Abstract
The productive replication of human immunodeficiency virus type 1 (HIV-1) occurs exclusively in defined cells of human or chimpanzee origin, explaining why heterologous animal models for HIV replication, pathogenesis, vaccination, and therapy are not available. This lack of an animal model for HIV-1 studies prompted us to examine the susceptibility of feline cells in order to evaluate the cat (Felis catus) as an animal model for studying HIV-1. Here, we report that feline cell lines harbor multiple restrictions with respect to HIV-1 replication. The feline CD4 receptor does not permit virus infection. Feline T-cell lines MYA-1 and FeT-1C showed postentry restrictions resulting in low HIV-1 luciferase reporter activity and low expression of viral Gag-Pol proteins when pseudotyped vectors were used. Feline fibroblastic CrFK and KE-R cells, expressing human CD4 and CCR5, were very permissive for viral entry and HIV-long terminal repeat-driven expression but failed to support spreading infection. KE-R cells displayed a profound block with respect to release of HIV-1 particles. In contrast, CrFK cells allowed very efficient particle production; however, the CrFK cell-derived HIV-1 particles had low specific infectivity. We subsequently identified feline apolipoprotein B-editing catalytic polypeptide 3 (feAPOBEC3) proteins as active inhibitors of HIV-1 particle infectivity. CrFK cells express at least three different APOBEC3s: APOBEC3C, APOBEC3H, and APOBEC3CH. While the feAPOBEC3C did not significantly inhibit HIV-1, the feAPOBEC3H and feAPOBEC3CH induced G to A hypermutations of the viral cDNA and reduced the infectivity ∼10- to ∼40-fold.
Like many retroviruses, human immunodeficiency virus type 1 (HIV-1) has a very limited host range; spreading replication is seen only in Homo sapiens and by artificial inoculation in the close relative the chimpanzee (Pan troglodytes) (2, 16), preventing the setup of an efficient small animal model for HIV-1 research. Many reasons argue against the widespread use of chimpanzees in research, including the ethical problems involved in the use of an endangered species, budgetary problems, and the very low induction of simian AIDS either from HIV-1 or its ancestor simian immunodeficiency virus cpz (SIVcpz) in infected chimpanzees (25, 26, 30, 57, 58, 60, 63).
In general, the tropism of HIV-1 in human tissue is determined by the expression of its receptor protein CD4 together with CCR5 or CXCR4 chemokine receptors. Simian CD4 but not murine CD4 supports entry of HIV-1 (15, 33). HIV-1 entry through receptor-mediated membrane fusion is required for reverse transcription of the viral genomic RNA into a double-stranded DNA molecule. Murine T cells show early postentry restriction of HIV-1 at reverse transcription (3). In simian cells, a related restriction of HIV-1, but not of SIVs (5, 10, 53), involves the simian TRIM5α protein, which leads to increased viral uncoating and thereby suppresses reverse transcription (73). Since HIV-1 does not show spreading replication in nonhuman cells, cell type- and tissue-specific tropism was studied mostly using human cells. Soon after identifying the relevance of the chemokine coreceptors for HIV infection, it was realized that certain receptor- and coreceptor-positive human cells, mostly nondividing, were still resistant to full HIV-1 replication. HIV-1 can infect human cells arrested in the cell cycle at the G0/1a or G2 stage (13, 21, 37). Remarkably, in a few quiescent and nonactivated human cells, such as monocytes and resting peripheral T lymphocytes, HIV-1 is restricted soon after membrane fusion. It has been discussed that a limiting nucleotide pool (31, 83) and/or a unique and uncharacterized activity of low-molecular-weight complexes of a protein called apolipoprotein B-editing catalytic polypeptide 3G (APOBEC3G) (see below) exclusively found in resting cells perturbs reverse transcription in these cells (9). In some experimental systems using nondividing human cells, the nuclear import and/or the integration of viral DNA into chromosomal DNA is also very inefficient (55, 69, 74). This is, however, not a general feature of resting cells in vivo, since human resting T cells residing within lymphoid tissues are permissive for HIV-1 infection (13), indicating that subtle differences in cell physiology play a crucial role in whether a given cell is permissive to HIV-1 replication or not.
Beside these so-called early replication blocks, nondividing human cells also show late blocks (for a review, see reference 81). In dividing murine cells, low levels of transcription by nonfunctional p-TEFb complexes (consisting of cyclin T1 and CDK9) have been observed (7, 17, 32, 79). Murine cells show several additional late blocks of HIV replication, such as disturbed RNA export (47, 75, 85) and processing and assembly blocks of the viral proteins (6, 45). One of the best-characterized cellular proteins efficiently restricting HIV-1 is the cytidine-deaminase APOBEC3G (70). Encapsidation of APOBEC3G and other members of the APOBEC3 family in HIV-1 virus particles leads to deamination of cytosine residues to uracil in growing single-stranded DNA during reverse transcription (8, 24, 34, 42, 43, 84). HIV uses the viral infectivity protein (Vif) to prevent or at least reduce incorporation of APOBEC3G into progeny virions (43, 46, 71). Despite vif expression, low levels of APOBEC3-mediated cytidine deamination are detectable, indicating that even wild-type (wt) HIV-1 is weakly restricted by the presence of APOBEC3 proteins (61). Because the HIV-1 Vif protein exclusively binds and inactivates human APOBEC3 proteins in a species-specific way, HIV-1 is strongly inhibited by the simian and murine orthologues of APOBEC3G (43) that evade HIV-1 Vif counteraction.
Given that there is neither a small animal model nor a primate model available for HIV-1 investigations, rodent systems were developed to model specific steps of HIV-1 infection. Transgenic mice containing full-length or individual genes under the control of non-HIV promoters were used to study the postintegration phase of the viral life cycle. These models provide a means for assessing effects of HIV-encoded proteins in vivo and mammalian responses to different viral gene products (23, 35, 65, 76). In addition, severe combined immunodeficient (SCID) mice, engrafted with human peripheral blood mononuclear cells or fetal thymus and fetal liver cells, have been used as a model for HIV-1 (50, 54). With both SCID models uncontrolled HIV-1 infections, limited to the engrafted cells, peak at 3 to 4 weeks postinoculation and are characterized by variable depletions of the engrafted CD4+ T cells and a lack of humoral or cellular responses to the virus (1, 51). To increase the longevity of the engraftment with multilineage hematopoiesis and properties of a more functional human immune system, Rag2−/−γc−/− or NOD/SCID/IL2Rγ−/− mice engrafted with human hematopoietic stem cells were recently used for HIV-1 infection studies (4, 20, 78).
The potential usefulness of an animal with an intact immune system as a model of HIV-1 infection and disease warrants further efforts directed at an assessment of the limitations and blocks in the viral life cycle in animals that may serve for animal experimentation. The cat (Felis catus) is an established animal model for studies of the brain, genetics, pharmacology, nutrition, and virology (59). Unlike rodents, cats are permissive for infection by a lentivirus, the feline immunodeficiency virus (FIV). But FIV is only distantly related to HIV-1; thus, studies of FIV are suitable only to a limited extent for understanding HIV-1 pathogenesis. Initial observations of the host range of HIV-1 in feline cells showed a lack of reverse transcriptase (RT) production after virus inoculation (36), but subsequent experiments using vesicular stomatitis virus G protein (VSV-G)-pseudotyped HIV vectors containing cytomegalovirus (CMV) promoters demonstrated robust gene transfer into feline cell lines (28, 64, 66). Cellular factors restricting HIV-1 in the cat are currently unknown. However, in a recent study of feline foamy viruses (FFV), we characterized a feline APOBEC3 protein (39), demonstrating that in addition to primates and rodents, felines also express cytosine-deaminases restricting retroviral replication. Because feline cells are known to be highly permissive for reporter gene expression by HIV vectors, we were curious to extend this finding and to explore the use of the cat as a potential small-laboratory animal model for HIV-1 infection.
We show here that cell lines derived from Felis catus show several restrictions to HIV-1 replication regarding entry, particle release, and particle infectivity. The feline APOBEC proteins 3H and 3CH were identified as potent inhibitors of HIV-1 in feline cells.
MATERIALS AND METHODS
Cells and transfections.
The adherent human cell lines HOS (American Type Culture Collection [ATCC] CRL-1543), HOS.CXCR4 (National Institute for Biological Standards and Control [NIBSC] ARP5000), HOS.CCR5 (NIBSC ARP5001), HOS.CD4.CCR5 (NIBSC ARP078), HT1080 (ATCC CCL121), and 293T and feline cell lines CrFK (ATCC CCL-94; feline kidney cells) and KE-R (feline embryonic fibroblast cells; a gift of Roland Riebe, Friedrich-Loeffler Institut, Riems, Germany) were maintained in Dulbecco's high-glucose modified Eagle's medium (Dulbecco's modified Eagle's medium complete; Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 0.29 mg/ml l-glutamine, and 100 units/ml penicillin/streptomycin. Cells of the human T-cell line A3.01 (NIBSC ARP098) were cultured in complete RPMI 1640 medium-10% heat-inactivated FBS-0.29 mg/ml l-glutamine-100 units/ml penicillin/streptomycin. Cells of the feline T-cell lines MYA-1 (ATCC CRL-2417) and FeT-1C (ATCC CRL-11968) were cultured in complete RPMI 1640-0.29 mg/ml l-glutamine-10 mM HEPES-1.0 mM sodium pyruvate supplemented with 0.05 mM 2-mercaptoethanol, 100 units/ml human recombinant interleukin-2 and 10% heat-inactivated FBS, and 100 units/ml penicillin/streptomycin. Plasmid transfection into 293T and CrFK cells was done with Lipofectamine 2000 according to the instructions of the manufacturer (Invitrogen). Feline CD4 was amplified from cDNA of feline peripheral blood mononuclear cells after activation with phytohemagglutinin (3 μg/ml), the forward primer 5′fCD4-EcoRI (5′-GAATTCATGAATCAAGGAGCCGTTTTTAGG-3′), the reverse primer 3′fCD4-BglII (5′-AGATCTTCAAATGGGATTACATGTCTTCTG-3′), and Pwo polymerase (Roche Diagnostics). Thirty cycles were run at 94°C for 30 s, 58°C for 1 min, and 72°C for 2 min. PCR products were cloned into pMSCVneo (Clontech) by use of EcoRI and BglII restriction sites. The identity of the resulting pMSCVneo-fCD4 was confirmed by sequencing. pMSCVneo-hCD4 was generated by transferring hCD4 cDNA from T4-pMV7 (41) into pMSCVneo by use of EcoRI restriction sites. Retroviral vector stocks of pBABE-CCR5 (puro) (12), pMSCVneo-hCD4, and pMSCVneo-fCD4 were used to infect cells. After selection with puromycin for CCR5 and G418 for CD4 vectors, receptor expression was confirmed by flow cytometry. The cells were stained for human CD4 with SK3-PerCP (BD PharMingen), for feline CD4 with 3-4F4-PE (SouthernBiotech), for human CCR5 with 2D7-PE (BD PharMingen), and for human CXCR4 with 12G5-PE (BD PharMingen).
Viruses and infections.
Replication-competent HIV-1NL4.3 and HIV-1NL-BaL (NL4.3 with the env BaL) (44) virus stocks were prepared by harvesting the supernatant of transfected 293T cells. To generate VSV-G-pseudotyped HIV-1, 293T cells were cotransfected with HIV-1 DNA (pNL4.3; pNL-BaL) and pMD.G VSV-G expression plasmid (14). HIV-1 single-cycle luciferase reporter viruses (HIV-Luc) were produced by cotransfecting 293T cells with pNL-LucR−E− (a gift from Nathaniel R. Landau; 45), JR.FL (pcJR.FL; 12), L102, a variant of the C-terminally truncated (at amino acid 712) HIV-1 Env strain BH10 (pcL102), HXB2 (pSV7d; 62), BaL.01 (HIV-1 clone BaL.01; 38), BaL.26 (HIV-1 clone BaL.26; 38), or VSV-G expression vector. Δvif HIV-Luc was produced by cotransfecting 293T cells with pNL-LucR−E−Δvif (43) and VSV-G expression plasmid. Single-cycle HIV-1-green fluorescent protein (GFP) (VSV-G) expressing enhanced GFP (EGFP) by an internal CMV promoter were generated by transfection of 293T with pHIV.NL4-3ΔE-EGFP (52) together with pMD.G. EGFP vectors were titrated by serial dilutions using HT1080 cells as described previously (52), 4 × 105 cells were transduced using a multiplicity of infection (MOI) of 5, and GFP expression was determined by flow cytometry 3 days posttransduction. FIV single-cycle luciferase (FIV-Luc) vectors were produced by cotransfecting 293T cells with pFP93 (a gift of Eric M. Poeschla; 40), pvLucFIVΔenv, and pMD.G. pvLucFIVΔenv vector was derived from p34TF10, a replication-competent molecular clone of FIV, contains a 2 kb internal deletion in env, and has most of gag-pol region replaced by the firefly luciferase gene under the control of an internal CMV promoter. RT of viruses was determined by use of a Cavidi HS Lenti RT kit (Cavidi Tech). Alternatively, viruses were quantified using an HIV-1 p24 antigen enzyme-linked immunosorbent assay (ELISA) based on a previously published method (48, 49). Briefly, p24 antigen is captured from a detergent lysate of virions (1% Empigen; Calbiochem) by use of a polyclonal antibody (D7320 sheep anti-HIV-1-p24 gag; Aalto Bio Reagents, Dublin, Ireland) adsorbed to a solid phase. Bound p24 is detected using an alkaline phosphatase-conjugated anti-p24 monoclonal antibody (BC 1071-AP alkaline phosphatase conjugate of anti-HIV-1-p24 mouse monoclonal antibody; Aalto Bio Reagents, Dublin, Ireland) and a luminescence detection system (Tropix ELISA-Light immunoassay system; Applied Biosystems) and analyzed using a Berthold MicroLumat Plus luminometer. For reporter virus infections, the adherent cells were seeded at 2.0 × 103 cells/well per day before transduction and suspension cells were seeded at 5.0 × 104 cells/well on the day of transduction in 96-well plates and then infected with reporter virus stocks normalized for RT. Firefly luciferase activity was measured according to the manufacturer's directions 3 days later with a Steadylite HTS reporter gene assay system (PerkinElmer) on a Berthold MicroLumat Plus luminometer.
APOBEC3 expression and plasmids.
Feline APOBEC3C (previously termed feAPOBEC3 [fe3]; GenBank accession no. AY971954) was described previously (39). Feline APOBEC3H and feline APOBEC3CH cDNAs were identified by using 5′ and 3′ rapid amplification of cDNA ends (RACE) reactions (5′/3′-RACE kit; Roche Diagnostics) employing total RNA from CrFK cells. For full-length expression cloning of C-terminal hemagglutinin (HA)-tagged feline APOBEC3H, forward primer fAPO-29 (5′-TGCATCGGTACCTGGAGGCAGCCTGGGAGGTG-3′) and reverse primer fAPO-28 (5′-AGCTCGAGTCAAGCGTAATCTGGAACATCGTATGGATATTCAAGTTTCAAATTTCTGAAG-3′) and Pwo polymerase (Roche Diagnostics) were used; for feline APOBEC3CH, forward primer fAPO-30 (5′-TGCATCGGTACCACCAAGGCTGGAGAGAGGAATGG-3′) and reverse primer fAPO-28 and Pwo polymerase were used. Each of 30 cycles was run at 94°C for 30 s, 58°C for 1 min, and 72°C for 2 min, PCR products were cloned into the KpnI and XhoI sites of pcDNA3.1(+), and correct clones were identified by sequencing. Expression studies of feline APOBEC3 RNA of CrFK cells were done by RT-PCR using total RNA, Taq polymerase (QIAGEN), and forward primer fAPO3F-18 (5′-TAGAAGCTTACCAAGGCTGGCGAGAGGAATGG-3′) and reverse primer fAPO3F-19 (5′-AGCTCGAGTCAAGCGTAATCTGGAACATCGTATGGATACCTAAGGATTTCTTGAAGCTCTGC-3′) for feline APOBEC3C, forward primer fAPO3F-9 and reverse primer fAPO-26 (5′-CTGCCCGAAGGCACCCTAATTC-3′) for feline APOBEC3H, and forward primer fAPO3F-11 (5′-ACCAAGGCTGGCGAGAGGAATGG-3′) and reverse primer fAPO-27 (5′-TCGTACTCGAGGCAGTTTATGAAGCATTGAGATGC-3′) for feline APOBEC3CH. PCRs were run for 30 cycles of 94°C for 30 s, annealing for 1 min for feA3C at 62°C, for feA3H at 61°C, and for feA3CH at 60°C, and 72°C for 2 min.
Immunoblot analysis.
Cells were infected with HIV-1NL.BaL(VSV-G) or cotransfected with plasmids for HIV-Luc or ΔvifHIV-Luc, and APOBEC3-HA expression plasmids and lysates and virions were prepared 3 days later. Virions were pelleted by centrifugation of filtered culture supernatant through a 20% sucrose cushion at 35,000 rpm in an SW40Ti rotor for 1.5 h and lysed in lysis buffer (100 mM NaCl, 10 mM EDTA, 20 mM Tris [pH 7.5], 1% Triton X-100, 1% sodium deoxycholate). Cell lysates were prepared by removing infected cells from the medium, washing the cells with phosphate-buffered saline, and lysing the cells in lysis buffer. Protein in the lysates was quantitated using Coomassie blue reagent (Bio-Rad). Lysates containing 20 μg of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride filters. Filters were probed with mouse anti-capsid p24 hybridoma supernatant (α-p24 183-H12-5C; provided by Egbert Flory) (1:50 dilution) or HIV-1 Vif antiserum (HIV-1HXB2 Vif antiserum; 19) (1:2,000 dilution) or anti-HA antibody (MMS-101P; Covance) (1:6,000 dilution) or mouse anti-α-tubulin (clone B5-1-2; Sigma-Aldrich) (1:4,000 dilution) followed by horseradish peroxide-conjugated rabbit anti-mouse antibody (α-mouse-IgG-HRP; Amersham Biosciences) and developed with ECL chemiluminescence reagents (Amersham Biosciences).
Sequencing of viral reverse transcripts.
HOS cells (1 × 106) were infected with DNase I (Roche)-treated HIV-Luc(VSV-G) (1,000 pg RT). At 10 h postinfection, cells were washed with phosphate-buffered saline and DNA was isolated using a DNeasy DNA isolation kit (QIAGEN). A 600 bp fragment covering long terminal repeat gag (LTR-gag) was amplified using Taq DNA polymerase (QIAGEN) and the primers MH 531 (5′-TGTGTGCCCGTCTGTTGTGT-3′) and CM100 (5′-TGGAGGTTCTGCACTATAGGG-3′). Each of thirty cycles was run at 94°C for 30 s, 58°C for 1 min, and 72°C for 2 min, and PCR products were cloned into TOPO TA-cloning pCR4 vector (Invitrogen) and sequenced. The nucleotide sequences of at least 10 independent clones were analyzed.
Nucleotide sequence accession numbers.
The sequences reported in this paper have been deposited in the GenBank database as follows: feline APOBEC3H (accession no. EF173020) and feline APOBEC3CH (accession no. EF173021).
RESULTS
HIV-1(VSV-G) infects feline adherent cells but is restricted in feline T cells.
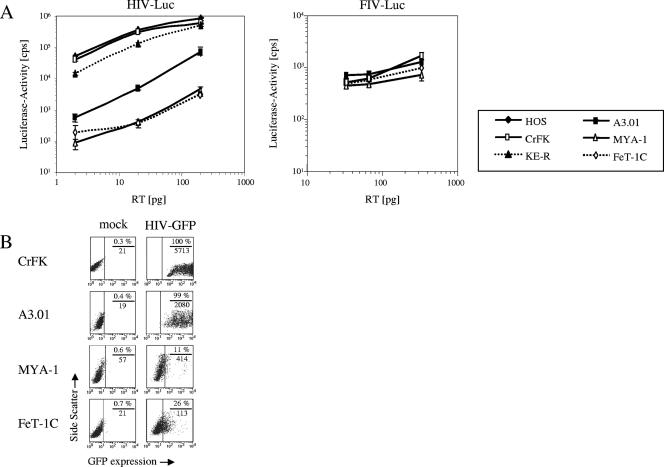
To evaluate the cat (Felis catus) as an animal model for HIV-1, established feline cell lines were used to characterize HIV-1 replication. Because Tat transactivation of the HIV-LTR in murine cells is inefficient (7, 17, 32, 79), we were interested in discovering whether HIV-LTR-driven reporter viruses in cat cells would generate high expression of the reporter protein. Feline adherent cell lines (KE-R and CrFK) and feline T-cell lines (MYA-1 and FeT-1C) were transduced with VSV-G-pseudotyped HIV-1 luciferase reporter viruses. To draw a comparison to results obtained in investigations of the feline cells, the permissive human cell lines HOS and A3.01 were coinoculated with the luciferase virus. Pseudotyping of the particles with VSV-G allowed infection of cells that were negative with respect to HIV receptor expression. The virus particles were produced by transient transfection in human 293T cells, and 2, 20, and 200 pg RT were used for transduction. At 3 days postinfection (dpi) luciferase activity in feline and human cells was analyzed. The results presented in Fig. 1A (left panel) show that CrFK cells had equal luciferase activity levels and that KE-R cells had two- to fourfold-lower luciferase counts than HOS cells. In contrast, the feline T-cell lines MYA-1 and FeT-1C showed 5- to 12-fold-lower luciferase activity levels than the human T-cell line A3.01. The results of these experiments indicate that the degree to which feline fibroblasts were permissive for HIV-1 transduction was similar to that seen with the human HOS cells, suggesting efficient LTR-driven transcription.
FIG. 1.
HIV-1 infects feline adherent cells but is restricted in feline T cells. (A) Left panel: adherent cell lines (human [HOS] and feline [CrFK, KE-R]) and T-cell lines (human [A3.01] and feline [MYA-1, FeT-1C]) were infected with increasing amounts of HIV-Luc(VSV-G). Luciferase activity (in counts per second) was determined at 3 dpi. Center panel: CrFK, A3.01, MYA-1, and FeT-1C cells were inoculated with increasing amounts of FIV-Luc(VSV-G). Luciferase activity was determined at 3 dpi. (B) CrFK, A3.01, MYA-1, and FeT-1C cells were transduced using HIV-GFP(VSV-G) at an MOI of 5. GFP fluorescence was determined 3 dpi by flow cytometry. The data are presented as side scatter versus the intensity of green. The quantity (percent) of GFP-positive cells over the threshold is indicated above of the horizontal line in each panel; mean fluorescence intensity is specified below the horizontal line.
To confirm that the feline T-cell lines used are susceptible to retrovirus transduction and luciferase reporter gene expression, a different lentiviral system, VSV-G-pseudotyped FIV-Luc was analyzed. The single-round replicating vector uses an internal CMV promoter for luciferase expression. At 3 days posttransduction we detected equal levels of luciferase activity in the human A3.01 and feline CrFK cells (Fig. 1A, center panel). FIV-Luc expression was only slightly (1.3- to 1.7-fold) higher in the human A3.01 cells than in the feline Fet-1C and MYA-1 cells. To rule out reduced HIV-LTR-driven expression in the feline T cells, we transduced the cells with a single-round HIV-1 vector which uses an internal CMV promoter to express the GFP gene (HIV-GFP). The HIV-GFP vector particles were also VSV-G pseudotyped. We inoculated the cells with at an MOI of 5 and analyzed the GFP expression by flow cytometry at 3 days posttransduction. The results obtained with the HIV-GFP vector resembled the previous results seen using the HIV-Luc transductions (Fig. 1B): 99% of A3.01 cells were positive for GFP expression, with a mean fluorescence intensity of 2,080, while in the culture of feline MYA-1 cells only 11% green cells were present, with a fivefold-lower mean fluorescence intensity of 414. We detected also a low mean GFP fluorescence intensity of 113, with 26% green cells, in the analyzed feline FeT-1C cells. Analogous results were obtained using a CMV promoter with HIV-2-GFP or SIVPBj-GFP vector (data not shown). Taken together, the data indicate that CMV promoter-driven expression of a reporter gene works in a FIV vector with similar levels of efficiency in human and feline T-cell lines. Apparently, HIV-1 vectors expressing GFP by use of an internal CMV promoter do not overcome the low level of expression of HIV in feline T-cell lines. The data also suggest that the HIV restriction in the feline T-cell lines is not related to cytoplasmic entry of the viral particles by VSV-G.
Feline cells expressing human CD4/CCR5 are permissive for HIV-1 reporter virus transduction.
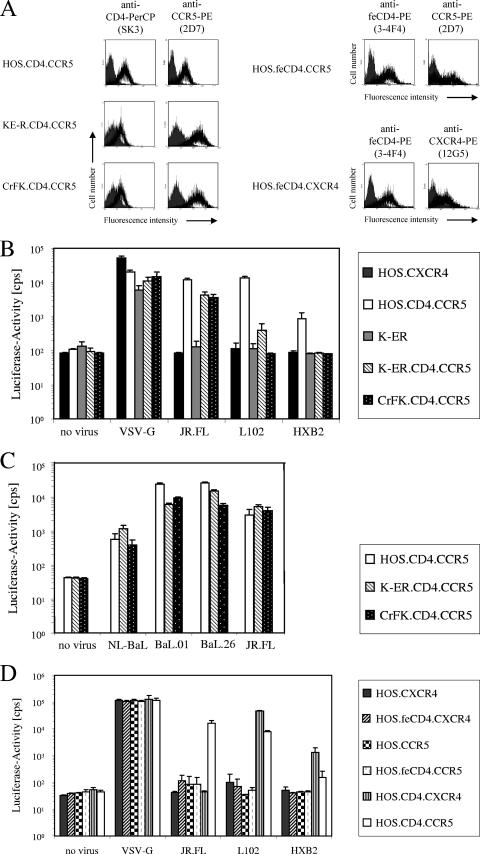
Because the feline CrFK and KE-R cells were more permissive for HIV-1 infection than the feline T-cell lines, we stably transduced CrFK and KE-R cells with retroviral vectors expressing human CD4 (huCD4) and huCCR5. Flow cytometry was used to confirm cell surface expression of huCD4 and huCCR5 in the CrFK.CD4.CCR5 and KE-R.CD4.CCR5 cells. The results demonstrated levels of expression of CD4 and CCR5 similar (CD4 with lower and CCR5 with higher expression) to those seen with the cell line HOS.CD4.CCR5 used as a reference (Fig. 2A). In order to find out whether the human receptors provide entry for HIV-1 into feline cells, we generated HIV-Luc reporter viruses pseudotyped with HIV-1 Env surface proteins. We used expression plasmids for CCR5-tropic JR.FL env and for env genes of HIV-1 strains L102 and HXB2 using CXCR4 as coreceptor. After transduction with JR.FL-pseudotyped reporter virus, feline CrFK.CD4.CCR5 and KE-R.CD4.CCR5 cells exhibited reporter activity as high as that found in human HOS.CD4.CCR5 cells (Fig. 2B and C). Because we planned to use replication-competent CCR5-tropic HIV-1 (NL-BaL; 44) for spreading-replication studies, we also pseudotyped HIV-Luc with BaL Env (Fig. 2C). Since there was no BaL env expression plasmid encoding the BaL gene of pNL-BaL available, we used BaL.01 and BaL.26; in addition, we cotransfected HIV-Luc with NL-BaL. The BaL gene in HIV-1NL-BaL differs from BaL.01 by 29 and from BaL.26 by 37 amino acid positions in gp160 (38). All BaL-pseudotyped HIV-Luc particles transduced the HOS.CD4.CCR5, CrFK.CD4.CCR5, and KE-R.CD4.CCR5 cells with similar levels of efficiency (Fig. 2C). Cells inoculated with the mixture of HIV-Luc/NL-Bal showed 10-fold-lower luciferase activity, most likely because the replication-competent virus induced massive syncytium formation at 2 to 3 dpi. The X4-tropic envelopes (L102, HXB2) did not allow infection of the huCD4-positive feline cell lines, while reporter viruses carrying either of these glycoproteins infected the CD4-expressing HOS.CD4.CCR5 cells. These results could imply that endogenously expressed CXCR4 protein in HOS cells, but not in feline cells, efficiently supports HIV-1 infection. The cell lines expressing no CD4 (HOS.CXCR4 and K-ER) showed luciferase activity only after inoculation with VSV-G-pseudotyped HIV-Luc particles. In experiments using human HOS cells expressing the feline CD4 protein together with huCCR5 or huCXCR4 (HOS.feCD4.CXCR4, HOS.feCD4.CCR5) (Fig. 2A), luciferase activity of HIV-Luc (JR.FL or L102 or HXB2) was not detectable (Fig. 2D). These results argue against the use of feline CD4 as a potential HIV-1 entry factor.
FIG. 2.
Feline cells expressing human CD4/CCR5 are permissive for HIV-1 reporter virus transduction. (A) Receptor expression of human CD4, feline CD4 (feCD4), human CCR5, and human CXCR4 on HOS, KE-R, and CrFK cells stably transduced by retroviral expression vectors (black) and mock transduced (grey). The cells were stained for human CD4 with SK3-PerCP, feline CD4 with 3-4F4-PE, human CCR5 with 2D7-PE, and human CXCR4 with 12G5-PE. Fluorescence intensity was analyzed by flow cytometry. (B and C) Human and feline cell lines coexpressing human CCR5 and human CD4 were infected with HIV-Luc pseudotyped with VSV-G- or HIV-1-derived envelopes (JR.FL, BaL.01, BaL.26, L102, HXB2). NL-BaL, virus generated by cotransfection of HIV-Luc and HIV-1NL-BaL. Luciferase activity (in counts per second) was determined 3 dpi. Cells expressing no CD4 (HOS.CXCR4 and K-ER) were used as controls. (D) Human HOS cells expressing feline CD4 (feCD4) or human CD4 (CD4) together with human CCR5 or human CXCR4 were infected with HIV-Luc pseudotyped with VSV-G- or HIV-1-derived envelopes (JR.FL, L102, HXB2). Luciferase activity (in counts per second) was determined 3 dpi. Cells expressing no CD4 (HOS.CXCR4 and HOS.CCR5) were used as controls.
Lack of spreading replication of HIV-1 in feline cells expressing human entry receptors.
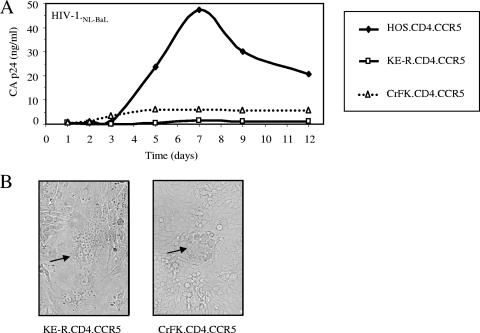
In order to further evaluate the capacity of the feline CrFK.CD4.CCR5 and KE-R.CD4.CCR5 cells to support HIV-1 replication, they were infected at a low MOI (0.05) with replication-competent HIV-1NL-BaL (44). Quantification of released CA.p24 in the supernatant of infected cells by ELISA showed a peak of viral replication in the human HOS.CD4.CCR5 cells at 7 dpi (Fig. 3A). Supernatants of infected feline cells yielded only background levels of p24, indicating the absence of spreading replication in the feline fibroblasts. Interestingly, the HIV-inoculated feline cells showed syncytium formation at 3 to 4 dpi (Fig. 3B), suggesting successful initial infection of some cells with HIV-1NL-BaL.
FIG. 3.
Feline cells expressing HIV receptors do not support spreading replication of HIV-1. (A) HOS.CD4.CCR5, KE-R.CD4.CCR5, and CrFK.CD4.CCR5 cells were infected with R5-tropic HIV-1NL-Bal at an MOI of 0.05. Supernatant virions were quantified by p24 ELISA at the indicated days. (B) HIV-1NL-Bal-infected feline cells, KE-R.CD4.CCR5 cells, and CrFK.CD4.CCR5 cells showed syncytium formation (arrows) at 3 to 4 dpi (magnification, ×10).
Processing and release of HIV-1 proteins in feline cells.
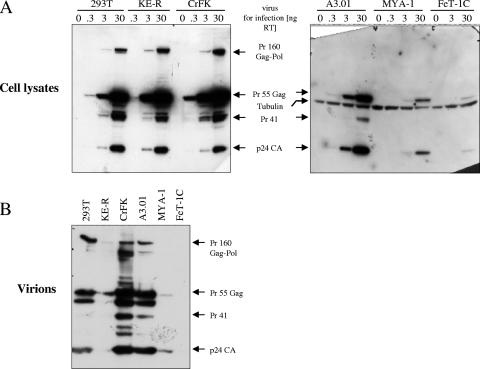
Since the feline CrFK.CD4.CC5 and KE-R.CD4.CCR5 cells were permissive with respect to transduction by HIV-Luc reporter virus whereas cultures infected with the replication-competent HIV-1NL-Bal did not show viral spreading, we wanted to investigate the molecular basis of this restriction. In a first step we characterized the expression patterns of viral proteins in feline and human cell lines. To ensure a single round of infection, receptor-negative feline cell lines (KE-R, CrFK, MYA-1, FeT-1C) and human cell lines (293T, A3.01) were used for infection with VSV-G-pseudotyped HIV-1 [NL-BaL(VSV-G)] and analyzed. We used 0.3, 3.0, and 30.0 ng RT for infection, carefully washed the cells to remove the input viral particles at 5 h postinfection, and studied the expression of the HIV-1 Gag/Gag-Pol proteins by anticapsid immunoblotting at 3 dpi. Cell lysates of the infected 293T, KE-R, and CrFK cells showed comparable high levels of expression of the HIV-1 proteins that were dose dependent (Fig. 4A). A significant but lower level of expression was detectable in the A3.01 cell line. Cell lysates of the feline T-cell lines MYA-1 and FeT-1C showed only weak signals with even the highest amount of input virus. The processing patterns in human and feline cells showed no obvious differences. In addition, viral particles released from cultures initially inoculated with 30 ng RT were collected and purified by centrifugation through a sucrose cushion and regular aliquots were analyzed by immunoblotting (Fig. 4B). While the two human cell lines (293T and A3.01) released similar amounts of HIV-1 particles, the feline CrFK cells showed two- to threefold more particles in the cell culture supernatant. In stark contrast, in repeated experiments the feline KE-R, MYA-1, and FeT-1C cells showed no or almost no particles released into the supernatants. This finding correlates with the modest amounts of intracellular HIV Gag in MYA-1 and FeT-1C cells, whereas KE-R cells clearly showed a substantial block in particle release.
FIG. 4.
Reduced HIV-1 Gag expression in feline T cells and restricted HIV-1 release from feline KE-R cells. The results of immunoblot analysis of HIV-1 Gag protein expression and processing by feline and human cells are shown. 293T, KE-R, CrFK, A3.01, MYA-1, and FeT-1C cells were infected with the indicated amounts of HIV-1NL-Bal(VSV-G). At 3 dpi, lysates of the cells were prepared (A) and virions pelleted from the supernatants were analyzed on immunoblots using p24-specific monoclonal antibody (B). For cell lysates of T cells, immunoblots were coprobed using anti-tubulin antibody to show equal sample concentrations. For virions, equal volumes corresponding to 0.5 ml supernatant of cells infected with 30 ng RT were loaded per lane. Gag precursor proteins (Pr 160 Gag-Pol, Pr 55 Gag, Pr 41) and p24 capsid protein (p24 CA) are indicated by arrows.
HIV-1 virions produced in CrFK cells show low infectivity.
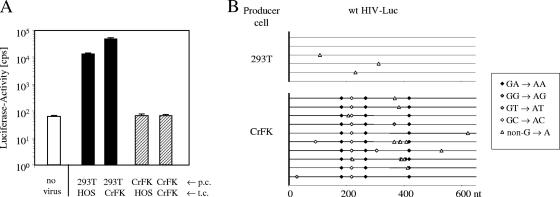
The lack of virus particle release in KE-R cells is probably a major reason for the replication block of HIV-1 in KE-R.CD4.CCR5 cells (Fig. 3A). Additional restrictions in feline cells must exist, since CrFK.CD4.CCR5 cells also did not allow spreading HIV-1 replication, although efficient release of particles occurred in infected CrFK cells. In order to study the infectivity of virus particles, we generated HIV-Luc(VSV-G) reporter virions in feline CrFK and human 293T cells by plasmid transfection. The collected viral vectors were normalized by RT activity and used to infect human HOS and feline CrFK cells. HIV-Luc(VSV-G) produced from 293T cells showed high luciferase activity. In contrast, particles from CrFK cells showed only background luciferase activity in human and in feline cells (Fig. 5A), suggesting that these particles had very low infectivity. Several attempts to produce HIV-Luc(VSV-G) in K-ER cells by plasmid transfection were unsuccessful (data not shown).
FIG. 5.
HIV-1 particles produced in feline CrFK cells show low infectivity. (A) HIV-Luc(VSV-G) was produced in human 293T and feline CrFK cells (producer cells [p.c.]) by plasmid transfection. Normalized viruses containing supernatants were used to infect human HOS and feline CrFK cells (target cells [t.c.]). Luciferase activity (in counts per second) was determined 3 dpi. no virus, uninfected cells. (B) Genome editing of HIV-Luc(VSV-G) produced in 293T or CrFK cells. A fragment in LTR-gag was amplified from reverse transcripts 10 h postinfection. At least 5 to 10 independent nucleotide sequences were determined. The mutations in the clones of each group are shown. Each mutation is indicated and coded with respect to the nucleotide substitution.
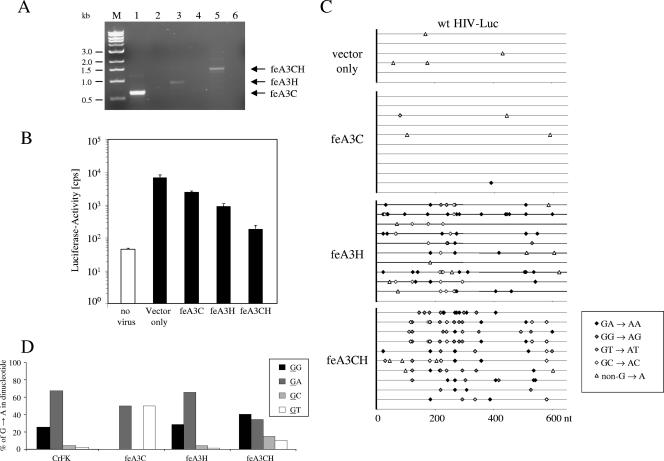
To identify the cause of the reduced infectivity of CrFK-derived HIV-1 particles, we treated the virus-containing supernatant with DNase I to remove potentially contaminating plasmid DNA and infected human HOS cells. Reverse transcription products were amplified using PCR primers specific for late-RT products (a 600 bp fragment covering LTR-gag) at 10 h postinfection, cloned, and analyzed by sequencing (Fig. 5B). HIV genomes derived from human 293T cells showed no G→A exchanges, but G→A substitutions were highly enriched (0.72%) in reverse transcripts of HIV particles made in feline CrFK cells (Table 1). The number of G→A exchanges ranged between four and seven per sequence (Fig. 5B). When we analyzed the positive strand for the sequence context which contained the G→A hypermutations, 25.5% were GG to AG changes, 68% were GA to AA exchanges, and only 6.5% were GC to AC or GT to AT mutations (Fig. 6D). Mutations of a guanosine-purine sequence to an adenosine-purine sequence on the DNA positive strand are typically found in APOBEC3-edited retroviral genomes (8, 24, 34, 39, 42, 43). In summary, the mutation rates showed a strong correlation with the infectivity levels of the HIV particles made in 293T and CrFK cells, explaining the lack of virus spread in CrFK.CD4.CCR5 cells.
TABLE 1.
Sequence characteristics of HIV-Luc DNA genomes of virions derived from CrFK cells and 293T-expressing feline APOBEC3 proteins (feA3C, feA3H, feA3CH) or empty expression plasmid
| Producer cell and protein or plasmid (vector) | No. of sequences analyzed | No. of G→A mutations/no. of other mutations | No. of mutations per 100 nucleotides | No. of clones without G→A editing | Minimal no. of G→A per clone | Maximal no. of G→A per clone | Average no. of G→A per clone | No. of G→A exchanges per 100 Gs | No. of G→A exchanges per 100 nucleotides |
|---|---|---|---|---|---|---|---|---|---|
| CrFK | |||||||||
| Vector | 10 | 47/14 | 0.94 | 0 | 4 | 7 | 4.7 | 2.7 | 0.72 |
| 293T | |||||||||
| Vector | 10 | 0/7 | 0.11 | 10 | 0 | 0 | 0 | 0 | 0 |
| feA3C | 10 | 2/3 | 0.08 | 8 | 1 | 1 | 0.2 | 0.1 | 0.03 |
| feA3H | 10 | 70/9 | 1.22 | 1 | 4 | 16 | 7 | 4.0 | 1.08 |
| feA3CH | 10 | 99/5 | 1.60 | 0 | 4 | 17 | 9.9 | 5.6 | 1.52 |
FIG. 6.
Defined feline APOBEC3 proteins inhibit HIV-1 infectivity to different degrees. (A) Analysis of feline APOBEC3C (feA3C; lane 1), APOBEC3H (feA3H; lane 3), and APOBEC3CH (feA3CH; lane 5) expression by RT-PCR of total RNA from feline CrFK cells. In lanes 2, 4, and 6, PCRs using the primers specific for feA3C (lane 2), feA3H (lane 4), and feA3CH (lane 6) were performed without template cDNA added. (B) HIV-Luc(VSV-G) was produced in 293T cells in the presence or absence of the indicated feline APOBEC3. Infectivity of the viruses was determined by quantification of luciferase activity in HOS cells infected with equal amounts of viruses 3 dpi. (C) A fragment in LTR-gag was amplified from reverse transcripts of HIV-Luc generated in the presence of the indicated feline APOBEC3 10 h postinfection. A total of 5 to 10 independent nucleotide sequences were determined. The mutations in the clones of each group are shown. Each mutation is indicated and coded with respect to nucleotide mutation. (D) Comparison of the dinucleotide sequence context of G (underlined)→A mutations in the positive-strand DNA of HIV-Luc derived from CrFK cells and from feAPOBEC3-expressing 293T cells.
Feline CrFK cells express several APOBEC3 RNAs.
It has been shown that wt HIV-1 can replicate in the presence of human APOBEC3G; however, its infectivity is dramatically reduced by the presence of heterologous, nonhuman APOBEC3 proteins (43). Based on this observation and the presence of typical G→A mutations in cDNAs from CrFK-derived particles, we assumed that feline APOBEC3 deaminases are a major reason for the low infectivity of HIV-1 produced in CrFK cells. Recently, we described feAPOBEC3C (feA3C; previously termed feAPOBEC3 [fe3]) (39) as being expressed in CrFK cells. For the present study we cloned two additional novel feline cDNAs, termed feAPOBEC3H and feAPOBEC3CH (feA3H and feA3CH), from CrFK-derived RNA by a combination of RT-PCR and 5′- and 3′-RACE techniques. The feA3CH cDNA is composed of the fused open reading frames of feA3C and feA3H. We propose a nomenclature classification based on the overall consensus and positions identical between feline and human APOBEC3s: feline APOBEC3C has 43.8% amino acid identity to human APOBEC3C, and feline APOBEC3H shares 44.4% identical amino acids with human APOBEC3H. Using diagnostic PCR primers we confirmed expression of the feA3s in CrFK cells (Fig. 6A). The sizes of the detected amplification products are in line with the structure of the cDNAs. In several experiments using different primer and PCR protocols for amplification of the three feA3 cDNAs, we always observed significantly more PCR product for feA3C than for feA3H and feA3CH (Fig. 6A and data not shown). We used the cloned feA3H and feA3CH cDNAs to generate HA-tagged expression plasmids.
Feline APOBEC3 proteins inhibit the infectivity of HIV-1.
To assess the antiviral activity of feA3 proteins, HIV-Luc reporter viruses were generated in human 293T cells in the presence of cotransfected feA3 expression plasmids and equal amounts of particles were used for infection experiments. The results depicted in Fig. 6B show that two of the three feline APOBEC3 proteins are inhibitors of HIV-1-mediated gene transfer: feA3H and feA3CH reduced the infectivity of HIV-1 by 7- and 37-fold, respectively. Only marginally reduced titers were observed in experiments using feA3C. The suppression of reporter gene transfer clearly correlated with a significantly increased G→A mutation rate in viral reverse transcription products (Fig. 6C, Table 1): cotransfection of feA3H or feA3CH resulted in 1.08% and 1.52% G→A substitutions, respectively. Viral genomes derived from transfections omitting an feA3 expression plasmid showed no G→A editing; using the feA3C expression plasmid only 0.03% G→A exchanges were detectable (Fig. 6C, Table 1). The sequence context of the majority of the G→A exchanges in viral genomes derived from 293T cells coexpressing feA3H was similar to that seen with CrFK cell-derived particles (Fig. 6D): feA3H induced 28% GG→AG and 65.7% GA→AA exchanges in the positive strand of the DNA; similarly, viral genomes of CrFK cell-released virions showed 25.5% GG→AG and 68% GA→AA mutations. The editing context of the more antiviral feA3CH showed a different distribution of dinucleotides containing G→A exchanges: 40.4% were GG→AG changes, 34% were GA→AG mutations, and GC or GT to AC or AT changes were found in 15% or 10%, respectively. These findings support the idea that the HIV-1 genome editing of CrFK-derived particles may be attributable to feA3H or to a closely related feline cytidine deaminase.
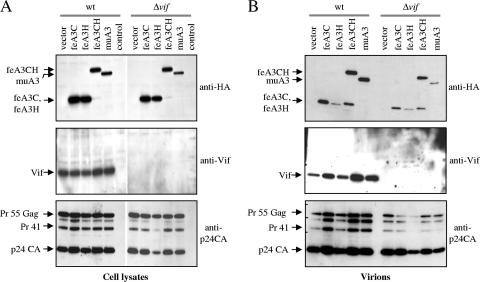
All three feline APOBEC3C proteins were detectable in purified particles of wt and Δvif HIV1-Luc reporter viruses by immunoblotting (Fig. 7B), suggesting that the Vif protein of HIV-1 cannot induce their exclusion from virions as it does with huAPOBEC3G (data not shown). Particles generated in the presence of feA3CH showed in addition to the 53 kDa protein a second smaller protein of ∼23 kDa, which was detectable in protein lysates of the producer cells only as a very faint band (compare the results shown for anti-HA in lane fe3ACH of Fig. 7A with those shown for anti-HA in lane feA3CH of Fig. 7B). We do not know whether this 23 kDa protein is generated in the 293T cells and preferentially encapsidated or produced in virus particles by cleavage of the feA3CH with the viral- or a particle-associated protease. In conclusion, it is likely that feline APOBEC3 proteins identical or related to feA3H and eventually to feA3CH significantly contribute to the block of spreading replication of HIV-1 in the CrFK.CD4.CCR5 cells.
FIG. 7.
Feline APOBEC3 proteins are encapsidated into HIV-1 virions. Virions were generated by cotransfection of 293T cells with wt HIV-Luc or Δvif HIV-Luc and HA-tagged feline APOBEC3C (feA3C), APOBEC3H (feA3H), APOBEC3CH (feA3CH), and murine APOBEC3 (muA3) expression plasmids or pcDNA3.1 (vector). (A) APOBEC3 expression in the transfected cells was detected by immunoblotting using anti-HA monoclonal antibody, Vif expression was detected using Vif antiserum, and expression of HIV-1 gag proteins (Pr 55 Gag, Pr 41, p24 CA) was detected using p24CA-specific monoclonal antibody. control, lysate of 293T cells not transfected. (B) Encapsidated APOBEC3 in virions normalized by RT activity was detected by probing with anti-HA antibody on a parallel immunoblot. Immunoblots of virions were also probed with Vif antiserum and anti-p24CA monoclonal antibody.
DISCUSSION
In this study, we characterized the ability of different feline cell lines to support HIV-1 replication with the long-term aim to use cats (Felis catus) or genetically modified cats as an animal model for HIV-1 studies. We found that the expression of functional CD4 and coreceptor molecules in feline cell lines was insufficient to constitute a permissive environment for HIV-1 replication. In fact, additional blocks to HIV-1 are present in feline cells. We identified the feline cytidine deaminases of the APOBEC3 family as one of the factors which exert a strong inhibitory effect on HIV-1 replication in feline cells.
To study the transduction of and entry into defined feline cells by HIV-1, single-round, replication-deficient HIV-Luc was used. In these assays with VSV-G-pseudotyped HIV-Luc, two adherent feline cell lines, CrFK and KE-R, were shown to be very permissive towards transduction by HIV vectors: HIV-LTR-driven expression generated a level of luciferase activity as high as in the human reference cell line HOS. In contrast, two feline T-cell lines used showed a restriction, with ∼10-fold-lower luciferase counts compared to the human T-cell line A3.01. Infection with HIV-1NL-BaL(VSV-G) followed by immunoblotting revealed a strong correlation of the expression of the luciferase reporter and viral Gag proteins: the feline adherent cells showed high expression of Gag-Pol, whereas, in contrast, the feline T-cell lines expressed low levels of Gag-Pol. Using FIV-based reporter vectors, we could show that the VSV-G-mediated entry pathway for lentiviruses works equally in human and feline T-cell lines, ruling out a trap of the HIV(VSV-G) particles in endosomal vesicles. Because HIV vectors expressing an EGFP gene driven by an internal CMV promoter were, like HIV-Luc, also clearly restricted in the feline T cells, our data suggest that the restriction to HIV-1(VSV-G) virions is not because of VSV-G pseudotyping and LTR-driven transcription but is a cytoplasmic postentry block due to the presence of HIV proteins. This observation is reminiscent of studies of HIV-1 restriction in rodent cells. In transgenic rat lymphocytes, HIV-1-luciferase levels were three- to sevenfold lower than those seen in parallel infections of primary human T lymphocytes (29). Baumann et al. (3) reported that murine T-cell lines had a nonsaturable block at an early, postentry replication stage which was not present in murine fibroblastic cell lines. While murine and feline T cells are more restrictive towards HIV-1 replication than fibroblastic cell lines, studies of simian cells also revealed a postentry restriction in fibroblastic cells, which was identified as an early saturable block at reverse transcription (5, 10, 53). The discovery of the cytoplasmic-body protein TRIM5α was a milestone in characterizing this cellular inhibitory activity in simian cells (72). It is currently unknown whether members of the Felidae carry a TRIM5 gene. Interestingly, however, Mus musculus seems to be devoid of it. Since the adherent feline cells did not show a TRIM5α-like restriction to HIV-1, the HIV-1 restriction in the feline T-cell lines could be TRIM5α dependent only if TRIM5α or a different inhibitor was expressed in a cell type-specific way. Although feline and rodent T cells have a postentry restriction for HIV-1 in common, we do not know whether the underlying mechanisms for this reduced susceptibility are related to each other. The involvement of a TRIM5α-like protein in the restriction of HIV-1 in feline T-cell lines is possible, but the involvement of a missing factor cannot be ruled out.
HIV-1 can enter cells expressing CD4 and CCR5 molecules of human and simian origin (15). We generated feline CrFK and KE-R cells expressing huCD4 and huCCR5. HIV-Luc viruses pseudotyped with HIV-Env efficiently transduced these cells, indicating that the human proteins are functional as HIV receptors or coreceptors in feline cells. In addition, we used HIV-1 to test the ability of feline CD4 in human cells to support entry of CXCR4 or CCR5. Our results suggest that feline CD4, in similarity to mouse and rabbit CD4 molecules (22, 33), cannot support the entry of HIV-1. Willett et al. (80) reported that feline CXCR4 functions as a coreceptor for Env-mediated cell fusion for the HIV-1 strain LAI. In our study we did not address the role of feline CCR5 or CXCR4, mostly because an antibody against feCCR5 was not available and attempts to detect feCXCR4 at the cell surface were unsuccessful.
Expression of huCD4 or huCCR5 did not render the feline CrFK or KE-R cells susceptible to spreading replication of CCR5-tropic HIV-1. In contrast to results seen with CrFK cells, KE-R cells in repeated experiments showed a profound block in HIV-1 particle release, suggesting differential expression of required and/or inhibitory cellular factors. But in contrast to the results previously obtained regarding the assembly and release block of HIV-1 in murine cells (6, 45), KE-R cells efficiently processed the Gag precursor. In primate cells, a similar cell type- and species-specific block in the release of progeny virions was described as dependent on the presence or absence of the viral vpu gene. Vpu facilitates the release of particles. Some cells are “permissive” and others are “nonpermissive” of viral replication in the absence of Vpu (18, 67, 68). In nonpermissive cells, Vpu is considered to counteract a dominant-negative factor whose identity remains to be determined (56, 77). It is possible that levels and/or species-specific variants of the ion channel TASK-1 are responsible for the block in release of virions in primate and feline cells (27). Clearly, more studies of the HIV-1 release block in KE-R cells are required to support or rule out the relevance of Vpu for HIV-1 replication in feline cells.
While the feline CrFK cells efficiently released HIV-1 particles, these virions showed a strongly suppressed level of infectivity. The viral cDNAs of these virions were mutated and preferentially showed G→A mutations, which are typically found when APOBEC3 proteins are encapsidated into retroviral particles, transferred into the newly infected cell, and deaminate single-stranded reverse transcription intermediates (8, 24, 34, 39, 42, 43, 82, 84). HIV-1 Vif can only counteract APOBEC3 cytidine deaminases after species-specific molecular interactions (43). In consequence, HIV-1 is able to replicate in the presence of the human APOBEC3G but is strongly suppressed by the orthologous proteins of African green monkeys or mice (43). Therefore, we analyzed the effect of the activity of three CrFK cell-derived feline APOBEC3s on the infectivity of HIV-1. We found that feA3C, a potent inhibitor of FFV (39), showed only a mild inhibitory effect on HIV-1. In contrast, feA3H and feA3CH were strong inhibitors of HIV-1. Only feA3H and feA3CH induced significant number of G→A mutations in the viral cDNA during the infection of target cells. The feA3H and feA3CH proteins were encapsidated in HIV particles, suggesting that the HIV-Vif protein does not bind and exclude them from released virions or that it targets them for proteasome-mediated destruction (compare the results shown for anti-HA in the wt lanes in Fig. 7B with the results shown in the Δvif lanes). Interestingly, feA3C is also packaged into HIV particles but fails to mutate the HIV genome. A very similar observation was described for the huAPOBEC3C, which is also encapsidated by HIV-1 but does not reduce its infectivity (82). Currently we are characterizing the retrovirus restriction activity of the feline APOBEC3s by use of FIV-, FFV-, and feline leukemia virus-based reporter systems.
The development of a transgenic animal model permissive for HIV-1 infection would aid studies of HIV transmission and pathogenesis and would allow testing of therapeutic strategies that include vaccines. A major obstacle to this end has been the inability of cells from present transgenic rabbit or rodent models to support a robust productive HIV-1 infection (6, 11, 45). Our study extents the list of multiple restriction mechanisms in nonhuman cells by describing the presence of antiretroviral factors in feline cells. In particular, components of the genome-deaminating machinery have been identified and characterized in this study. A detailed analysis of how FIV can overcome the cellular barriers active against HIV will likely generate knowledge showing how to genetically adjust HIV-1 to feline cells.
Acknowledgments
We thank Sylvia Panitz for expert technical assistance and Nathaniel R. Landau, Eric M. Poeschla, Roland Plesker, Roland Riebe, and Egbert Flory for the gift of reagents. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: T4-pMV7 from Richard Axel, HXB2-env from Kathleen Page and Dan Littman, HIV-1 clone BaL.01 and clone BaL.26 from John R. Mascola, and HIV-1HXB2 Vif antiserum from Dana Gabudzda.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. [DOI] [PubMed] [Google Scholar]
- 2.Alter, H. J., J. W. Eichberg, H. Masur, W. C. Saxinger, R. Gallo, A. M. Macher, H. C. Lane, and A. S. Fauci. 1984. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science 226:549-552. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, J. G., D. Unutmaz, M. D. Miller, S. K. Breun, S. M. Grill, J. Mirro, D. R. Littman, A. Rein, and V. N. KewalRamani. 2004. Murine T cells potently restrict human immunodeficiency virus infection. J. Virol. 78:12537-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berges, B. K., W. H. Wheat, B. E. Palmer, E. Connick, and R. Akkina. 2006. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−γc−/− (RAG-hu) mouse model. Retrovirology 3:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 99:11920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieniasz, P. D., and B. R. Cullen. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74:9868-9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieniasz, P. D., T. A. Grdina, H. P. Bogerd, and B. R. Cullen. 1998. Recruitment of a protein complex containing Tat and cyclin T1 to TAR governs the species specificity of HIV-1 Tat. EMBO J. 17:7056-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop, K. N., R. K. Holmes, A. M. Sheehy, N. O. Davidson, S. J. Cho, and M. H. Malim. 2004. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr. Biol. 14:1392-1396. [DOI] [PubMed] [Google Scholar]
- 9.Chiu, Y. L., V. B. Soros, J. F. Kreisberg, K. Stopak, W. Yonemoto, and W. C. Greene. 2005. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature 435:108-114. [DOI] [PubMed] [Google Scholar]
- 10.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Muesing, H. G. Gottlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 99:11914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutiño-Moguel, T., and A. Fassati. 2006. A phenotypic recessive, post-entry block in rabbit cells that results in aberrant trafficking of HIV-1. Traffic 7:978-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 13.Eckstein, D. A., M. L. Penn, Y. D. Korin, D. D. Scripture-Adams, J. A. Zack, J. F. Kreisberg, M. Roederer, M. P. Sherman, P. S. Chin, and M. A. Goldsmith. 2001. HIV-1 actively replicates in naive CD4+ T cells residing within human lymphoid tissues. Immunity 15:671-682. [DOI] [PubMed] [Google Scholar]
- 14.Emi, N., T. Friedmann, and J. K. Yee. 1991. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J. Virol. 65:1202-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fomsgaard, A., P. R. Johnson, C. Nielsen, F. J. Novembre, J. Hansen, S. Goldstein, and V. M. Hirsch. 1995. Receptor function of CD4 structures from African green monkey and pig-tail macaque for simian immunodeficiency virus, SIVsm, SIVagm, and human immunodeficiency virus type-1. Viral Immunol. 8:121-133. [DOI] [PubMed] [Google Scholar]
- 16.Gajdusek, D. C., H. L. Amyx, C. J. Gibbs, Jr., D. M. Asher, P. Rodgers-Johnson, L. G. Epstein, P. S. Sarin, R. C. Gallo, A. Maluish, L. O. Arthur, et al. 1985. Infection of chimpanzees by human T-lymphotropic retroviruses in brain and other tissues from AIDS patients. Lancet i:55-56. [DOI] [PubMed] [Google Scholar]
- 17.Garber, M. E., P. Wei, V. N. KewalRamani, T. P. Mayall, C. H. Herrmann, A. P. Rice, D. R. Littman, and K. A. Jones. 1998. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 12:3512-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraghty, R. J., K. J. Talbot, M. Callahan, W. Harper, and A. T. Panganiban. 1994. Cell type-dependence for Vpu function. J. Med. Primatol. 23:146-150. [DOI] [PubMed] [Google Scholar]
- 19.Goncalves, J., P. Jallepalli, and D. H. Gabuzda. 1994. Subcellular localization of the Vif protein of human immunodeficiency virus type 1. J. Virol. 68:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorantla, S., H. Sneller, L. Walters, J. G. Sharp, S. J. Pirruccello, J. T. West, C. Wood, S. Dewhurst, H. E. Gendelman, and L. Poluektova. 2007. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2−/−γc−/− mice. J. Virol. 81:2700-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groschel, B., and F. Bushman. 2005. Cell cycle arrest in G2/M promotes early steps of infection by human immunodeficiency virus. J. Virol. 79:5695-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hague, B. F., S. Sawasdikosol, T. J. Brown, K. Lee, D. P. Recker, and T. J. Kindt. 1992. CD4 and its role in infection of rabbit cell lines by human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 89:7963-7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 24.Harris, R. S., K. N. Bishop, A. M. Sheehy, H. M. Craig, S. K. Petersen-Mahrt, I. N. Watt, M. S. Neuberger, and M. H. Malim. 2003. DNA deamination mediates innate immunity to retroviral infection. Cell 113:803-809. [DOI] [PubMed] [Google Scholar]
- 25.Heeney, J., W. Bogers, L. Buijs, R. Dubbes, P. ten Haaft, W. Koornstra, H. Niphuis, P. Nara, and V. Teeuwsen. 1996. Immune strategies utilized by lentivirus infected chimpanzees to resist progression to AIDS. Immunol. Lett. 51:45-52. [DOI] [PubMed] [Google Scholar]
- 26.Heeney, J., R. Jonker, W. Koornstra, R. Dubbes, H. Niphuis, A. M. Di Rienzo, M. L. Gougeon, and L. Montagnier. 1993. The resistance of HIV-infected chimpanzees to progression to AIDS correlates with absence of HIV-related T-cell dysfunction. J. Med. Primatol. 22:194-200. [PubMed] [Google Scholar]
- 27.Hsu, K., J. Seharaseyon, P. Dong, S. Bour, and E. Marban. 2004. Mutual functional destruction of HIV-1 Vpu and host TASK-1 channel. Mol. Cell 14:259-267. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda, Y., M. K. Collins, P. A. Radcliffe, K. A. Mitrophanous, and Y. Takeuchi. 2002. Gene transduction efficiency in cells of different species by HIV and EIAV vectors. Gene Ther. 9:932-938. [DOI] [PubMed] [Google Scholar]
- 29.Keppler, O. T., F. J. Welte, T. A. Ngo, P. S. Chin, K. S. Patton, C. L. Tsou, N. W. Abbey, M. E. Sharkey, R. M. Grant, Y. You, J. D. Scarborough, W. Ellmeier, D. R. Littman, M. Stevenson, I. F. Charo, B. G. Herndier, R. F. Speck, and M. A. Goldsmith. 2002. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 195:719-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kestens, L., J. Vingerhoets, M. Peeters, G. Vanham, C. Vereecken, G. Penne, H. Niphuis, P. van Eerd, G. van der Groen, P. Gigase, et al. 1995. Phenotypic and functional parameters of cellular immunity in a chimpanzee with a naturally acquired simian immunodeficiency virus infection. J. Infect. Dis. 172:957-963. [DOI] [PubMed] [Google Scholar]
- 31.Korin, Y. D., and J. A. Zack. 1999. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J. Virol. 73:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwak, Y. T., D. Ivanov, J. Guo, E. Nee, and R. B. Gaynor. 1999. Role of the human and murine cyclin T proteins in regulating HIV-1 tat-activation. J. Mol. Biol. 288:57-69. [DOI] [PubMed] [Google Scholar]
- 33.Landau, N. R., M. Warton, and D. R. Littman. 1988. The envelope glycoprotein of the human immunodeficiency virus binds to the immunoglobulin-like domain of CD4. Nature 334:159-162. [DOI] [PubMed] [Google Scholar]
- 34.Lecossier, D., F. Bouchonnet, F. Clavel, and A. J. Hance. 2003. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science 300:1112. [DOI] [PubMed] [Google Scholar]
- 35.Leonard, J. M., J. W. Abramczuk, D. S. Pezen, R. Rutledge, J. H. Belcher, F. Hakim, G. Shearer, L. Lamperth, W. Travis, T. Fredrickson, et al. 1988. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science 242:1665-1670. [DOI] [PubMed] [Google Scholar]
- 36.Levy, J. A., J. Shimabukuro, T. McHugh, C. Casavant, D. Stites, and L. Oshiro. 1985. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology 147:441-448. [DOI] [PubMed] [Google Scholar]
- 37.Lewis, P., M. Hensel, and M. Emerman. 1992. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 11:3053-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, Y., K. Svehla, N. L. Mathy, G. Voss, J. R. Mascola, and R. Wyatt. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 80:1414-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Löchelt, M., F. Romen, P. Bastone, H. Muckenfuss, N. Kirchner, Y. B. Kim, U. Truyen, U. Rosler, M. Battenberg, A. Saib, E. Flory, K. Cichutek, and C. Münk. 2005. The antiretroviral activity of APOBEC3 is inhibited by the foamy virus accessory Bet protein. Proc. Natl. Acad. Sci. USA 102:7982-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loewen, N., R. Barraza, T. Whitwam, D. T. Saenz, I. Kemler, and E. M. Poeschla. 2003. FIV vectors. Methods Mol. Biol. 229:251-271. [DOI] [PubMed] [Google Scholar]
- 41.Maddon, P. J., A. G. Dalgleish, J. S. McDougal, P. R. Clapham, R. A. Weiss, and R. Axel. 1986. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47:333-348. [DOI] [PubMed] [Google Scholar]
- 42.Mangeat, B., P. Turelli, G. Caron, M. Friedli, L. Perrin, and D. Trono. 2003. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99-103. [DOI] [PubMed] [Google Scholar]
- 43.Mariani, R., D. Chen, B. Schröfelbauer, F. Navarro, R. König, B. Bollman, C. Münk, H. Nymark-McMahon, and N. R. Landau. 2003. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114:21-31. [DOI] [PubMed] [Google Scholar]
- 44.Mariani, R., B. A. Rasala, G. Rutter, K. Wiegers, S. M. Brandt, H. G. Krausslich, and N. R. Landau. 2001. Mouse-human heterokaryons support efficient human immunodeficiency virus type 1 assembly. J. Virol. 75:3141-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mariani, R., G. Rutter, M. E. Harris, T. J. Hope, H. G. Krausslich, and N. R. Landau. 2000. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 74:3859-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marin, M., K. M. Rose, S. L. Kozak, and D. Kabat. 2003. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 9:1398-1403. [DOI] [PubMed] [Google Scholar]
- 47.Marques, S. M., J.-L. Veyrune, R. R. Shukla, and A. Kumar. 2003. Restriction of human immunodeficiency virus type 1 Rev function in murine A9 cells involves the Rev C-terminal domain. J. Virol. 77:3084-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore, J. P., J. A. McKeating, W. A. Norton, and Q. J. Sattentau. 1991. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J. Virol. 65:1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore, J. P., J. A. McKeating, R. A. Weiss, and Q. J. Sattentau. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250:1139-1142. [DOI] [PubMed] [Google Scholar]
- 50.Mosier, D. E., R. J. Gulizia, S. M. Baird, D. B. Wilson, D. H. Spector, and S. A. Spector. 1991. Human immunodeficiency virus infection of human-PBL-SCID mice. Science 251:791-794. [DOI] [PubMed] [Google Scholar]
- 51.Mosier, D. E., R. J. Gulizia, P. D. MacIsaac, B. E. Torbett, and J. A. Levy. 1993. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science 260:689-692. [DOI] [PubMed] [Google Scholar]
- 52.Mühlebach, M. D., N. Wolfrum, S. Schule, U. Tschulena, R. Sanzenbacher, E. Flory, K. Cichutek, and M. Schweizer. 2005. Stable transduction of primary human monocytes by simian lentiviral vector PBj. Mol. Ther. 12:1206-1216. [DOI] [PubMed] [Google Scholar]
- 53.Münk, C., S. M. Brandt, G. Lucero, and N. R. Landau. 2002. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. USA 99:13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Namikawa, R., H. Kaneshima, M. Lieberman, I. L. Weissman, and J. M. McCune. 1988. Infection of the SCID-hu mouse by HIV-1. Science 242:1684-1686. [DOI] [PubMed] [Google Scholar]
- 55.Neil, S., F. Martin, Y. Ikeda, and M. Collins. 2001. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J. Virol. 75:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neil, S. J., S. W. Eastman, N. Jouvenet, and P. D. Bieniasz. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novembre, F. J., M. Saucier, D. C. Anderson, S. A. Klumpp, S. P. O'Neil, C. R. Brown, 2nd, C. E. Hart, P. C. Guenthner, R. B. Swenson, and H. M. McClure. 1997. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J. Virol. 71:4086-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nyambi, P. N., P. Lewi, M. Peeters, W. Janssens, L. Heyndrickx, K. Fransen, K. Andries, M. Vanden Haesevelde, J. Heeney, P. Piot, and G. van der Groen. 1997. Study of the dynamics of neutralization escape mutants in a chimpanzee naturally infected with the simian immunodeficiency virus SIVcpz-ant. J. Virol. 71:2320-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Brien, S. J., M. Menotti-Raymond, W. J. Murphy, and N. Yuhki. 2002. The feline genome project. Annu. Rev. Genet. 36:657-686. [DOI] [PubMed] [Google Scholar]
- 60.O'Neil, S. P., F. J. Novembre, A. B. Hill, C. Suwyn, C. E. Hart, T. Evans-Strickfaden, D. C. Anderson, J. deRosayro, J. G. Herndon, M. Saucier, and H. M. McClure. 2000. Progressive infection in a subset of HIV-1-positive chimpanzees. J. Infect. Dis. 182:1051-1062. [DOI] [PubMed] [Google Scholar]
- 61.Pace, C., J. Keller, D. Nolan, I. James, S. Gaudieri, C. Moore, and S. Mallal. 2006. Population level analysis of human immunodeficiency virus type 1 hypermutation and its relationship with APOBEC3G and vif genetic variation. J. Virol. 80:9259-9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Page, K. A., N. R. Landau, and D. R. Littman. 1990. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J. Virol. 64:5270-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peeters, M., W. Janssens, M. Vanden Haesevelde, K. Fransen, B. Willems, L. Heyndrickx, L. Kestens, P. Piot, G. Van der Groen, and J. Heeney. 1995. Virologic and serologic characteristics of a natural chimpanzee lentivirus infection. Virology 211:312-315. [DOI] [PubMed] [Google Scholar]
- 64.Price, M. A., S. S. Case, D. A. Carbonaro, X. J. Yu, D. Petersen, K. M. Sabo, M. A. Curran, B. C. Engel, H. Margarian, J. L. Abkowitz, G. P. Nolan, D. B. Kohn, and G. M. Crooks. 2002. Expression from second-generation feline immunodeficiency virus vectors is impaired in human hematopoietic cells. Mol. Ther. 6:645-652. [PubMed] [Google Scholar]
- 65.Priceputu, E., I. Rodrigue, P. Chrobak, J. Poudrier, T. W. Mak, Z. Hanna, C. Hu, D. G. Kay, and P. Jolicoeur. 2005. The Nef-mediated AIDS-like disease of CD4C/human immunodeficiency virus transgenic mice is associated with increased Fas/FasL expression on T cells and T-cell death but is not prevented in Fas-, FasL-, tumor necrosis factor receptor 1-, or interleukin-1β-converting enzyme-deficient or Bcl2-expressing transgenic mice. J. Virol. 79:6377-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saenz, D. T., W. Teo, J. C. Olsen, and E. M. Poeschla. 2005. Restriction of feline immunodeficiency virus by Ref1, Lv1, and primate TRIM5α proteins. J. Virol. 79:15175-15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakai, H., K. Tokunaga, M. Kawamura, and A. Adachi. 1995. Function of human immunodeficiency virus type 1 Vpu protein in various cell types. J. Gen. Virol. 76:2717-2722. [DOI] [PubMed] [Google Scholar]
- 68.Schubert, U., K. A. Clouse, and K. Strebel. 1995. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J. Virol. 69:7699-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schuitemaker, H., N. A. Kootstra, R. A. Fouchier, B. Hooibrink, and F. Miedema. 1994. Productive HIV-1 infection of macrophages restricted to the cell fraction with proliferative capacity. EMBO J. 13:5929-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646-650. [DOI] [PubMed] [Google Scholar]
- 71.Sheehy, A. M., N. C. Gaddis, and M. H. Malim. 2003. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 9:1404-1407. [DOI] [PubMed] [Google Scholar]
- 72.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 73.Stremlau, M., M. Perron, M. Lee, Y. Li, B. Song, H. Javanbakht, F. Diaz-Griffero, D. J. Anderson, W. I. Sundquist, and J. Sodroski. 2006. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. USA 103:5514-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun, Y., L. M. Pinchuk, M. B. Agy, and E. A. Clark. 1997. Nuclear import of HIV-1 DNA in resting CD4+ T cells requires a cyclosporin A-sensitive pathway. J. Immunol. 158:512-517. [PubMed] [Google Scholar]
- 75.Swanson, C. M., B. A. Puffer, K. M. Ahmad, R. W. Doms, and M. H. Malim. 2004. Retroviral mRNA nuclear export elements regulate protein function and virion assembly. EMBO J. 23:2632-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toggas, S. M., E. Masliah, E. M. Rockenstein, G. F. Rall, C. R. Abraham, and L. Mucke. 1994. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367:188-193. [DOI] [PubMed] [Google Scholar]
- 77.Varthakavi, V., R. M. Smith, S. P. Bour, K. Strebel, and P. Spearman. 2003. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc. Natl. Acad. Sci. USA 100:15154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watanabe, S., K. Terashima, S. Ohta, S. Horibata, M. Yajima, Y. Shiozawa, M. Z. Dewan, Z. Yu, M. Ito, T. Morio, N. Shimizu, M. Honda, and N. Yamamoto. 2007. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109:212-218. [DOI] [PubMed] [Google Scholar]
- 79.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451-462. [DOI] [PubMed] [Google Scholar]
- 80.Willett, B. J., L. Picard, M. J. Hosie, J. D. Turner, K. Adema, and P. R. Clapham. 1997. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J. Virol. 71:6407-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams, S. A., and W. C. Greene. 2005. Host factors regulating post-integration latency of HIV. Trends Microbiol. 13:137-139. [DOI] [PubMed] [Google Scholar]
- 82.Yu, Q., D. Chen, R. König, R. Mariani, D. Unutmaz, and N. R. Landau. 2004. APOBEC3B and APOBEC3C are potent inhibitors of simian immunodeficiency virus replication. J. Biol. Chem. 279:53379-53386. [DOI] [PubMed] [Google Scholar]
- 83.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]
- 84.Zhang, H., B. Yang, R. J. Pomerantz, C. Zhang, S. C. Arunachalam, and L. Gao. 2003. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng, Y. H., H. F. Yu, and B. M. Peterlin. 2003. Human p32 protein relieves a post-transcriptional block to HIV replication in murine cells. Nat. Cell Biol. 5:611-618. [DOI] [PubMed] [Google Scholar]