Abstract
Langerhans cells (LCs) are a subset of dendritic cells (DCs) that reside within epidermal and mucosal tissue. Because of their location, LCs are potentially the first cells to encounter human immunodeficiency virus (HIV) during sexual transmission. We report that LCs purified from CD34+-derived DCs can facilitate the transinfection of target cells but only after activation. Virions were observed in an intracellular compartment that contains several tetraspanins, in addition to the unique LC markers langerin and CD1a. This reveals that the trafficking of HIV within LCs is reminiscent of that which occurs in mature monocyte-derived DCs and that it varies with the activation state of the cell. The observation that activated LCs can mediate transinfection suggests a potential role for these cells in the known increase in HIV transmission associated with sexually transmitted infections that would cause inflammation of the genital lining.
Dendritic cells (DCs) are potent antigen-presenting cells that can efficiently stimulate T cells to protect the body from invading pathogens (37). While migrating through the lymphatic system, DCs progress from immature cells in peripheral tissues to mature antigen-presenting DCs in the lymphatic system. As part of its ability to disrupt the immune system, human immunodeficiency virus (HIV) has evolved mechanisms of avoiding immune detection by using DCs as a safe haven for transport from the periphery directly to targets of infection (9). Interestingly, DCs do not need to be infected themselves in order to facilitate viral replication and disseminate infection. Rather, they facilitate the transfer of captured virus to target cells. Initially it was believed that the virus was required to be internalized before transfer. However, more recent work suggesting that virus captured on the cell surface could also be efficiently transferred clearly indicates that more analysis is required (4, 6). The conjugation of DCs harboring virus with target cells can result in both an immunological and an infectious interaction (20, 34).
Considerable research has focused on the role of DC-SIGN, a C-type lectin expressed by many DC subsets, in harboring pathogens and mediating infection. The discovery of DC-SIGN, combined with previous studies examining DCs, led to the development of a model for DCs in the sexual transmission of HIV. In this model, DCs capture virions in the periphery via DC-SIGN and transport infectious virus to T-cell-rich lymphatic regions. Ultimately, productive infection is initiated after DCs transfer virus to target cells. The DCs themselves never become infected in this model. Cells expressing DC-SIGN, however, are confined to the submucosal compartment, making a direct encounter between virus and DC-SIGN-expressing cells an unlikely initial event in sexual transmission.
Langerhans cells (LCs) comprise a distinct subset of DCs that reside within the genital epithelium and mucosal tissues. LCs can extend cellular processes into these mucosal layers and are some of the first cells to confront and recognize sexually transmitted pathogens (21). Distinct from other DCs, LCs express the surface proteins langerin and E-cadherin and high levels of CD1a (3). Additionally, they form unique intracellular structures called Birbeck granules, which are considered subdomains for the endosomal recycling of langerin and CD1a (18, 31). While LCs do express CD4 and HIV coreceptors, a key consideration is that LCs do not express DC-SIGN (33). Specific to HIV, individual studies have described the ability of langerin to bind gp120 and the identification of langerin and HIV virions within Birbeck granules (7, 33). A recent report suggests that the binding of HIV to langerin leads to its degradation (7). However, experiments on LCs blocking the langerin receptor reveal that these cells must express other proteins that can capture HIV. Therefore, LC-specific markers can potentially play key roles in antigen recognition and presentation and act as mediators of HIV entry and/or infection.
While previous studies have investigated HIV infection of LCs, the ability of uninfected LCs to mediate transinfection of target cells remains unknown (2, 22, 23, 30). We observed that activated LCs incubated with HIV were able to stimulate transinfection of target cells, while unactivated LCs were not. We also examined the interaction of HIV with LCs using fluorescent deconvolution microscopy. This analysis revealed that activated LCs demonstrated clustering of virions within a distinct multivesicular body (MVB) that contains tetraspanin markers along with CD1a and langerin. CD1a was also transferred with virus to conjugated T cells. Overall, these experiments identify LCs as targets of HIV. Additionally, when they are activated, LCs allow HIV to maintain its infectivity and act as mediators of viral transmission to target cells.
MATERIALS AND METHODS
Primary cell cultures and cell lines.
Monocyte-derived DCs (MDDCs) were purified from peripheral blood of healthy, consenting donors. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by centrifugation with lymphocyte separation medium (Biowhittaker). CD14+ monocytes were obtained by incubating PBMCs with CD14 magnetic beads and separating them using the MACS system (Miltenyi Biotech). Purified monocytes were cultured in RPMI supplemented with 15% fetal bovine serum (FBS), granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/ml), and interleukin-4 (IL-4; 100 ng/ml) for 5 days to allow differentiation into immature MDDCs. Mature MDDCs were obtained by adding lipopolysaccharide (LPS; 100 ng/ml) to cell culture for 2 days.
CD4+ T cells were isolated from PBMCs through negative selection by an indirect magnetic labeling system (CD4+ T-Cell Isolation Kit II; Miltenyi Biotech). Purified CD4+ T cells were cultured in RPMI supplemented with 15% FBS.
DC-100 cells were generated from CD34+ progenitor cells derived from umbilical cord blood at MatTek Corporation (Ashland, MA) and shipped overnight with DC-MM medium (MatTek). DC-MM is an RPMI 1640-based medium supplemented with cytokines to maintain the LC phenotype (MatTek).
p4-2 cells (HeLa cells expressing CD4) and 293T cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS.
Characterization of DC-100 cells.
DC-100 cells were characterized by first washing them with washing buffer and then resuspending 2 × 105 cells in cold phosphate-buffered saline (PBS) containing 2% FBS. Fluorescent dye-conjugated monoclonal antibody (BD Pharmingen; DC-SIGN from R&D Systems) was added to the respective wells, and cells were incubated for 30 min on ice, washed, fixed with formaldehyde, and analyzed by flow cytometry at the Dana-Farber Cancer Institute core facility (Boston, MA).
Isolation of CD1ahi LCs.
CD1ahi LCs were isolated from the DC-100 population by flow cytometry (UIC Flow Cytometry Service, Chicago, IL). Cells were incubated with fluorescein isothiocyanate (FITC)-conjugated human anti-CD1a antibody (BD Pharmingen) for 30 min at 37°C. Cells were washed with RPMI plus 20% FBS, and CD1ahi and CD1alo/neg populations were isolated and cultured in DC-MM medium (MatTek). Sorted LCs were activated by culturing them in medium containing LPS (5 μg/ml) and tumor necrosis factor alpha (TNF-α; 30 ng/ml) for 2 days.
Virus stocks.
HIV type 1 (HIV-1) stocks for luciferase transmission assays were generated by calcium phosphate transfection of 293T cells with a CXCR4-tropic proviral plasmid. NL43-R−-luc is deficient in viral vpr and carries the firefly luciferase reporter gene in place of HIV nef. CCR5-tropic virus was generated by cotransfecting 293T cells with the envelope-deficient NL43-R−Env−-luc vector and a plasmid carrying the JRFL env gene. Transfectants were washed 16 h posttransfection, and medium was replaced 24 h posttransfection. Infectious virus was harvested after 48 h. Virus concentration was determined by enzyme-linked immunosorbent assay for HIV-1 p24 capsid protein.
CXCR4-tropic HIV-1 used in microscopy assays was generated by transfection of 293T cells using polyethyleneimine reagent (Polysciences, Inc.). Cells were cotransfected with NL43-R−-luc vector and red fluorescent protein (RFP)-Vpr plasmid at a 40:1 ratio. Medium was changed approximately 16 h posttransfection, and virus was harvested by filtration 48 to 56 h thereafter. Viral titer was estimated based on the level of RFP-Vpr signal observed in MDDCs incubated with virus.
Transmission assays.
p4-2 cells were plated in a 96-well plate and cultured overnight in DMEM. Prior to incubation with virus, medium was replaced with RPMI supplemented with 15% FBS. MDDCs, DC-100 cells, and LCs were exposed to various concentrations of virus at 37°C for 2 h (10,000 cells/100 μl). Following preincubation, medium was removed from target wells and DC-100 cells, LCs, or MDDCs, along with their respective viral supernatants, were aliquoted onto target cells (100 μl/well). Additionally, p4-2 target cells were directly exposed to virus alone to define the background level of infection. For pulse experiments, cells were washed twice with RPMI to remove free virions prior to addition to p4-2 cells. The final volume in these wells was adjusted to 100 μl/well with RPMI plus 15% FBS. Medium in the plate was replaced with fresh DMEM plus 10% FBS after 16 to 17 h, and luciferase activity was measured 40 h later. For removal of surface virions, cells were treated with 0.05% trypsin (Invitrogen) for 5 min at 37°C before coculture.
Luciferase assay.
Luciferase assay reagent (LAR; Promega) was prepared according to the manufacturer's instructions. LAR was allowed to sit at room temperature for 30 min prior to completion of the assay. To prepare cell lysates, target cells were washed with PBS and lysed with 20 μl of 1× cell culture lysis reagent (Promega), prepared according to instructions. Cells were lysed at room temperature for 10 to 15 min. Cell lysates were added to LAR and vortexed briefly, and the resulting light was measured in a single-tube luminometer (FB12 luminometer; Berthold Detection Systems).
Virus incubations and staining.
Approximately 1 × 105 unsorted DC-100 cells were incubated with CXCR4-tropic HIV-1 expressing RFP-Vpr for 2.5 h. Cells were washed with PBS and incubated with an equal number of nonautologous CD4+ T cells for 15 min. Cells were fixed to CellTak-coated coverslips (BD Biosciences) with formaldehyde. Coverslips were incubated with block solution (10% normal donkey serum, 0.1% Triton X-100, 1% bovine serum albumin, 0.01% NaN3) for 5 minutes prior to antibody staining. CD1a (Leinco Technologies) and langerin (Immunotech) antibodies were used at a 1:200 dilution for 2 h at 4°C. For experiments with the CD1a blocking antibody, fixed cells were first stained with a Cy5 donkey anti-mouse secondary antibody prior to exposure to the FITC-conjugated CD1a antibody.
T cells were identified with an anti-CD4 biotin-conjugated antibody, followed by incubation with Cy5-conjugated streptavidin (Jackson Immunolabs) at dilutions of 1:100 and 1:200, respectively. Alexa Fluor phalloidin 350 was used at a dilution of 1:40 to identify actin. Coverslips were mounted onto slides using GelMount (Biomeda Corporation) and allowed to dry prior to viewing via deconvolution microscopy.
Microscopy.
Images were acquired with an Olympus IX71 microscope and a CoolSnap HQ camera using a 100× oil objective and DeltaVision deconvolution microscopy software. Fluorescent filters used included (excitation/emission) Cy5 (640/685), RFP (555/617), FITC (490/528), and UV (360/457). Thirty z sections, 0.2 μm apart, were collected per field at room temperature and 1×1 binning. Images were deconvolved and analyzed using SoftWorx software, and virus was pseudocolored green, CD4 was pseudocolored red, and langerin or CD1a was pseudocolored blue. Individual z sections were saved to generate figures subsequently composed in Adobe Photoshop.
CD1a blocking antibody preparation and experiments.
OKT6 hybridoma (ATCC) was cultured in Iscove's modified Dulbecco's medium supplemented with 20% FBS. The supernatant was harvested periodically and filtered through a 0.22-μm filter to purify the CD1a blocking antibody. For experiments, prior to incubation with virus, LCs were treated with approximately 200 μl of the blocking antibody per 100,000 cells for 30 min at 37°C. Untreated and treated LCs were then exposed to X4-tropic virus for 2 h. In transmission assays, treated LCs were kept with antibody throughout coculture with target cells and luciferase assays were conducted as described above. For immunofluorescence analysis, virus and antibody were washed from cells prior to fixing and staining (as described above).
RESULTS
Characterization of CD34+-derived DC-100 cells.
Although LCs are commonly found within normal mucosal epithelium and throughout skin, it is difficult to isolate a large number of primary LCs from tissue. Therefore, we used LCs derived from CD34+ hematopoietic progenitors. Briefly, CD34+ cells were isolated from umbilical cord blood by magnetic separation. Cells were then cultured in DC-MM medium (MatTek, Ashland, MA), an RPMI-based medium, supplemented with GM-CSF, TNF-α, and transforming growth factor β to obtain what are designated DC-100 cells (MatTek, Ashland, MA). The CD34-derived DC-100 cells were characterized by flow cytometry to determine expression levels of relevant surface markers. We detected a subset of the cells that expressed both langerin and high levels of CD1a, characteristic traits of endogenous LCs (Fig. 1A). Additionally, DC-100 cells contained both cytoplasmic and plasma membrane-bound Birbeck granules, which are unique to LCs (Fig. 1B).
FIG. 1.
Characterization of LC-specific traits for CD34+-derived DC-100 cells. (A) Flow cytometry of DC-100 cells to determine surface expression of langerin and CD1a. Unstained cells were used as a control for comparison to DC-100 cells stained with antibodies to langerin and CD1a. (B) Electron micrograph of LC within DC-100 population. An example of Birbeck granules is boxed and enlarged.
Previous studies have shown that mature MDDCs are much more potent in their ability to mediate transinfection of HIV. Mature MDDCs are characterized by increased expression of HLA-DR, CD83, and CD86. We examined the expression of these cell surface markers on DC-100 cells before and after stimulation with either LPS, TNF-α, or both. A subset of the unstimulated DC-100 cells expressed HLA-DR (87%), CD80 (54%), CD40 (37%), CD206, and mannose receptor (42%) (Fig. 2A and data not shown). Cells expressing the maturation markers CD83 and CD86 appeared at relatively low levels in untreated DC-100 cells, 13% and 38%, respectively (Fig. 2A). Upon activation with LPS and TNF-α, DC-100 cells had an increase in the number of cells expressing HLA-DR and a marked increase in the total mean fluorescence of HLA-DR. Likewise, expression of the activation markers CD80 (78%), CD83 (70%), and CD86 (76%) increased (Fig. 2A). Treatment of the DC-100 cells with both LPS and TNF-α caused a twofold downregulation of cells double positive for CD1a and langerin (Fig. 2B). Additionally, activated DC-100 cells expressed and released high levels of cytokines and chemokines such as IL-12, IL-1β, IL-6, RANTES, and MIP-1α and upregulated intercellular adhesion molecule 1 expression (data not shown). This demonstrates that treatment with LPS and TNF-α induces activation of the DC-100 cell population. In addition to characterization related to activation state, expression of the lectin DC-SIGN on DC-100 cells was also analyzed. As previously documented for LCs, DC-SIGN expression was not observed in the DC-100 cell population, regardless of activation (Fig. 2C). Collectively, these features clearly identified a subset of cells with characteristics of tissue-derived LCs within the DC-100 population.
FIG. 2.
Characterization of DC-100 cells following activation. (A) Surface expression of maturation markers HLA-DR, CD80, CD83, and CD86 was measured in untreated cells (first column) and after activation with LPS (second column), TNF-α (third column), or both (fourth column). Marker expression was compared to that of unstained controls (first row). (B) Expression of CD1a and langerin was measured in unactivated (untreated) and activated (LPS plus TNF-α) DC-100 cells. (C) Expression of DC-SIGN in untreated and LPS-plus-TNF-α-treated DC-100 cells.
DC-100 cells mediate transinfection after activation.
Previous studies have demonstrated that infected LCs are capable of efficiently disseminating HIV infection (24, 29). This is an important finding, due to the location of LCs at the surface of epidermal tissue and their high potential for being primary targets of initial pathogen exposure. However, even though other DC subtypes have been shown to promote transinfection, it remains unclear whether LCs have this ability (20, 27). Therefore, we investigated whether LCs could stimulate HIV infection of target cells in trans, without becoming infected themselves. For these studies, we used two luciferase-expressing reporter viruses; either an NL43-luc viral plasmid encoding its own CXCR4-tropic envelope or an envelope-minus NL43-luc plasmid pseudotyped with the JRFL envelope (CCR5-tropic). Consistent with previous reports, measurable luciferase activity was observed in both DC-100 cells and MDDCs exposed to CCR5-pseudotyped virus (Fig. 3A). However, CXCR4-exposed cells did not demonstrate any luciferase activity, indicating that the CXCR4-tropic virus was unable to infect either DC-100 cells or MDDCs (Fig. 3A). Therefore, CXCR4-tropic virus was used in subsequent experiments to determine whether the LCs within the DC-100 cell population could mediate transinfection of target cells.
FIG. 3.
Infectivity profiles of unsorted DC-100 cells. (A) Unactivated and activated LCs were exposed to X4-tropic or R5-tropic HIV, and infection of the cells was determined by level of luciferase activity (relative light units). The level of infection was compared to that occurring in target p4-2 cells directly exposed to virus. (B) Unactivated (open circles) and activated (open squares) DC-100 cells were exposed to increasing concentrations of X4-tropic HIV prior to coculture with target cells. Infection was determined by measuring luciferase activity of samples (relative light units). p4-2 targets exposed to virus (asterisks) defined background infectivity. Cocultures with mature MDDCs acted as a positive control (open triangles). Any infection of effector cells was determined by analysis of cells cultured with virus and not exposed to targets. These controls include unactivated DC-100 cells alone (closed circles), activated DC-100 cells (closed squares), and mature MDDCs (closed triangles). Each data point represents the average of three samples with error bars representing the standard errors of the means. Representative results of four independent experiments are shown.
It has previously been shown that activation of MDDCs can influence the efficiency of transinfection. Therefore, we compared transfer of infectious virus between unactivated DC-100 cells and those activated with LPS. Viral transmission was slightly enhanced in cocultures of LPS-activated DC-100 cells and target cells; however, a decrease in viability of activated DC-100 cells was observed. Recent studies suggest that the maturation and viability of cultured LCs can be enhanced upon treatment with TNF-α (1). Therefore, DC-100 cells were treated with LPS (5 μg/ml) and TNF-α (30 ng/ml) in subsequent experiments to fully activate and increase viability of the culture.
DC-100 cells activated with LPS and TNF-α were found to increase HIV-1 infection of target cells in a manner similar to that of mature MDDCs (Fig. 3B). As has previously been shown, cocultures of mature MDDCs, NL43-luc virus, and target cells displayed greater luciferase activity than p4-2 cells alone, in a dose-dependent manner (20). Interestingly, LPS- and TNF-α-activated DC-100 cells also demonstrated a dose-dependent increase in luciferase activity when cocultured with p4-2 cells and virus. In contrast to activated cells, cocultures including unactivated DC-100 cells did not increase luciferase activity above the level of infection occurring in target cells cultured with virus alone. No luciferase activity was detected when target cells were not included, indicating that CXCR4-tropic virus did not infect MDDCs or DC-100 cells. Note that these data points (for DC-100 cells or MDDCs alone) directly overlap each other on the graph along the x axis. Therefore, activated DC-100 cells are able to stimulate the infection of target cells without becoming infected themselves.
Activated CD1ahi LCs transfer virus in coculture.
Activation of the DC-100 cells clearly demonstrated the potential to enhance HIV infection of target cells. These cells consist of a mixture of cells with markers of different DC subtypes, including LCs and plasmacytoid DCs. We wanted to focus our studies specifically on LCs within this population and determine the role that these cells may play in mediating transinfection. We utilized the elevated CD1a expression on primary LCs to isolate CD1ahi- and CD1alo/neg-expressing cells by flow cytometry (Fig. 4A).
FIG. 4.
Sorting and infectivity profiles of CD1ahi and CD1alo/neg DC-100 cells. (A) DC-100 cells were stained with FITC-conjugated CD1a antibody and sorted to isolate CD1alo/neg and CD1ahi DC-100 cells. From left to right, dot plots show unsorted, CD1ahi, and CD1alo/neg DC-100 cells. (B and C) Luciferase activity measured in cocultures of unactivated (closed squares) and activated (open squares) CD1ahi LCs with target cells. Background levels were defined by virus-exposed p4-2 targets (asterisks). (B) Cocultures of unactivated (closed diamonds) and activated (open diamonds) CD1alo/neg cells; (C) cocultures of immature (closed triangles) and mature (open triangles) MDDCs. Each data point represents the average of three samples with error bars representing the standard errors of the means. Each graph is representative of three independent experiments.
Activated CD1ahi and CD1alo/neg cells were both observed to mediate transinfection of HIV (Fig. 4B). Activated and unactivated cells of each isolate were exposed to virus for 2 hours prior to coculture of the cells and virus with target cells. p4-2 target cells cultured with virus alone provided a background infectivity profile. As expected, unactivated cells were unable to increase luciferase activity in cocultures above the level of virus alone on target cells. Cocultures with activated CD1alo/neg cells demonstrated a dose-dependent increase in luciferase activity, attributable to the presence of DC subsets other than LCs in this population. Interestingly, activated CD1ahi LCs also increased luciferase activity of cocultures in a dose-dependent manner and were slightly more efficient than CD1alo/neg cells. LCs had not previously been shown to mediate virus transfer in the absence of cis infection; therefore, we were especially interested in the efficiency of activated CD1ahi LCs in transferring virus in cocultures.
Activated CD1ahi LCs were nearly as efficient as mature MDDCs in enhancing viral transmission to target cells (Fig. 4C). In this experiment, both activated and unactivated CD1ahi cells were incubated with virus for 2 hours prior to direct addition to target cells. As described above, p4-2 target cells were cultured with virus alone to establish a level of background infection. As controls, we included immature and mature MDDCs exposed to virus and cocultured with p4-2 targets. Cocultures with mature MDDCs expressed high levels of luciferase activity that increased in a dose-dependent manner (Fig. 4C, open triangles). Immature MDDCs did not significantly increase luciferase activity of cocultures above levels of control virus cultured alone with targets (Fig. 4C, filled triangles). Activated CD1ahi LC cocultures also exhibited high levels of luciferase activity that increased in a dose-dependent manner and were nearly as efficient as mature MDDCs in mediating transinfection. Overall, the ability of CD1ahi cells of the DC-100 population to mediate transinfection suggests a role for activated LCs in viral presentation and infection of target cells.
Enhanced transinfection by activated LCs involves internalized virions.
In the experiments described above, LCs cultured with HIV were directly added to target cells without a wash to remove free virions. These data show an enhancement of infection in the presence of activated LCs over control cultures of p4-2 targets with virus alone. However, we were also interested in determining if cells could mediate transinfection after a 2-hour pulse with virus. Therefore, after incubation of untreated and treated LCs with HIV expressing the luciferase reporter gene, virus was washed from cells with RPMI and then cocultured with target p4-2 cells in DC-MM medium. The next day the DC-MM medium was replaced with DMEM to maintain the viability of the target cells. As in Fig. 4, we observed an enhancement of transinfection by the activated LCs more than 35 times higher than that occurring in unactivated LCs (Fig. 5A). This is comparable to the enhanced transinfection observed in mature MDDCs compared to that in immature cells.
FIG. 5.
Enhanced transinfection by activated LCs involves internalized virions. (A) Luciferase activity measured in cultures of p4-2 target cells with unactivated and activated CD1ahi LCs that were previously exposed to virus and washed to remove unbound virus. Immature and mature MDDCs were also pulsed with virus and served as negative and positive controls, respectively. (B) Unactivated and activated LCs were pulsed with virus for 2 h, washed to remove unbound virus, and then exposed to 0.05% trypsin plus 0.53 mM EDTA for 5 min at 37°C to remove surface-bound virions. Luciferase activity was measured as described above. Each data point represents the average of three samples with error bars representing the standard errors of the means. Representative results from four independent experiments are shown.
These experiments suggest that HIV virions already associated with activated LCs maintain their infectivity, and it is these virions that are transmitted to target cells. Recent reports from other laboratories have indicated a critical role for surface-bound virions in transinfection mediated by DCs (4, 6). Therefore, LCs pulsed with virus were exposed to trypsin treatment to help decipher roles for surface and internalized virions in transinfection. This method has previously been used to cleave virions or cellular surface molecules that promote binding (12, 15, 32). Unactivated and activated LCs were pulsed with virus for 2 h, at which point the cells were washed with RPMI to remove free virions. Cells were resuspended in media and cultured for an additional hour. This incubation was performed to allow bound virions an opportunity to internalize into the LC. Finally, cells were either cultured with media as a mock control or incubated with trypsin to remove any remaining surface-bound virions that had not been internalized into a trypsin-resistant compartment. Subsequently, cells were cocultured with p4-2 targets as described above.
A reduction in transinfection was observed for both unactivated and activated LCs after exposure to trypsin. Activated cells treated with trypsin, however, were still able to transmit virus at almost 20% of the level of mock-treated cells (Fig. 5B). Additionally, activated LCs elevated transinfection above that of unactivated LCs regardless of exposure to trypsin. We also observed that transinfection by internalized virions doubled if cells were allowed to internalize virions for an additional hour (data not shown). This suggests that at least some virions are internalized by activated LCs into a trypsin-resistant compartment where they are able to maintain their infectivity at a greater level than unactivated cells.
Characterization of HIV localization in LCs.
Evidence presented above and previously indicates that DCs can harbor infectious virus prior to transmission to target cells (15, 20, 34). Very little, however, is known about the specific intracellular location of virus within the various subtypes of DCs. Since their ability to transinfect differed with activation state, we were interested in determining whether the location of HIV within LCs also varied. For these studies, DC-100 cells were either activated with LPS and TNF-α or remained untreated prior to incubation with CXCR4-tropic HIV-1. In order to visualize the virions, fluorescently labeled HIV was generated by cotransfection of viral DNA and a plasmid coding for HIV Vpr was fused to mouse RFP (see Materials and Methods). This construct is analogous to green fluorescent protein-Vpr previously used by our lab for imaging of HIV virions in MDDCs and labels individual virions just as the green fluorescent protein construct does (5, 19). After 3 hours of incubation, free virus was washed from the cells with PBS. Cells were then briefly cocultured with nonautologous CD4+ T cells to allow the formation of LC:T-cell conjugates prior to fixing and staining. LCs were identified within the DC-100 population by elevated CD1a expression, a trait unique to the DC subset. T cells were identified by CD4 and distinguished from LCs by size and lack of CD1a expression.
Activation of cells resulted in a clustering of virus within a single small area of the cell. Additionally, a greater number of viral particles were typically observed within the activated cells than within unactivated controls (data not shown). Previous work by our laboratory and others has shown compartmentalization of HIV to an MVB within MDDCs which contains tetraspanins and major histocompatibility complex class II (10). To determine if LCs trafficked virus to a similar compartment, we stained unactivated and activated LCs that were exposed to X4-tropic HIV with antibodies to recognize the tetraspanins CD81, CD9, and CD63. Just as with mature MDDCs, we observed a significant increase in the association of each of the tetraspanins with HIV in activated LCs over that in unactivated cells. Overlap of the tetraspanins with virions was minimal in unactivated cells, and the increase observed in activated LCs varied depending on the marker being analyzed. Stimulated LCs showed a four- to sixfold increase for CD81 and CD63; however, the most significant difference was observed with CD9, where the overlap with virus was elevated approximately 30-fold in activated LCs (Fig. 6A).
FIG. 6.
Characterization of MVB components in activated LCs. Unsorted unactivated and activated DC-100 cells were incubated with X4-tropic HIV with RFP-Vpr prior to fixing and staining. (A) Percentage of unactivated (light bars) and activated (dark bars) LCs with the representative tetraspanin associated with HIV. Error bars show standard errors of the means. (B) LCs were identified by positive staining for CD1a (blue). Virus was identified by expression of fluorescently tagged Vpr (pseudocolored green), and cells were then stained for CD81 (red). The boxed area is enlarged and color separated to show individual stains.
A representative image in Fig. 6B shows the presence of both CD1a and CD81 in the same MVB as HIV virions. This confirms that the virus-containing compartment in activated LCs is similar to that identified in mature MDDCs exposed to HIV. Accumulation of CD1a would not have been observed in this MVB in MDDCs, as expression of this protein is relatively low in all DC subsets, except for LCs.
While staining cells to analyze tetraspanin expression in MVBs, we observed a difference in CD1a localization between unactivated and activated cells. Surface expression of CD1a occurred over the entire LC, regardless of activation state. Interestingly, the receptor was frequently observed to be concentrated in the same intracellular location as labeled virus and tetraspanins, but only in the activated cultures (Fig. 7A). Over 100 conjugates were analyzed to determine the frequency at which concentrated CD1a protein was observed in the same discrete area of the cell as the internalized HIV virions. Overall, association of virus with CD1a was observed in over 80% of activated cells. This differed significantly from the less than 20% association occurring in unactivated LCs (Fig. 7C).
FIG. 7.
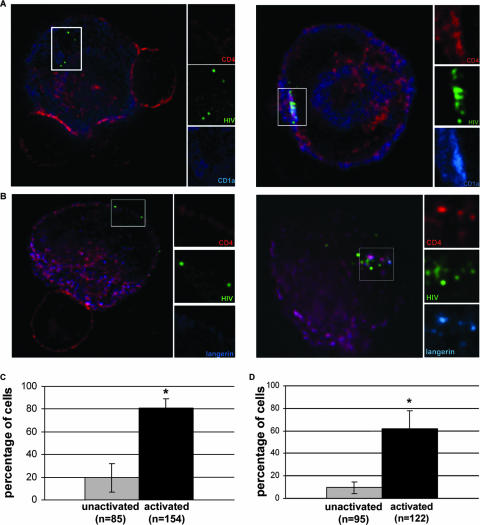
Association of CD1a and langerin in an MVB in activated LCs. (A) Deconvolution microscopy of DC-100 cells. Unsorted unactivated (left) and activated (right) DC-100 cells were incubated with X4-tropic HIV with RFP-Vpr (pseudocolored green) prior to coculture with CD4+ T cells. LCs were identified by positive staining for CD1a (blue) and CD4 (red), while T cells were only CD4 positive. The boxed area is enlarged and color separated to show individual stains. (B) DC-100 cells were treated as for panel A; however, LCs were identified by staining for langerin (blue). (C) Percentages of unactivated and activated LCs with CD1a associated with HIV. Error bars show standard errors of the means from four individual experiments; a P value of <0.05 is marked with an asterisk. (D) Percentages of cells with langerin associated with HIV in unactivated and activated LCs. Error bars show standard errors of the means from four individual experiments; a P value of <0.05 is marked with an asterisk.
Along with elevated CD1a expression, the lectin langerin is a surface receptor specific to LCs. Due to reports of HIV interacting with cellular lectins and the ability of langerin to bind HIV gp120, we were particularly interested in whether virions were also associating with langerin as with CD1a (33). Unactivated or activated DC-100 cells were exposed to virus, followed by coculture with CD4+ T cells, as described above. For these experiments, LCs were distinguished from other cells in the population by positive langerin staining. We observed a concentration of langerin in a discrete region of the cell similar to that observed with CD1a staining. Further, the clustering of virions that occurs in activated cells appears to occur in the same area as the intensified langerin signal (Fig. 7B). There was a significant difference in the association of HIV with concentrated langerin. Overlap of a subset of HIV and langerin was observed in almost 62% of activated cells and less than 10% of unactivated LCs (Fig. 7D).
In addition to a concentration of LC-specific markers and tetraspanins in a compartment containing HIV virions in activated LCs, clustering of CD4 also occurred in the same area (Fig. 7A and 7B). Therefore, since it is observed across DC subsets, this compartmentalization of virions likely plays a critical role in intracellular trafficking of HIV in DCs.
LCs transfer HIV virions to CD4+ T cells.
As demonstrated in the luciferase assays described above, we observed transinfection of HIV from LCs to target cells. The transfer of virions has been visualized between MDDCs and target cells (20). We sought to determine if LCs could function in a similar manner. To study this transfer, we used the LC:T-cell cocultures described above. LCs interacting with T cells were identified by actin staining, and the identity of LCs was confirmed by elevated CD1a expression. HIV virions were observed in T cells conjugated to either activated or unactivated LCs (Fig. 8A). Because free virus was washed from LCs, the virus present in T cells is likely to have been transferred from LCs. Although activated cells did transfer virions more frequently, the difference was not significant (Fig. 8B).
FIG. 8.
Transfer of CD1a from activated LCs to T cells. (A) Unsorted unactivated (left) and activated (right) DC-100 cells were incubated with X4-tropic HIV with RFP-Vpr (pseudocolored green) prior to coculture with CD4+ T cells. LCs were identified by positive staining for CD1a (blue) and CD4 (red), while T cells were only CD4 positive. The boxed area includes a single, transferred virion within a T cell. Small panels show magnifications of virions and individual stains. (B) Percentages of unactivated and activated LCs that have transferred virions to T cells. (C) Percentages of virions transferred to T cells that show an association with CD1a after coculture with unactivated or activated LCs. Error bars show standard errors of the means from two individual experiments; a P value of <0.05 is marked with an asterisk.
Activated LCs transfer CD1a with HIV to T cells.
During analysis of HIV transfer to T cells we observed that, in addition to virus, CD1a protein was also transferred to the target cells (Fig. 8A). CD1a not only was visible in these T cells but also clearly overlapped with the labeled virions. Because this protein is not expressed in T cells, it must be originating from the activated LCs. While HIV was observed to be associated with T cells incubated with either unactivated or activated LCs, CD1a associated with transferred virus was observed only in T cells cocultured with activated LCs (Fig. 8A and C). Conjugates that exhibited transfer of HIV from an LC to a T cell were analyzed to determine how frequently this overlap occurred. The continued association of CD1a with transferred virions occurred in 56% of T cells that received virus from an activated LC (Fig. 8C). This is in contrast to less than 3% overlap of CD1a with virus that was transferred from an unactivated LC.
CD1a antibody does not block HIV transmission.
Because of the continued association of CD1a with virions transferred to T cells, we investigated if CD1a binding to HIV was required for the compartmentalization and enhanced transmission of HIV by activated cells. We treated cells with OKT6 antibody. This antibody has previously been used against CD1a and was cultured with cells here so that CD1a would potentially be blocked from interacting directly with HIV (11, 16, 26). Transmission assays were performed to assay transinfection in the presence or absence of antibody, and no difference in luciferase activity was detected (data not shown). Untreated and treated LCs were also fixed and stained with a secondary antibody to visualize the CD1a blocking antibody. CD1a appeared in the same intracellular compartment as virus in both sets of cells (data not shown). Therefore, these experiments suggest that the anti-CD1a antibody OKT6 alters transinfection with HIV mediated by activated LCs.
DISCUSSION
LCs are a subset of DCs that reside within the skin and genital epithelium. Due to their proximity to mucosal surfaces, these are some of the first cells of the immune system to encounter invading pathogens. Previous work with tissue-derived LCs has demonstrated that they can be infected by CCR5-tropic HIV. The data presented here demonstrate that activated LCs derived from CD34+ stem cells have the ability to mediate the transinfection of target cells with HIV.
For the present studies, we utilized LCs derived from CD34+-derived stem cells through treatment with GM-CSF, TNF-α, and transforming growth factor β. This treatment generated the DC-100 population which contains cells with the morphological, ultrastructural, and cell surface markers of LCs (Fig. 1 and 2). Importantly, a subset of these cells express high levels of langerin and CD1a, do not express DC-SIGN, and contain Birbeck granules, all known features of primary tissue-derived LCs. It was possible to purify the CD1ahi cells from the DC-100 population to generate a highly enriched population of LCs in sufficient quantities for subsequent analysis. It is worth noting that the isolation of LCs from skin and tissue samples can be problematic (25). For example, isolation of LCs from tissue can lead to maturation and phenotypic alteration of the LC population. Additionally, emigrated LCs isolated from skin represent only a subset of tissue-resident LCs, and it is difficult to obtain a sufficient number of cells for desired experiments. Therefore, the CD34+ stem cell-derived LCs represent a reasonable model system for the analysis of the interaction of HIV with LCs in vivo.
Several groups have shown that LCs can be infected with R5-tropic HIV (13, 24). It has also been reported that LCs, and DCs in general, are not infected with X4-tropic HIV (8) (Fig. 3A). Therefore, we chose to extend these studies to focus on the ability of the LCs to stimulate transinfection of target cells. We utilized only X4-tropic HIV in our studies of transinfection to avoid infection of LCs that could confound the interpretation of our results.
Results published by several groups have shown that mature MDDCs are much more efficient at mediating transinfection of target cells than are their immature counterparts (20, 27). Due to their location in the body, it was important to determine if LCs also possessed this ability. We found that unactivated DC-100 cells and isolated LCs do not enhance transinfection above background levels of target cells with virus alone. However, activation with LPS and TNF-α stimulated LCs in such a manner that they could now efficiently mediate transinfection at levels comparable to those for LPS-stimulated MDDCs (Fig. 4). Activation of LCs with LPS alone was not sufficient to allow the cells to mediate transinfection even though the expected increase in levels of cell surface activation markers such as HLA-DR and CD86 was observed. It appears that the requirements for activation of LCs to facilitate transinfection are more stringent than those of mature MDDCs. This requirement may be a reflection on the localization of LCs at the boundary of epithelial and mucosal barriers. As they are the first line of defense, constitutively responding to foreign antigens might lead to inappropriate immune responses.
Alternatively, a recent report showing that langerin acts as an antiviral factor may provide further insight into why activated LCs are more efficient at mediating transinfection (7). It was shown that the use of a langerin-blocking antibody increased the infection of primary unactivated LCs. As shown in Fig. 2B, activation of LCs led to a downregulation of cell surface langerin expression. This could alter the interaction of HIV with the activated LCs, allowing the virus to traffic to the CD1a-containing MVB described here and be available for transinfection.
Experiments described here using trypsin treatment of LCs to remove surface-bound virions suggest a role for internalized virions in the transinfection of cells by LCs. To gain further insights into the interaction of LCs and HIV, we analyzed the interactions of LCs and HIV using fluorescent deconvolution microscopy. We found HIV associated with both unactivated and activated LCs; however, the localizations of HIV in the two sets of LCs were quite different. HIV was dispersed throughout unactivated LCs. In contrast, in LPS- and TNF-α-activated LCs, HIV was found to accumulate within a small region of the cell enriched in cell surface markers such as CD81, CD9, and CD63 (Fig. 6). These tetraspanin markers have previously been shown to be enriched in endosomal compartments such as MVBs (17). These MVBs also contain the LC-specific markers langerin and CD1a, along with CD4 (Fig. 7). The accumulation of HIV within an MVB containing high levels of CD81 and other tetraspanins in MDDCs has recently been reported (10). Therefore, it appears that HIV is trafficked through an MVB of similar composition across different DC subtypes.
While imaging experiments demonstrated that both unactivated and activated LCs could transfer virions, the transmission assays revealed an underlying difference in the infectivities of these virions. Two differences clearly observed in activated LCs and absent in unactivated cells are (i) the efficient trafficking of HIV to the MVB and (ii) the fact that virions transferred by activated LCs are often also stained with an anti-CD1a antibody (Fig. 8). The correlation of HIV association with CD1a and the ability to mediate transinfection suggest an inherent difference in the ways in which unactivated and activated LCs interact with HIV. To explain this difference, we consider a model where HIV is trafficked through the CD1a-rich MVB present in activated LCs and then more efficiently transferred to target cells through the infectious synapse formed between LCs and target cells.
While more study is required to prove this model, the transfer of CD1a together with virus is similar to the recent report by Wiley and Gummuluru, which found that HIV becomes associated with exosomes after internalization by immature MDDCs (36). This exosome association was found to increase the infectivity of the virus, even in the absence of MDDCs. Both the transfer of CD1a with HIV and mediation of transinfection take place only in conjugates containing activated LCs. Therefore, the observations of transinfection and CD1a association are consistent with the model proposed by Gummuluru and coworkers that exosome-associated virus is more infectious than cell-free virus.
A recent paper from the Greene laboratory, which analyzed the DC-100 population from MatTek, suggested that only surface-bound HIV is transferred to target cells by LCs (6). However, because the authors utilized the entire population and not LCs purified from the culture, it is not really possible to directly compare their results to those presented here. In Fig. 5 we demonstrate that at least a subset of the virus analyzed is protected from trypsin digestion before transinfection. This supports a role for internalized virions in the observed transinfection of HIV by LCs. In interpreting the result of trypsin treatment, it is also important to consider the digestion of other proteins from the surface of the LCs that may also decrease the efficiency of transinfection. An additional possibility is the continuous recycling of HIV between the internalized compartment and the cell surface of LCs. This process would further obscure the interpretation of protease treatment of cells as a way to differentiate between these two different cell-associated virus populations. A final determination of the exact role of internalized virus in transinfection mediated by LCs and DCs will require higher-resolution techniques. Current results, however, suggest that both internalized and cell surface HIV play a role.
The ability of activated LCs to mediate transinfection has potential implications for the mechanism of the sexual transmission of HIV. Because of their resident localization within mucosal squamous epithelium, the LCs would likely be among the first cells interacting with HIV entering the outermost layers of this tissue (14). This interaction could have multiple consequences. First, the LC could become directly infected, albeit such infection is inefficient. Alternatively, the LC could internalize HIV for later transfer. Here we show that activated LCs have the ability to mediate transinfection. Therefore, especially under conditions where there is local inflammation, the LCs resident in the mucosal epithelium could capture the virus and migrate inward. This captured virus could be involved in transinfection of either resident T cells or cells in a local lymph node after LCs migrate from the epithelium. Alternatively, the captured virus could be transferred to the DC-SIGN-expressing DCs located in the submucosal tissue. It has recently been proposed that LCs primarily function to capture antigens, which are then transferred to DCs that are more efficient at antigen presentation through the process of antigen sharing (35). Although at first glance this model would appear to conflict with the recent report suggesting that LCs and langerin are sentinels preventing HIV transmission, these studies did not examine the potential role of activated LCs. In fact, consistent with the Geijtenbeek group report, we find that unactivated LCs do not stimulate HIV infection of target cells in coculture experiments (Fig. 4B) (7).
The strongest correlate associated with the sexual transmission of HIV is infection with sexually transmitted pathogens. Infection and/or inflammation of the genital epithelium in humans is known to increase the probability of a person acquiring HIV infection (28). The inflammation occurring in these situations would lead to activation of resident LCs, making them permissive for viral transfer and transinfection. The localization of LCs at the mucosal barrier, along with the abilities of LCs to be infected by HIV and of activated LCs to mediate HIV transinfection, suggests that these cells likely play a role in certain cases of HIV sexual transmission.
Acknowledgments
This work was supported by National Institutes of Health grants RO1 AI052051 and PO1 HD40539 to T.J.H. and the Elizabeth Glaser Pediatric AIDS Foundation. T.J.H. is an Elizabeth Glaser Scientist.
Footnotes
Published ahead of print on 18 April 2007.
REFERENCES
- 1.Berthier-Vergnes, O., F. Bermond, V. Flacher, C. Massacrier, D. Schmitt, and J. Peguet-Navarro. 2005. TNF-alpha enhances phenotypic and functional maturation of human epidermal Langerhans cells and induces IL-12 p40 and IP-10/CXCL-10 production. FEBS Lett. 579:3660-3668. [DOI] [PubMed] [Google Scholar]
- 2.Blauvelt, A., S. Glushakova, and L. B. Margolis. 2000. HIV-infected human Langerhans cells transmit infection to human lymphoid tissue ex vivo. AIDS 14:647-651. [DOI] [PubMed] [Google Scholar]
- 3.Blauvelt, A., S. I. Katz, and M. C. Udey. 1995. Human Langerhans cells express E-cadherin. J. Investig. Dermatol. 104:293-296. [DOI] [PubMed] [Google Scholar]
- 4.Boggiano, C., N. Manel, and D. R. Littman. 2007. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J. Virol. 81:2519-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, E. M., O. Perez, M. Melar, and T. J. Hope. 2007. Labeling HIV-1 virions with two fluorescent proteins allows identification of virions that have productively entered the target cell. Virology 360:286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavrois, M., J. Neidleman, J. F. Kreisberg, and W. C. Greene. 2007. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathogens 3:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Witte, L., A. Nabatov, M. Pion, D. Fluitsma, M. A. W. P. de Jong, T. de Gruijl, V. Piguet, Y. van Kooyk, and T. B. H. Geijtenbeek. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13:367-371. [DOI] [PubMed] [Google Scholar]
- 8.Doms, R. W., and S. C. Peiper. 1997. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology 235:179-190. [DOI] [PubMed] [Google Scholar]
- 9.Frank, I., and M. Pope. 2002. The enigma of dendritic cell-immunodeficiency virus interplay. Curr. Mol. Med. 2:229-248. [DOI] [PubMed] [Google Scholar]
- 10.Garcia, E., M. Pion, A. Pelchen-Matthews, L. Collinson, J. F. Arrighi, G. Blot, F. Leuba, J. M. Escola, N. Demaurex, M. Marsh, and V. Piguet. 2005. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6:488-501. [DOI] [PubMed] [Google Scholar]
- 11.Garcia, F., B. Galocha, J. A. Villadangos, J. R. Lamas, J. P. Albar, A. Marina, and J. A. Lopaz de Castro. 1997. HLA-B27 (B*2701) specificity for peptides lacking Arg2 is determined by polymorphism outside the B pocket. Tissue Antigens 49:580-587. [DOI] [PubMed] [Google Scholar]
- 12.Gummuluru, S., M. Rogel, L. Stamatatos, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 77:12865-12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamura, T., F. O. Gulden, M. Sugaya, D. T. McNamara, D. L. Borris, M. M. Lederman, J. M. Orenstein, P. A. Zimmerman, and A. Blauvelt. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. USA 100:8401-8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura, T., S. E. Kurtz, A. Blauvelt, and S. Shimada. 2005. The role of Langerhans cells in the sexual transmission of HIV. J. Dermatol. Sci. 40:147-155. [DOI] [PubMed] [Google Scholar]
- 15.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 16.Leslie, D. S., M. S. Vincent, F. M. Spada, H. Das, M. Sugita, C. T. Morita, and M. B. Brenner. 2002. CD1-mediated gamma/delta T cell maturation of dendritic cells. J. Exp. Med. 196:1575-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantegazza, A. R., M. M. Barrio, S. Moutel, L. Bover, M. Weck, P. Brossart, J.-L. Teillaud, and J. Mordoh. 2004. CD63 tetraspanin slows down cell migration and translocates to the endosomal-lysosomal-MIICs route after extracellular stimuli in human immature dendritic cells. Blood 104:1183-1190. [DOI] [PubMed] [Google Scholar]
- 18.McDermott, R., U. Ziylan, D. Spehner, H. Bausinger, D. Lipsker, M. Mommaas, J. P. Cazenave, G. Raposo, B. Goud, H. de la Salle, J. Salamero, and D. Hanau. 2002. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol. Biol. Cell 13:317-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald, D., M. A. Vodicka, G. Lucero, T. M. Svitkina, G. G. Borisy, M. Emerman, and T. J. Hope. 2002. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 159:441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 21.Miller, C. J., and R. J. Shattock. 2003. Target cells in vaginal HIV transmission. Microbes Infect. 5:59-67. [DOI] [PubMed] [Google Scholar]
- 22.Popov, S., A. L. Chenine, A. Gruber, P. L. Li, and R. M. Ruprecht. 2005. Long-term productive human immunodeficiency virus infection of CD1a-sorted myeloid dendritic cells. J. Virol. 79:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramazzotti, E., A. Marconi, M. C. Re, G. Girolomoni, G. Cenacchi, M. Vignoli, G. Zambruno, G. Furlini, M. La Placa, and A. Giannetti. 1995. In vitro infection of human epidermal Langerhans' cells with HIV-1. Immunology 85:94-98. [PMC free article] [PubMed] [Google Scholar]
- 24.Reece, J. C., A. J. Handley, E. J. Anstee, W. A. Morrison, S. M. Crowe, and P. U. Cameron. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romani, N., S. Holzmann, C. H. Tripp, F. Koch, and P. Stoitzner. 2003. Langerhans cells-dendritic cells of the epidermis. APMIS 111:725-740. [DOI] [PubMed] [Google Scholar]
- 26.Sabet, S., M.-T. Ochoa, P. A. Sieling, T. H. Rea, and R. L. Modlin. 2007. Functional characterization of a T-cell receptor BV6+ T-cell clone derived from a leprosy lesion. Immunology 120:354-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76:7812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 29.Sivard, P., W. Berlier, B. Picard, O. Sabido, C. Genin, and L. Misery. 2004. HIV-1 infection of Langerhans cells in a reconstructed vaginal mucosa. J. Infect. Dis. 190:227-235. [DOI] [PubMed] [Google Scholar]
- 30.Sugaya, M., K. Lore, R. A. Koup, D. C. Douek, and A. Blauvelt. 2004. HIV-infected Langerhans cells preferentially transmit virus to proliferating autologous CD4+ memory T cells located within Langerhans cell-T cell clusters. J. Immunol. 172:2219-2224. [DOI] [PubMed] [Google Scholar]
- 31.Sugita, M., P. J. Peters, and M. B. Brenner. 2000. Pathways for lipid antigen presentation by CD1 molecules: nowhere for intracellular pathogens to hide. Traffic 1:295-300. [DOI] [PubMed] [Google Scholar]
- 32.Turville, S. G., J. Arthos, K. MacDonald, G. Lynch, H. Naif, G. Clark, D. Hart, and A. L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood 98:2482-2488. [DOI] [PubMed] [Google Scholar]
- 33.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 34.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 35.Villadangos, J. A., and W. R. Heath. 2005. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Semin. Immunol. 17:262-272. [DOI] [PubMed] [Google Scholar]
- 36.Wiley, R. D., and S. Gummuluru. 2006. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. USA 103:738-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zambruno, G., A. Giannetti, U. Bertazzoni, G. Girolomoni, E. Ramazzotti, A. Marconi, M. C. Re, G. Cenacchi, M. Vignoli, G. Furlini, and M. La Placa. 1995. Langerhans cells and HIV infection. In vitro infection of human epidermal Langerhans' cells with HIV-1. Immunol. Today 16:520-524.7495488 [Google Scholar]