factor alpha, or NF-κB. This MDA5 upregulation is largely lost in IRF3 knockout DCs and is achieved in type I IFN-deficient cells expressing constitutively active IRF3.
Virus infection of conventional dendritic cells (DCs) can be detected by cytosolic RNA helicases. Specifically, RIG-I and MDA5 have emerged as primary sensors for viruses belonging to diverse families such as paramyxoviruses, orthomyxoviruses, rhabdoviruses, flaviviruses, and picornaviruses, leading to the production of type I interferons (IFNs) (4, 8, 9, 20). RIG-I has been shown to detect the majority of these viruses through recognition of uncapped 5′ triphosphates on viral genomic RNA (6, 15). MDA5 has been shown to be essential for the recognition of encephalomyocarditis virus, a picornavirus that encodes a protein that caps the 5′ end of its genome but generates secondary RNA structures (4, 9). Activation of RIG-I and MDA5 leads to the nuclear translocation of transcription factors such as IRF3 and NF-κB that are crucial for the transcriptional induction of type I IFNs and other cytokines and chemokines (19, 20).
Upregulation of RIG-I and MDA5 upon viral infection is thought to be important in optimizing the sensitivity of virus detection (17). As these molecules are known to be induced by type I IFN (7, 20), it is likely that type I IFN feedback signaling involving STAT1 is required for maximum activation of DCs through RIG-I and MDA5 triggering. Accordingly, viruses encoding antagonists of type I IFN signaling are often poorly detected by infected DCs (9). Likewise, maturation of DCs by Newcastle disease virus (NDV), which is unable to antagonize type I IFN signaling in mammalian cells, is lost in DCs lacking the type I IFN receptor (5, 12). However, our previously published data demonstrated that secretion of cytokines induced by another paramyxovirus, Sendai virus strain Cantell (SeV-C), was normal in type I IFN receptor knockout DCs but not in wild-type DCs (10). SeV-C is known to produce unique defective interfering (DI) genomes responsible for its potent stimulatory activity (18, 21). In an attempt to understand the dichotomy in the requirement for IFN signaling in the DC response to paramyxoviruses such as NDV and SeV-C, we examined the upregulation of RIG-I and MDA5 known to be critical for the initiation of conventional DC maturation upon viral infection.
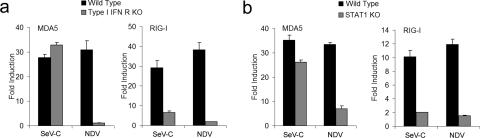
In order to examine the dependence on type I IFN for RIG-I and MDA5 induction, DCs derived from wild-type and type I IFN receptor knockout mice (SV129 background; B & K Universal) prepared as described elsewhere (10, 21) were infected with SeV-C or NDV at a multiplicity of infection (MOI) of 1.5 and the upregulation of these viral sensors was analyzed at 6 h postinfection (hpi) by quantitative reverse transcriptase PCR (qRT-PCR). RIG-I induction was largely dependent on type I IFN receptor signaling regardless of the virus used (Fig. 1a). MDA5 expression was increased independently of the presence of type I IFN by SeV-C infection whereas MDA5 upregulation by NDV infection was lost in type I IFN receptor knockout DCs (Fig. 1a). It has been reported that in some instances, SeV is able to directly induce the phosphorylation of STAT1 (3), a molecule critical to the type I IFN signaling pathway. However, we observed that MDA5 upregulation by SeV-C occurred normally in STAT1 knockout DCs (SV129 background; Taconic Farms) (Fig. 1b) and confirmed the dependence of RIG-I upregulation on the presence of the type I IFN signaling pathway in response to both SeV-C and NDV infections (Fig. 1b).
FIG. 1.
MDA5 upregulation in response to SeV-C is independent of type I IFN signaling. Type I IFN receptor knockout (KO) DCs (a) and STAT1 knockout DCs (b) as well as wild-type DCs were infected with SeV-C or NDV at an MOI of 1.5 or were mock infected. RNA was extracted at 6 hpi and analyzed by qRT-PCR for expression of MDA5 and RIG-I. Induction values (n-fold) represent comparisons to the values obtained for mock-infected cells. Error bars represent the standard deviations of values obtained in triplicate measurements in a representative experiment.
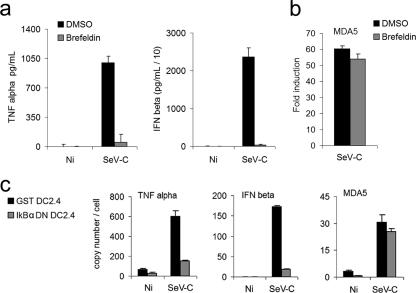
MDA5 is known to be weakly induced by tumor necrosis factor alpha (TNF-α) signaling (7), and SeV-C is known to be a strong inducer of TNF-α secretion from infected DCs (10, 11, 21). Thus, the type I IFN-independent induction of MDA5 by SeV-C could be explained by the presence of TNF-α signaling. To address this possibility, DCs were treated for 1 h prior to infection with 1 μg/ml brefeldin A (Golgi Plug; BD Pharmingen) to disrupt the Golgi apparatus, thereby blocking secretion of cytokines such as TNF-α and type I IFNs (Fig. 2a). This inhibition of cytokine secretion was unable to prevent upregulation of MDA5 by SeV-C (Fig. 2b). Furthermore, DC2.4 cells stably transduced with the IκBα dominant-negative inhibitor of NF-κB kindly provided by Adrian Ting and Bruno Moltedo (Mount Sinai School of Medicine, New York, NY) lost their ability to highly upregulate TNF-α and beta IFN (IFN-β) whereas MDA5 induction was similar to that seen with control cells expressing glutathione S-transferase (Fig. 2c). Overall, these data demonstrate that MDA5 upregulation by SeV-C occurs through a previously undescribed mechanism that is independent of TNF-α and type I IFN signaling as well as NF-κB activation.
FIG. 2.
MDA5 is upregulated by SeV-C independently of the presence of TNF-α, type I IFN, and NF-κB. (a and b) DCs were pretreated for 1 h with brefeldin (1 μg/ml) or an equal volume of dimethyl sulfoxide (DMSO) prior to infection with SeV-C at an MOI of 1.5 or mock infection (Ni). (a) Supernatants were analyzed by enzyme-linked immunosorbent assay for TNF-α and IFN-β secretion 6 hpi. (b) RNA was extracted 6 hpi for analysis of MDA5 expression by qRT-PCR. Induction values (n-fold) represent comparisons to the values obtained for mock-infected cells. (c) DC2.4 cells transduced with retroviruses encoding either glutathione S-transferase (GST) or an IkBα dominant-negative inhibitor of NF-κB were infected with SeV-C at an MOI of 1.5 or mock infected for 6 h, and RNA was extracted for qRT-PCR analysis of TNF-α, IFN-β, and MDA5 expression. Error bars represent the standard deviations of values obtained in triplicate measurements in representative experiments.
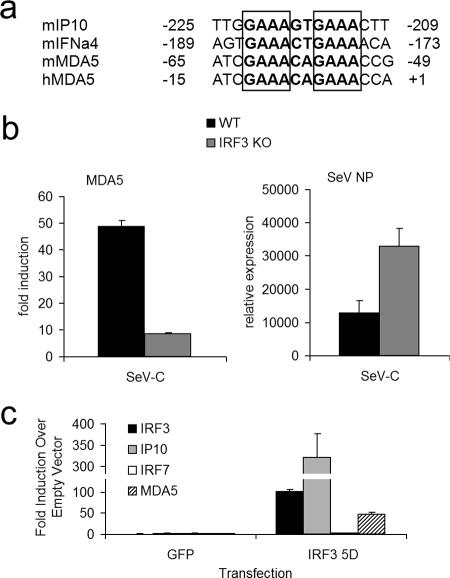
In order to understand the cytokine-independent induction of MDA5 by SeV-C, regions upstream of the transcriptional start site of both murine and human MDA5 were analyzed for the presence of transcription factor binding sites that might explain its pattern of induction. Particularly, we searched for motifs required for the binding of IRF3, as this transcription factor is known to be activated early in viral infections. Putative IRF3 binding sites were found in the MDA5 promoters (Fig. 3a) that matched consensus IRF3 binding sites in the promoters of genes such as murine IP10 and IFN-α4 (13, 14, 16). Accordingly, the induction of MDA5 by SeV-C was decreased in IRF3 knockout DCs (Japan CLEA) whereas viral replication as measured by SeV NP mRNA levels was increased (Fig. 3b). To confirm the existence of IRF3-dependent upregulation of MDA5, we transfected K562 cells using an Amaxa nucleofection system and 2.5 μg of pCAGGS empty vector, pCAGGS-GFP, or pCAGGS-IRF3(5D), kindly provided by Adolfo Garcia-Sastre and Luis Martinez-Sobrido (Mount Sinai School of Medicine, New York, NY). The overexpression of the constitutively active IRF3 mutant (5D) in human K562 cells unable to produce type I IFNs resulted in the upregulation of MDA5 as measured by qRT-PCR (Fig. 3c). IP10 served as a positive control for a gene induced by IRF3, while IRF7 expression served as a negative control (Fig. 3c). Thus, the cytokine-independent transcriptional upregulation of MDA5 by SeV-C results from the activation of IRF3 by virus infection.
FIG. 3.
IRF3-dependent upregulation of MDA5. (a) Known IRF3 binding sites in the murine IP10 and IFN-α4 promoters were compared with putative IRF3 binding sites in the murine and human MDA5 promoter regions. Boxes highlight GAAA motifs required for IRF3 binding. (b) Wild-type and IRF3 knockout DCs were infected with SeV-C at an MOI of 1.5 or were mock infected for 6 h. RNA was extracted and analyzed by qRT-PCR for MDA5 expression and production of SeV NP mRNA. Induction values (n-fold) represent comparisons to the values obtained for mock-infected cells. Relative expression values indicate induction levels (n-fold) relative to the values obtained for background levels of the SeV NP PCR in mock-infected cells. (c) Human K562 cells were transfected with 2.5 μg of empty pCAGGS, pCAGGS-GFP, or pCAGGS-IRF3(5D) using an Amaxa Nucleofector kit V. RNA was extracted 18 h after transfection and analyzed by qRT-PCR for expression of IRF3 (the analysis technique used also detects transfected IRF3), IP10, IRF7, and MDA5. Induction values (n-fold) represent comparisons to the values obtained for cells transfected with empty pCAGGS. Error bars represent the standard deviations of values obtained in triplicate measurements in representative experiments.
The potent DC stimulus provided by SeV-C has allowed us to show a direct role for IRF3 signaling in the upregulation of MDA5, previously thought to be upregulated only by type I IFN and to a minor extent by TNF-α (7). The initial activation of IRF3 during viral infection is likely to occur after viral detection by RIG-I or MDA5 present at low constitutive levels in immature DCs, as Toll-like receptor signaling has been shown to be dispensable for the triggering of DC maturation by SeV-C (11). Recent work demonstrated that RIG-I, but not MDA5, plays a significant role in the triggering of type I IFN expression in response to SeV lacking its antagonistic proteins (9). The relative contributions of RIG-I and MDA5 in recognition of the stimulus provided by SeV-C that contains DI particles and maintains type I IFN signaling antagonism (21) remain to be studied. Here we show potent transcription of MDA5 in response to SeV-C, while NDV, known to trigger DC activation through RIG-I (9), was unable to upregulate MDA5 independently of type I IFN signaling. Interestingly, it was demonstrated that the SeV V protein interacts with MDA5 and inhibits its activation (1). This interaction and inhibition of MDA5 by the V protein was also demonstrated for at least 12 other paramyxoviruses (2), suggesting that a currently unappreciated role exists for MDA5 in the antiviral response to paramyxoviruses. We can hypothesize the involvement of MDA5 in the recognition of paramyxovirus replication byproducts like DI particles, whereas RIG-I detects standard paramyxovirus genomes (9). The cytokine-independent MDA5 upregulation could explain the ability of SeV-C to mature DCs independently of the presence of type I IFN (10) and may be particularly significant in the detection of DI particles that interfere with viral protein production, including that of the V protein (18, 21). Overall, this report highlights a previously unrecognized level of regulation of MDA5 through IRF3 activation independently of cytokine signaling and suggests a role for MDA5 in the detection of paramyxovirus replication products.
Acknowledgments
The work was supported by grants 1R01AI41111, U19AI062623-01, and HHSN266200500021C (to T.M.M.) from the National Institute of Allergy and Infectious Diseases.
We thank Luis Muñoz for technical assistance and Bruno Moltedo, Adrian Ting, Luis Martinez-Sobrido, and Adolfo Garcia-Sastre for providing reagents critical to the study. We also acknowledge the Quantitative PCR Shared Resource Facility of the Mount Sinai School of Medicine for performing qRT-PCR on human RNA samples from K562 cells.
Footnotes
Published ahead of print on 2 May 2007.
REFERENCES
- 1.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Childs, K., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. Randall, and S. Goodbourn. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359:190-200. [DOI] [PubMed] [Google Scholar]
- 3.Garcin, D., J. B. Marq, S. Goodbourn, and D. Kolakofsky. 2003. The amino-terminal extensions of the longer Sendai virus C proteins modulate pY701-Stat1 and bulk Stat1 levels independently of interferon signaling. J. Virol. 77:2321-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103:8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994-997. [DOI] [PubMed] [Google Scholar]
- 7.Kang, D. C., R. V. Gopalkrishnan, Q. Wu, E. Jankowsky, A. M. Pyle, and P. B. Fisher. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 99:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 9.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 10.López, C. B., A. Garcia-Sastre, B. R. Williams, and T. M. Moran. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 11.López, C. B., B. Moltedo, L. Alexopoulou, L. Bonifaz, R. A. Flavell, and T. M. Moran. 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 173:6882-6889. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Sobrido, L., N. Gitiban, A. Fernandez-Sesma, J. Cros, S. E. Mertz, N. A. Jewell, S. Hammond, E. Flano, R. K. Durbin, A. Garcia-Sastre, and J. E. Durbin. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J. Virol. 80:1130-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morin, P., J. Braganca, M. T. Bandu, R. Lin, J. Hiscott, J. Doly, and A. Civas. 2002. Preferential binding sites for interferon regulatory factors 3 and 7 involved in interferon-A gene transcription. J. Mol. Biol. 316:1009-1022. [DOI] [PubMed] [Google Scholar]
- 14.Nakaya, T., M. Sato, N. Hata, M. Asagiri, H. Suemori, S. Noguchi, N. Tanaka, and T. Taniguchi. 2001. Gene induction pathways mediated by distinct IRFs during viral infection. Biochem. Biophys. Res. Commun. 283:1150-1156. [DOI] [PubMed] [Google Scholar]
- 15.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 16.Schafer, S. L., R. Lin, P. A. Moore, J. Hiscott, and P. M. Pitha. 1998. Regulation of type I interferon gene expression by interferon regulatory factor-3. J. Biol. Chem. 273:2714-2720. [DOI] [PubMed] [Google Scholar]
- 17.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25:373-381. [DOI] [PubMed] [Google Scholar]
- 18.Strahle, L., D. Garcin, and D. Kolakofsky. 2006. Sendai virus defective-interfering genomes and the activation of interferon-beta. Virology. 351:101-111. [DOI] [PubMed] [Google Scholar]
- 19.Yoneyama, M., M. Kikuchi, K. Matsumoto, T. Imaizumi, M. Miyagishi, K. Taira, E. Foy, Y. M. Loo, M. Gale, Jr., S. Akira, S. Yonehara, A. Kato, and T. Fujita. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175:2851-2858. [DOI] [PubMed] [Google Scholar]
- 20.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 21.Yount, J. S., T. A. Kraus, C. M. Horvath, T. M. Moran, and C. B. Lopez. 2006. A novel role for viral-defective interfering particles in enhancing dendritic cell maturation. J. Immunol. 177:4503-4513. [DOI] [PubMed] [Google Scholar]