Abstract
The abundant R2 mRNA encoded by the single left-end promoter of Aleutian mink disease parvovirus is tricistronic; it not only expresses the capsid proteins VP1 and VP2 but is also the major source for the nonstructural protein NS2. A cis-acting sequence within the NS2 gene was shown to be required for efficient capsid protein production, and its effect displayed a distinct location dependence. Ribosome transit through the upstream NS2 gene region was necessary for efficient VP1 and VP2 expression; however, neither ablation nor improvement of the NS2 initiating AUG had an effect on capsid protein production, suggesting that the translation of the NS2 protein per se had little influence on VP1 and VP2 expression. Thus, proper control of the alternative translation of the tricistronic R2 mRNA, a process critical for viral replication, is governed in a complex manner.
Aleutian mink disease parvovirus (AMDV) is an autonomous parvovirus (genus Amdovirus) (8, 12) that causes a number of important clinical and pathological syndromes in mink, including abortion, acute pneumonia in neonatal mink, and chronic immune complex-mediated glomerulonephritis and arteritis in adult mink (1, 3, 4, 14, 23). Strain AMDV-G is a variant of strain Utah 1, which was adapted to grow permissively in Crandell feline kidney cells (CrFK) at 32°C and is nonpathogenic in adult mink (8). It has been demonstrated that AMDV-G, similar to human parvoviruses in the genus of Erythrovirus (20, 22, 33), uses a single promoter at the left-hand end of the genome (24). This promoter encodes a pre-mRNA that is processed by alternative splicing and alternative polyadenylation to generate six mRNAs (24) (Fig. 1). Three different splicing patterns are used for AMDV-G RNA, and each type is found polyadenylated either at the 3′ end of the genome [the distal site (pA)d] or at a site in the center of the genome [the proximal site (pA)p] (24). Use of the internal polyadenylation [poly(A)] site (pA)p precludes the inclusion of the capsid-encoding open reading frame (ORF), and so the decision to either polyadenylate at (pA)p or read through to the distal site is critical to capsid protein production and hence the viral life cycle. The relative abundance of AMDV-G mRNAs does not change throughout infection (24). The R1 and R1′ RNAs retain the NS1 coding region intact, while this region is spliced out of mRNAs R2 and R2′ and in a different manner from that of Rx and Rx′, as shown in Fig. 1. R2 mRNA is the predominant mRNA found throughout productive AMDV-G infection of the CrFK cells (24, 31). Furthermore, R2 mRNA contains ORFs for the viral capsid proteins VP1 and VP2 as well as the NS2 protein (24). VP1 is initiated from what, by inspection, seems to be a weak initiating AUG (18, 19), while the initiating AUG for VP2 would be predicted to be stronger. We previously demonstrated that the accumulation of R2 mRNA correlates with the level of VP1 and VP2 expression during infection of CrFK cells and that only R2 mRNA expresses VP1 and VP2 (24).
FIG. 1.
Genetic map of AMDV-G. The genome of AMDV-G is shown to scale, with the major transcription units indicated, including the inverted hairpin termini (TR), promoters, splice donors (D1, D2, and D3), and acceptors (A1, A2, and A3) as well as the internal proximal and distal polyadenylation sites [(pA)p and (pA)d, respectively]. The AMDV-G proteins that are predicted to be encoded from each RNA are indicated. The sizes of all RNAs, minus polyadenylated tails, are given in parentheses (in nucleotides). Within the nonstructural region, the black bar indicates NS1-encoding ORF1, the striped box indicates NS3-encoding ORF2, and the gray box indicates NS2-encoding ORF3. The large capsid protein-encoding ORF shared by VP1 and VP2 in the right-hand end of the genome is read in ORF3, which is indicated by an open box.
In this study, we demonstrate that R2 mRNA not only expresses the capsid proteins VP1/VP2 but is also the major source for the nonstructural protein NS2. A cis-acting sequence within the NS2 gene was found to be required for efficient capsid protein production, and this element displayed a distinct location dependence. While ribosome transit through the NS2 gene region was necessary for efficient capsid protein production, translation of NS2 gene ORF had little effect on VP1 and VP2 production. Since VP1, VP2, and NS2 are all generated by R2 mRNA, their relative levels of production must be governed by a highly controlled mechanism of translation initiation.
MATERIALS AND METHODS
Cells and transfection methods.
CrFK cells (ATCC CCL-94) were maintained in Dulbecco's modified Eagle's medium with 10% fetal calf serum at 37°C in 5% CO2. Cells were transfected with 1 μg of DNA/well of a six-well plate with Lipofectamine Plus reagent (Gibco BRL) as previously described (20).
Plasmids. (i) Construction of AMDV expression plasmids.
All nucleotide numbers for AMDV-G used in the paper refer to GenBank accession no. M20036 (6). CMV-NS2-Cap and CMV-HA-NS2-Cap were renamed for simplicity and refer to plasmids CMVΔD1A2Cap and CMVΔD1A2CapNSHA, respectively, which have been previously described (24). CMV-HA-NS2-Cap(pA)p(−) was created by ablating the AAUAAA signal of (pA)p. CMV-λ330-Cap was constructed by replacing the NS2 coding region with the λ phage DNA sequence (nucleotides [nt] 10950 to 11279 containing no ATG in CMV-NS2-Cap, as diagrammed in Fig. 3C) (the region surrounding VP1 ATG from nt 2191 was left intact). CMV2081Cap and CMV2121 were constructed by deleting AMDV-G nt 180 to 2080 and nt 180 to 2120, respectively, from CMV-NS2-Cap, as diagrammed in Fig. 3C. Insertion of λ DNA sequences at nt 24561 to 24763 and nt 24390 to 24763 (containing no ATG) into nt 2205 in CMV-NS-Cap generated CMV-NS2-λ200-Cap and CMV-NS2-λ370-Cap, respectively, as diagrammed in Fig. 4C (the region surrounding VP1 ATG from nt 2191 was left intact). CMV-NS2mATG-Cap was generated by the mutation of the NS2 AUG to GGG, and CMV-NS2ATG(+)-Cap was generated by changing the region upstream of the NS2 AUG to improve its Kozak consensus signal (AGT AAC AUG G to GCC ACC AUG G [initiating AUG underlined]) in the parent construct CMV-NS2-Cap. Insertion of five repeats of the hairpin-forming sequence GGATCC (28, 35) into nt 200, 320, 380, and 2059 in CMV-NS-Cap generated CMV-5×Bam/nt200-NS2-Cap, CMV-NS2-5×Bam/nt320-Cap, CMV-NS2-5×Bam/nt380-Cap, and CMV-NS2-5×Bam/nt2059-Cap, respectively, as diagrammed in Fig. 6. The T7-driven constructs T7-NS2-Cap and T7-NS2(-ATG)-Cap were similar to the cytomegalovirus (CMV) constructs but contain the T7 promoter and have the intron (D3-A3) removed, as diagrammed in Fig. 5B. T7mNS2-Cap and T7 mNS2(-ATG)-Cap contain the same λ ORF insertion as CMV-λ330-Cap described above. For in vitro transcription, the plasmid was linearized by XbaI digestion.
FIG. 3.
Substitution or deletion of NS2 reduces VP1 and VP2 expression. The constructs used for this experiment are shown in C and are designated a to d, as are the relevant ORFs used and the potential products generated. The protein products generated by these constructs, as indicated above the lanes, are shown in the Western blot in A. VP1, VP2, β-actin, VPx, and GFP are indicated. (B) RNase protection assays using probe A3 (lanes 1 to 4) and probe P(pA)p (lanes 5 to 8) were performed on cytoplasmic RNA taken from the same experiments, showing that all constructs generate similar amounts of cytoplasmic RNA and a similar ratio of (pA)p to (pA)d. Probes are diagrammed in C. Expression levels of HA-tagged GFP and β-actin (in lanes 1 and 2) were used as an internal control; protein expression is shown at the bottom of A, and RNA accumulation is shown as the GFP-HA band in B. AMDV-G RNA is indicated as unspliced (Unspl), spliced (Spl), RNA polyadenylated at (pA)p [(pA)p], and RNA polyadenylated at (pA)d [(pA)d].
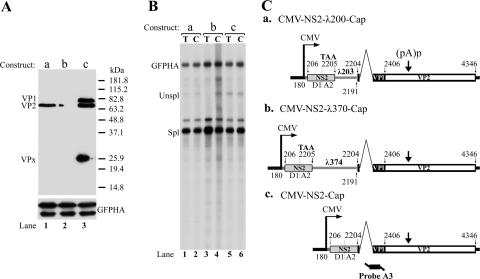
FIG. 4.
Effect of the NS2 cis element on the expression of VP1 and VP2 is location dependent. The constructs used, as described in the text, are shown in C and are designated a to c. The relevant ORFs used, the inserted heterologous λ DNA fragments, and an indication of the improved NS2 AUG signal are also shown. The protein products generated by these constructs are shown in the Western blot in A. VP1, VP2, and VPx are indicated. B shows an RNase protection assay of total and cytoplasmic RNA taken from the same experiment using probe A3, which is diagrammed at the bottom of C. Expression of HA-tagged GFP was used as an internal control, protein expression is shown at the bottom of A, and RNA accumulation is shown as the GFP-HA band in B. Unspl, unspliced; Spl, spliced; T, total RNA; C, cytoplasmic RNA.
FIG. 6.
Hairpin structures within the NS2 gene region block VP1 and VP2 expression. The parent plasmid (CMV-NS2-Cap) and mutants described in the text with hairpin insertions in the 5′ UTR (CMV-5×Bam/nt200-NS2-Cap), the beginning of the NS2 second exon (CMV-NS2-5×Bam/nt2059-Cap), the middle of the NS2 first exon (CMV-NS2-5×Bam/nt320-Cap), and the end of the NS2 first exon (CMV-NS2-5×Bam/nt380-Cap) are shown in C and are designated a to e, respectively. A cartoon showing where a ribosome would initiate at the 5′ end of the message is also depicted. The protein products generated following transfection of these constructs are shown in the Western blot in A. VP1, VP2, and VPx are indicated. B shows an RNase protection assay of cytoplasmic RNA taken from the same experiment, using probe A3, which is diagrammed in C (panel e). Expression of HA-tagged GFP was used as an internal control, protein expression is shown at the bottom of A, and cytoplasmic RNA accumulation is shown as the GFP-HA band in B. Unspl, unspliced; Spl, spliced.
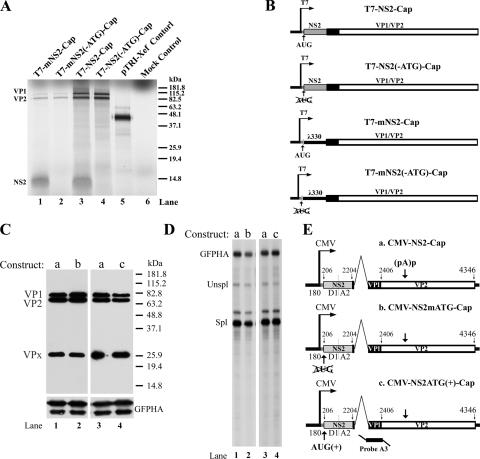
FIG. 5.
R2 mRNA generates NS2 and VP1/VP2 in vitro, and translation of the NS2 ORF does not affect proper capsid protein production. T7-driven constructs used for the generation of mRNA, as described in the text, are shown in B. RNA was transcribed in vitro and was capped and poly(A) tailed. Rabbit reticulocyte lysate was used for in vitro translation in the presence of [35S]methionine. Translated proteins were resolved by SDS-10% PAGE gels (A). The gel was dried and imaged by autoradiography. Individual bands of VP1, VP2, and NS2 are indicated along with their respective sizes. Lane 5 shows a positive in vitro translation control (Xef mRNA; supplied by the manufacturer), and lane 6 is a mock control. CMV-driven R2-expressing construct CMV-NS2-Cap (E, panel a) and the NS2 ATG mutants described in the text (E, panels b and c) were transfected into CrFK cells. Forty-eight hours posttransfection, total cell lysates from transfections using plasmids as indicated were resolved on SDS-10% PAGE gels and analyzed by Western blotting using antibody against the capsid proteins (C). The VP1, VP2, and VPx protein bands are indicated. Cytoplasmic RNA extracted from the same experiment was analyzed by RNase protection assay (D) using probe A3, which is diagrammed in E (panel c). An HA-tagged GFP expression plasmid was cotransfected as a control, and protein expression and RNA expression are indicated (C and D).
(ii) Clones used to generate probes for RNase protection assay.
Probe P(pA)p was described previously (24). The clone for the homologous P(pA)p probe used for the detection of RNAs generated from CMV-HA-NS2-Cap(pA)p(−) was constructed by cloning nt 2518 to 2669 from that construct into BamHI-HindIII-digested pGEM4Z (Promega). Probe A3 was constructed by cloning nt 2241 to 2445 of AMDV-G into BamHI-HindIII-digested pGEM4Z (Promega).
All of the DNA constructs were sequenced at the DNA core facility of the University of Missouri—Columbia to ensure that they were as predicted.
RNA isolation and RNase protection assay.
Total RNA or cytoplasmic RNA was isolated using guanidine isothiocyanate and purified by CsCl ultracentrifugation as previously reported (27). RNase protection assays were performed with 10 μg of total RNA as previously described (21, 27), and signals were quantified with Fuji FLA 3000 and Fuji Multi Gauge v2.3 software (FUJIFILM Medical Systems USA, Inc.). Relative molar ratios of individual species of RNAs were determined after adjustment for the number of 32P-labeled uridines in each protected fragment as previously reported (27).
Western blot analysis.
Western blotting was performed on cell lysates taken 48 h posttransfection as previously described (20). A monoclonal antibody to AMDV VP2 amino acids 428 to 446 (hybridoma cell line 282.20.1.4) (7) was used to detected AMDV capsid proteins, and monoclonal antibody HA-7 (Sigma, St. Louis, MO) was used to detect all hemagglutinin (HA)-tagged proteins. Monoclonal antibody mAbCam8224 (AbCam, Ltd.) was used to detect β-actin as an internal control.
In vitro translation.
Capped R2 RNA was generated by T7 RNA polymerase from 1 μg of XbaI-linearized DNA of T7-NS2-Cap, T7NS2(-ATG)-Cap, T7-mNS2-Cap, T7-mNS2(-ATG)-Cap, and the control pTRI-Xef (see Fig. 5) using the mMESSAGE mMACHINE High Yield Capped RNA transcription kit (Ambion). A poly(A) tail was added to these capped R2 RNAs using a poly(A)-tailing kit (Ambion). Finally, 5 μg of this R2 mRNA was incubated with rabbit reticulocyte lysate in the presence of [35S]methionine according to the manufacturer's instructions (Ambion). Five microliters of the translation reaction mixture was dissolved in loading buffer, resolved by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE), and detected by autoradiography.
RESULTS
The AMDV-G NS2, VP1, and VP2 proteins are all encoded by R2 mRNA.
As mentioned above, we previously demonstrated that all AMDV-G RNAs are encoded by a single promoter and that the abundant R2 mRNA, which we showed was capable of encoding NS2, is the sole source of the capsid proteins VP1 and VP2 (24). For those experiments, we used a construct that expressed two RNAs: the R2 mRNA and the internally polyadenylated R2′ mRNA, which, although lacking the capsid protein-encoding ORF, was also theoretically capable of encoding NS2 (CMV-HA-NS2-Cap) (Fig. 2C, panel a). To determine which of these RNAs was the primary source of NS2, we debilitated the internal poly(A) site by mutation in the R2-plus-R2′ expression vector [CMV-HA-NS2-Cap-(pA)p(−)] (Fig. 2C, panel b) to preclude the expression of R2′. As can be seen in Fig. 2B (lane 4), this mutant construct now generated little to no R2′ RNA but generated as much capsid protein (Fig. 2A, lane 2) and NS2 (Fig. 2A, lane 4) as did the wild-type expression construct (compare Fig. 2A and B, lanes 1 and 3). This result demonstrated that capsid protein-encoding R2 mRNA generated the majority of the NS2 in the cell, along with being the sole source of VP1 and VP2. Accumulation of cytoplasmic RNA, assayed by RNase protection assay (Fig. 2B, lanes 1 and 2) (probe A3), was unchanged. In addition, we have verified that there is no additional splicing in this area that could generate an additional mRNA, nor is there an additional promoter in the area that could account for these results (24; data not shown).
FIG. 2.
The R2 mRNA encodes both NS2 and capsid proteins VP1 and VP2. CMV-driven AMDV-G R2-expressing constructs CMV-HA-NS2-Cap and CMV-HA-NS2Cap(pA)p(−), diagrammed in C and designated a and b, respectively, were transfected into CrFK cells. The location of the inserted HA tag is shown. The predicted RNA products that they generate, their relevant ORFs, and their protein products are indicated. Two days following transfection, total cell lysates were resolved on SDS-10% PAGE gels and analyzed by Western blotting using antibodies against either VP1/VP2 (lanes 1 and 2) or the HA-tagged NS2 protein (lanes 3 and 4) (A). An HA-tagged GFP plasmid was cotransfected as a control (lanes 3 and 4). VP1, VP2, GFP, and NS2 protein bands are indicated. The small band at approximately 25 kDa in lanes 1 and 2, which also appears in other figures, marked VPx, is a specific caspase cleavage product of the capsid proteins, which will be described elsewhere (Qiu and Pintel, unpublished). (B) RNase protection analysis of total RNA protected by probe A3 (lanes 1 and 2) and probe P(PA)p (lane 3 and 4). Probes and their protected bands are diagramed at the bottom of C (panel b), with their respective sizes given in nucleotides. Unspl, unspliced; Spl, spliced.
A location-dependent cis-acting sequence within the NS2 gene is required for proper production of VP1 and VP2.
What role did the upstream NS2 gene play in expression from the tricistronic R2 of the downstream capsid protein genes? When the NS2 gene was replaced with an ORF from phage lambda DNA (CMV-λ330-Cap), capsid protein production was reduced to less than 10% (Fig. 3A, compare the mutant [lane 2] to the parent CMV-NS2-Cap [lane 1]). The lambda ORF (containing no termination codons) was inserted immediately downstream of the NS2 initiating AUG, extending in frame until the authentic NS2 terminating TGA. The initiating AUG of VP1 was left intact. Neither cytoplasmic accumulation of mRNA (Fig. 3B, lanes 1 and 2) nor the ratio of (pA)p to (pA)d (Fig. 3B, lanes 5 and 6) was affected by the insertion. In addition, a second inserted sequence had the same effect (data not shown), ruling out the possibility that our mutation had introduced a negative element.
Deletions of the NS2 gene sequences were also made. When the CMV promoter was brought to nt 2081 or nt 2121, leaving either 123 nt or 83 nt upstream of the VP1 AUG, VP1 and VP2 expression was drastically decreased (Fig. 3A, lanes 4 and 5), although the accumulation of cytoplasmic RNA remained normal (Fig. 3B, lanes 2 to 4). Importantly, these NS2 mutations had no effect on the ratio of (pA)p to (pA)d (Fig. 3B, lanes 6 to 8). These results demonstrated that the NS2 gene contains an element required for efficient downstream capsid protein translation.
We next sought to determine whether the element within the NS2 gene required for capsid protein production exhibited a location dependence for its effect. When the NS2 gene was separated from the VP1 initiation site by insertions of either 203 or 374 nt (CMV-NS2-λ200-Cap and CMV-NS2-λ370-Cap, respectively), capsid protein production was reduced proportionally to the size of the insertion (Fig. 4, lanes 1 and 2). The inserted sequence left the TGA of NS2 and the initiating codon for VP1 intact and contained no termination codons. The splicing efficiency of the small intron and cytoplasmic accumulation of mRNA were unaffected (Fig. 4B), and the (pA)p-to-(pA)d ratio was not changed (data not shown). These results suggested that the element within the NS2 gene required for efficient VP1/2 translation exhibited a location dependence and was more effective when near the initiating AUG for VP1.
R2 mRNA generates NS2 and VP1/VP2 in vitro, and translation of the NS2 ORF does not affect proper capsid protein production.
As another way to confirm that the R2 mRNA is tricistronic, we examined its ability to program the production of NS2, VP1, and VP2 in an in vitro translation assay. Capped R2 RNA was generated by the T7 promoter from construct T7-NS2-Cap (diagrammed in Fig. 5B) using the T7 in vitro transcription kit, and a poly(A) tail was added. This R2 mRNA was incubated with rabbit reticulocyte lysate in the presence of [35S]methionine. After autoradiography, the bands of VP1, VP2, and NS2 were abundantly evident (Fig. 5A, lane 3). The relative ratio of expression was determined to be approximately 1:1:2 by phosphorimage analysis. The ratio of VP1 to VP2 was similar to levels seen following transfection of CrFK cells. The identity of the NS2 band was confirmed by the absence of its expression from mRNA generated from the template T7-NS2(-ATG)-Cap in which the NS2 AUG was mutated (Fig. 5A, lane 4, and construct diagrammed in Fig. 5B), and the identity of VP1 and VP2 was confirmed by Western blot analysis (data not shown). These results demonstrated that both the nonstructural protein NS2 and the structural proteins VP1 and VP2 are expressed in vitro from the tricistronic R2 mRNA and also indicated that the translation of the NS2 ORF was not essential for the translation of VP1 and VP2 in vitro.
The same AUG knockout mutation was then introduced into the CMV-NS2-Cap expression construct (Fig. 5E, panel a) to generate CMV-NS2mATG-Cap (Fig. 5E, panel b). As can be seen in Fig. 5C, similar to results obtained in vitro, this mutant generated levels of capsid proteins equivalent to those of the wild-type parent vector following transfection of CrFK cells. The accumulation of cytoplasmic RNA assayed by RNase protection assay was unchanged (Fig. 5D, lanes 1 and 2, using probe A3; diagrammed in Fig. 5E, panel c). In addition, improvement of the NS2 AUG [CMV-NS2ATG(+)-Cap] (Fig. 5E, panel c) had little effect on capsid protein production (Fig. 5C, lane 4). Together, these results suggested that the translation of the NS2 ORF per se had little effect on capsid protein accumulation, and thus, it was unlikely that a simple leaky-scanning-type model governed the translation of VP1 from NS2-containing R2.
NS2 cis-acting sequences were also found to be important for the translation of VP1 and VP2 in vitro. Translation in vitro of VP1 and VP2 from RNA generated by the T7-NS2-Cap and T7-NS2(-ATG)-Cap constructs, which had been modified such that their NS2 genes had been replaced with the same ORF from phage λ as described above [to generate T7-mNS2-Cap and T7-mNS2(-ATG)-Cap, respectively] (diagrammed in Fig. 5B), was significantly reduced (∼10-fold) compared to the translation of RNA generated by the parent constructs (Fig. 5A, compare lanes 1 and 2 to 3 and 4), consistent with our previous observation in vivo. Translation of the substituted phage λ ORF in these constructs was inconsequential for the expression of VP1 and VP2 (Fig. 5A, compare lanes 1 and 2).
Transit of ribosomes from the 5′ end of R2 mRNA is required for translation of both VP1 and VP2.
Although the translation of the NS2 gene per se had little effect on the expression of VP1 and VP2, we found that ribosome transit across the NS2 gene was necessary for the efficient translation of the downstream capsid proteins. A stable hairpin structure composed of five copies of the sequence GGATCC, which has a ΔG of −69.2 kcal/mol (28, 35), was inserted into the 5′ untranslated region (UTR) of the NS2 gene (CMV-5×Bam/nt200-NS2-Cap), within the NS2 coding region in the second exon immediately after the splice junction (CMV-NS2-5×Bam/nt2059-Cap), within the NS2 coding region in the middle of first exon (CMV-NS2-5×Bam/nt320-Cap), or within the NS2 coding region at the end of first exon (CMV-NS2-5×Bam/nt380-Cap) (Fig. 6C). All four mutations greatly reduced the production of the capsid proteins, with the insertion in the 5′ UTR having the greatest effect (Fig. 6A), although neither insertion had effects on the splicing or cytoplasmic accumulation of mRNAs (Fig. 6B). These results suggested that the transit of ribosomes from the 5′ end of the message was likely required for efficient translation downstream of both VP1 and VP2 and argued against the presence of a ribosome shunting-type mechanism, as seen for the translation of adenovirus structural proteins (35) and duck hepatitis B virus reverse transcriptase (28), or the presence of an internal ribosome entry site (IRES) in this region.
DISCUSSION
In this study, we have demonstrated that in CrFK cells, R2 mRNA of AMDV-G expresses not only the capsid proteins VP1 and VP2 but also the nonstructural NS2 protein. Thus, R2 is a tricistronic mRNA. R2 also programmed the production of NS2, VP1, and VP2 in vitro at levels similar to those seen following transfection. Deletion or replacement of the NS2 gene with a heterologous prokaryotic sequence dramatically reduced VP1/VP2 expression following transfection of CrFK cells in a manner independent of the cytoplasmic accumulation of RNA, and this effect was also manifest in vitro. These results suggested that there was a cis-acting element within the NS2 coding region that is important for the translation of the downstream capsid protein ORFs from R2. Neither ablation nor improvement of the NS2 AUG had detectable effects on capsid protein translation; however, the introduction of a stable hairpin into AMDV-G RNA in the 5′ UTR of the NS2 gene, and within the NS2 gene itself, prevented the generation of VP1 and VP2. This suggested that although the translation of the NS2 gene per se had little effect, ribosome transit through this region was important for capsid protein production.
Viruses often use unusual translation strategies to expand the coding capacity of the viral genome. This has been characterized primarily for RNA viruses. For example, the leaky-scanning model is used for the translation of the C, C/C′, and P proteins of Sendai virus (11); influenza B virus utilizes a translation reinitiation scheme to express the M1 and BM2 proteins from segment 7 mRNA (17); and a number of viruses, including picornavirus and hepatitis C virus, have well-characterized IRESs (15). DNA viruses also employ unusual translational strategies. Ribosome shunting has been well characterized as a mechanism regulating the translation of the adenovirus major late capsid protein gene cluster (35). The capsid proteins of the parvovirus adeno-associated virus and the densoviruses utilize leaky-scanning mechanisms to encode their capsid proteins (5, 32). Also, similar to what we have recently observed for the AMDV-G R2 mRNA, it has been reported that the E6, E7, and E1 ORFs of human papillomavirus type 18 have been shown to be translated from a single tricistronic mRNA (26, 29).
Expression of the AMDV-G capsid proteins VP1 and VP2 does not seem to fit either commonly used alternative translation mechanisms or the ribosome-shunting mechanism. While AMDV-G NS2, VP1, and VP2 are all abundantly expressed from the same R2 mRNA, the fact that making the NS2 AUG more of a consensus does not decrease abundant capsid protein translation suggests that the translation of the R2 mRNA does not proceed by a simple leaky-scanning model. Translation of NS2 is apparently not required for VP1 and VP2 expression, arguing against a translation reinitiation model. However, experiments introducing stable hairpin structures both within the 5′ UTR of R2 and in the middle of the NS2 ORF abolished the translation of VP1 and VP2. This observation argues strongly against either the presence of a ribosome-shunting mechanism that skips the NS2 AUG, of the type that is used for the regulated translation of the adenovirus structural proteins (35), or the presence of an IRES, although the transit of ribosomes from the 5′ end of the message is apparently required for the translation of both VP1 and VP2. It should be noted, however, that these results do not formally rule out the presence of an IRES, since it remains possible that the insertion of one of these hairpins may potentially destroy an IRES that may lie within the NS2 region.
How, then, is the NS2 AUG bypassed to initiate translation at the VP1 AUG? A modified leaky-scanning-type mechanism is most likely to be involved in VP1/VP2 expression. Such a mechanism would be unusual because it would involve (and require) the NS2 gene element to enhance leaky identification of the VP1 AUG. Formation of the 48S complex is critical for ribosome scanning through the 5′ UTR (13, 16), and perhaps the element within the NS2 gene that is required for enhancing capsid protein translation is ultimately required to enable the 48S complex to initiate translation at the capsid protein AUGs, perhaps by affecting its composition. This would represent a novel mechanism that may yield important insight into regulated mechanisms of translation initiation.
We have not yet completed the mapping of the capsid protein translation-enhancing NS2 gene element. Deletion of the upstream exon debilitates its function, but whether the element extends downstream into the 3′ NS2 exon, or whether the element might require splicing for its reconstitution, is yet to be determined.
There are significant differences among the five genera of the Parvovirinae in how their capsid protein-encoding RNAs are generated. Members of the Parvovirus and Dependovirus genera utilize an internal promoter to generate their capsid-encoding RNAs, which allows for the regulation of the accumulation of capsid protein-encoding mRNA during the synthesis phase of viral replication. These promoters have been shown to be activated at accelerating rates, as the levels of their transactivating inducers increase during infection (10, 34). This mechanism of regulation of the capsid genes is not available for three of the five genera of the Parvovirinae. Erythrovirus strain B19 was the first parvovirus genus to be shown to have a single promoter (22), and amdoviruses (24) and bocaviruses (J. Qiu and D. Pintel, unpublished data) also fall into this category. Parvoviruses, which have a single left-hand-end promoter, must govern their genetic diversity exclusively by posttranscriptional mechanisms.
The parvoviruses and dependoviruses, which utilize a separate promoter to generate their capsid-encoding RNAs, as well as the single-promoter erythroviruses, control the production of the appropriate relative levels of their individual capsid proteins via alternative splicing and, in some cases, alternative polyadenylation (25). These processing events generate the appropriate levels of individual capsid-encoding mRNAs that are necessary to program the production of proper relative levels of the two main capsid proteins. Thus, mRNAs that individually encode VP1 and VP2 (or VP3) are generated. Other than the expression of the minor VP2 protein of adeno-associated virus (5), no translation regulation of these monocistronic mRNAs has been detected or needs to be invoked to explain the observed levels of capsid proteins. These mRNAs are present at approximately the same relative levels seen for their encoded proteins. However, because following splicing, the single R2 mRNA encodes both AMDV-G capsid proteins VP1 and VP2 and, from its 5′ end, an essential small nonstructural protein, NS2, regulation of the steady-state levels of these critical proteins is governed at least to some extent by alternative translation.
The persistence of AMDV in host animals is characterized by, and perhaps requires, controlled low levels of capsid protein synthesis (2, 9, 30, 31), and how the translation of R2 mRNA is governed likely influences this process. How the NS2 sequence governs the translation of VP1 from a weak AUG signal and the translation of VP2 from a strong AUG signal and how the balance of expression among these three proteins is controlled remain critical issues in the regulation of AMDV-G capsid gene expression.
Acknowledgments
We thank Lisa Burger for excellent technical assistance. We are indebted to Marshall E. Bloom and Sonja Best (Rocky Mountain Laboratory, NIH, NIAID, Hamilton, MT) for providing cells, plasmids, and antibodies for this study.
This work was supported by PHS grants RO1 AI46458, RO1 AI56310, and RO1 AI21302 from the NIAID to D.P.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Alexandersen, S. 1986. Acute interstitial pneumonia in mink kits: experimental reproduction of the disease. Vet. Pathol. 23:579-588. [DOI] [PubMed] [Google Scholar]
- 2.Alexandersen, S., M. E. Bloom, and J. Wolfinbarger. 1988. Evidence of restricted viral replication in adult mink infected with Aleutian disease of mink parvovirus. J. Virol. 62:1495-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandersen, S., M. E. Bloom, J. Wolfinbarger, and R. E. Race. 1987. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J. Virol. 61:2407-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandersen, S., A. Uttenthal-Jensen, and B. Aasted. 1986. Demonstration of non-degraded Aleutian disease virus (ADV) proteins in lung tissue from experimentally infected mink kits. Arch. Virol. 87:127-133. [DOI] [PubMed] [Google Scholar]
- 5.Becerra, S. P., J. A. Rose, M. Hardy, B. M. Baroudy, and C. W. Anderson. 1985. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc. Natl. Acad. Sci. USA 82:7919-7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom, M. E., S. Alexandersen, S. Perryman, D. Lechner, and J. B. Wolfinbarger. 1988. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J. Virol. 62:2903-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom, M. E., S. M. Best, S. F. Hayes, R. D. Wells, J. B. Wolfinbarger, R. McKenna, and M. Gbandje-McKenna. 2001. Identification of Aleutian mink disease parvovirus capsid sequences mediating antibody-dependent enhancement of infection, virus neutralization, and immune complex formation. J. Virol. 75:11116-11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloom, M. E., R. E. Race, and J. B. Wolfinbarger. 1980. Characterization of Aleutian disease virus as a parvovirus. J. Virol. 35:836-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen, J., T. Storgaard, B. Viuff, B. Aasted, and S. Alexandersen. 1993. Comparison of promoter activity in Aleutian mink disease parvovirus, minute virus of mice, and canine parvovirus: possible role of weak promoters in the pathogenesis of Aleutian mink disease parvovirus infection. J. Virol. 67:1877-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotmore, S. F., and P. Tattersall. 2006. Structure and organization of the viral genome, p. 73-94. In J. R. Kerr, S. F. Cotmore, M. E. Bloom, M. E. Linden, and C. R. Parish (ed.), Parvoviruses. Hodder Arnold, London, United Kingdom.
- 11.Curran, J. A., C. Richardson, and D. Kolakofsky. 1986. Ribosomal initiation at alternate AUGs on the Sendai virus P/C mRNA. J. Virol. 57:684-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauquet, C. M., M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.). 2004. Virus taxonomy. Eighth report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, London, England.
- 13.Gingras, A. C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 14.Hadlow, W. J., R. E. Race, and R. C. Kennedy. 1983. Comparative pathogenicity of four strains of Aleutian disease virus for pastel and sapphire mink. Infect. Immun. 41:1016-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellen, C. U., and P. Sarnow. 2001. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 15:1593-1612. [DOI] [PubMed] [Google Scholar]
- 16.Hershey, J. W. B., and W. C. Merrick. 2000. Pathway and mechanism of initiation of protein synthesis, p. 33-88. In N. Sonenberg and J. W. B. Hershey (ed.), Translation control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 17.Horvath, C. M., M. A. Williams, and R. A. Lamb. 1990. Eukaryotic coupled translation of tandem cistrons: identification of the influenza B virus BM2 polypeptide. EMBO J. 9:2639-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozak, M. 1978. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell 15:1109-1123. [DOI] [PubMed] [Google Scholar]
- 19.Kozak, M. 1987. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol. Cell. Biol. 7:3438-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, Z., J. Qiu, F. Cheng, Y. Chu, Y. Yoto, M. G. O'Sullivan, K. E. Brown, and D. J. Pintel. 2004. Comparison of the transcription profile of simian parvovirus with that of the human erythrovirus B19 reveals a number of unique features. J. Virol. 78:12929-12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naeger, L. K., R. V. Schoborg, Q. Zhao, G. E. Tullis, and D. J. Pintel. 1992. Nonsense mutations inhibit splicing of MVM RNA in cis when they interrupt the reading frame of either exon of the final spliced product. Genes Dev. 6:1107-1119. [DOI] [PubMed] [Google Scholar]
- 22.Ozawa, K., J. Ayub, Y. S. Hao, G. Kurtzman, T. Shimada, and N. Young. 1987. Novel transcription map for the B19 (human) pathogenic parvovirus. J. Virol. 61:2395-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter, D. D., A. E. Larsen, and H. G. Porter. 1969. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J. Exp. Med. 130:575-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu, J., F. Cheng, L. R. Burger, and D. Pintel. 2006. The transcription profile of Aleutian mink disease virus (AMDV) in CRFK cells is generated by alternative processing of pre-mRNAs produced from a single promoter. J. Virol. 80:654-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu, J., Y. Yoto, G. E. Tullis, and D. Pintel. 2006. Parvovirus RNA processing strategies, p. 253-274. In J. R. Kerr, S. F. Cotmore, M. E. Bloom, M. E. Linden, and C. R. Parish (ed.), Parvoviruses. Hodder Arnold, London, United Kingdom.
- 26.Remm, M., A. Remm, and M. Ustav. 1999. Human papillomavirus type 18 E1 protein is translated from polycistronic mRNA by a discontinuous scanning mechanism. J. Virol. 73:3062-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoborg, R. V., and D. J. Pintel. 1991. Accumulation of MVM gene products is differentially regulated by transcription initiation, RNA processing and protein stability. Virology 181:22-34. [DOI] [PubMed] [Google Scholar]
- 28.Sen, N., F. Cao, and J. E. Tavis. 2004. Translation of duck hepatitis B virus reverse transcriptase by ribosomal shunting. J. Virol. 78:11751-11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stacey, S. N., D. Jordan, A. J. Williamson, M. Brown, J. H. Coote, and J. R. Arrand. 2000. Leaky scanning is the predominant mechanism for translation of human papillomavirus type 16 E7 oncoprotein from E6/E7 bicistronic mRNA. J. Virol. 74:7284-7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storgaard, T., J. Christensen, B. Aasted, and S. Alexandersen. 1993. cis-acting sequences in the Aleutian mink disease parvovirus late promoter important for transcription: comparison to the canine parvovirus and minute virus of mice. J. Virol. 67:1887-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storgaard, T., M. Oleksiewicz, M. E. Bloom, B. Ching, and S. Alexandersen. 1997. Two parvoviruses that cause different diseases in mink have different transcription patterns: transcription analysis of mink enteritis virus and Aleutian mink disease parvovirus in the same cell line. J. Virol. 71:4990-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tijssen, P., Y. Li, M. El-Far, J. Szelei, M. Letarte, and Z. Zadori. 2003. Organization and expression strategy of the ambisense genome of densonucleosis virus of Galleria mellonella. J. Virol. 77:10357-10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vashisht, K., K. S. Faaberg, A. L. Aber, K. E. Brown, and M. G. O'Sullivan. 2004. Splice junction map of simian parvovirus transcripts. J. Virol. 78:10911-10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward, P. 2006. Replication of adeno-associated virus DNA, p. 189-212. In J. R. Kerr, S. F. Cotmore, M. E. Bloom, M. E. Linden, and C. R. Parish (ed.), Parvoviruses. Hodder Arnold, London, United Kingdom.
- 35.Yueh, A., and R. J. Schneider. 1996. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev. 10:1557-1567. [DOI] [PubMed] [Google Scholar]