Abstract
The X gene of Borna disease virus (BDV) encodes a nonstructural 10-kDa protein that can interact with viral polymerase cofactor P, thus regulating polymerase activity. It remained unknown whether X is essential for virus multiplication. All our attempts to generate mutant BDV with a nonfunctional X gene proved unsuccessful. However, a mutant virus with an inactive X gene was able to replicate in Vero cells if an artificial gene cassette encoding X was inserted at a site near the 5′ end of the viral genome. These results indicate that X performs essential viral functions.
The genome of Borna disease virus (BDV) codes for at least six proteins, of which five are present in viral particles (3, 10, 11). The sixth protein, denominated X or p10, is a 10-kDa nonstructural polypeptide (15) that associates with the viral polymerase complex in infected cells (17) but is not required for the formation of an active polymerase complex in artificial minireplicon systems (7, 13). X can specifically bind to viral polymerase cofactor P with the help of an interaction domain located near its N terminus (18). Wild-type X, but not X mutants lacking a functional P interaction domain, inhibits polymerase activity in artificial minireplicon systems (8), indicating that X is a polymerase regulator. It remained unclear, however, whether X is essential for virus multiplication.
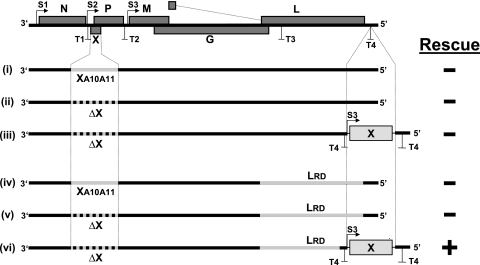
To address this question, we introduced specific mutations into the X gene of a full-length cDNA clone that was previously shown to yield infectious BDV upon transfection into suitable cells (6, 14). Since the X and P proteins are translated from overlapping reading frames of a single mRNA, analysis of X protein function is difficult, as mutations in X might influence the expression of P. In construct i, we intended to specifically inactivate the P interaction domain in X by mutating the amino acids at positions 10 and 11 (Fig. 1). Previous studies with a viral minireplicon system showed that mutant XA10A11 lost the ability to inhibit BDV polymerase activity (8). In construct ii, we inactivated the X initiation codon by changing AUG to GCG and blocked translation of the X open reading frame by further introducing termination codons at amino acid positions 11 and 12 (Fig. 1). At least five attempts were made to generate infectious virus from each of these two constructs. None of the attempts was successful (Fig. 1). The deleterious effect of X gene inactivation was not overcome if we used rescue constructs with a modified L polymerase gene (LRD) (Fig. 1, constructs iv and v), which strongly improves the multiplication of an attenuated virus expressing green fluorescent protein (GFP) (12). Appropriate wild-type constructs that were used in parallel yielded virus in each trial (data not shown), excluding trivial technical problems.
FIG. 1.
Schematic drawing showing the gene organization of wild-type BDV and the various genetic modifications introduced into full-length viral cDNA constructs. In constructs i and iv, the X protein was rendered nonfunctional by introducing alanine residues at positions 10 and 11 (XA10A11). In constructs ii, iii, v, and vi, the open reading frame of the X gene was destroyed by eliminating the initiation codon and further inserting termination codons at positions 11 and 12 (ΔX). For ectopic expression of X, the indicated gene cassette was inserted at a site near the 5′ end of the virus genome previously shown to tolerate insertion of a foreign gene (12). Two mutations that promote virus growth were introduced into the L gene (LRD) of constructs iv, v, and vi. Successful (+) and unsuccessful (−) attempts to rescue virus from the various constructs are indicated. Rescue attempts for the various constructs were repeated at least three times.
These results indicated that X serves essential functions and is required for virus multiplication in Vero cells. Since this preliminary conclusion was based on negative results, it remained possible that our failure to recover virus from the mutant constructs was not due to the lack of X gene expression but rather resulted from altered expression of P. It further remained possible that the few nucleotide changes introduced when the codons of X were altered had destroyed unrecognized structural elements in the viral RNA. If we had managed to rescue infectious virus from our mutant constructs by ectopic expression of X, these arguments would be greatly diminished.
For unknown reasons, all our attempts to generate a “helper” cell line that might express the X protein of BDV from a transgene were unsuccessful. We therefore considered the possibility of reconstituting the X protein function by inserting an X gene expression cassette at a site near the 5′ end of the viral genome as indicated in Fig. 1. Work published in the accompanying article by Schneider and coworkers (12) showed that this site can tolerate the insertion of a GFP expression unit. However, construct iii, encoding such an X gene expression cassette in combination with the wild-type L polymerase, failed to yield infectious BDV in several rescue attempts. In contrast, the analogous construct vi, encoding the LRD polymerase mutant instead of wild-type L, readily produced virus, although at strongly reduced titers, that could be isolated and transferred to new Vero and C6 cells. The corresponding recombinant virus was designated BDV-LRD-ΔX-X5′. The detection of comparable polymerase II-mediated transcription of antigenomic RNA from constructs v and vi in transfected 293T cells excluded the possibility of a trivial explanation for the lack of virus rescues from constructs i through v (data not shown).
Sequence analysis of PCR fragments derived from viral RNA confirmed the presence of all three disabling mutations in the endogenous X gene of BDV-LRD-ΔX-X5′ and further demonstrated that the ectopic X expression cassette was intact (data not shown). Northern blot analysis of RNA from Vero cells infected with either BDV-LRD-ΔX-X5′ or parental BDV-LRD virus was performed to verify the presence of transcripts derived from the ectopic expression cassette. Using a radiolabeled cDNA probe comprising the X open reading frame, we detected roughly normal levels of RNAs with sizes of 8.9 kb, 1.9 kb, and 0.8 kb (Fig. 2A). In cells infected with BDV-LRD-ΔX-X5′, we further observed a transcript of approximately 0.3 kb that was not present in cells infected with wild-type virus (Fig. 2A). The size of this transcript corresponded well to the expected size of mRNAs originating from the ectopic X expression cassette. Western blot analysis showed that cells infected with BDV-LRD-ΔX-X5′ contained easily detectable levels of X (Fig. 2B). When the signals were quantified and normalized to N levels, we noted that X was three- to fourfold less abundant in Vero cells infected with BDV-LRD-ΔX-X5′ than in cells infected with parental BDV-LRD. Interestingly, the P level was also about twofold reduced in BDV-LRD-ΔX-X5′-infected cells (Fig. 2B), indicating that the mutations which we introduced to eliminate the X open reading frame affected the translation efficacy of P.
FIG. 2.
Comparison of viral gene expression and growth characteristics of BDV-LRD and BDV-LRD-ΔX-X5′ in Vero cells. (A) Northern blot analysis of RNA (samples of 20 μg) from Vero cells infected with BDV-LRD or BDV-LRD-ΔX-X5′. The blot was hybridized with a radiolabeled DNA probe specific for the BDV-X open reading frame. Note the additional 0.3-kb transcript originating from the ectopic X gene in cells infected with BDV-LRD-ΔX-X5′. (B) Western blot analysis of extracts (samples of 5 μg) from Vero cells that were either not infected (n.i.) or infected with BDV-LRD and BDV-LRD-ΔX-X5′. Blots were stained with antibodies specific for actin or the viral proteins P, X, and N. (C) Cultures infected with either virus at a multiplicity of 0.01 per cell were split twice weekly, and the proportion of infected cells was determined by immunostaining for the BDV N protein as described previously (6).
Careful determination of virus growth showed that BDV-LRD-ΔX-X5′ spread substantially slower in Vero cell cultures than parental BDV-LRD (Fig. 2C). Due to the very low titers of BDV-LRD-ΔX-X5′, it was not possible to analyze the growth kinetics of BDV-LRD-ΔX-X5′ after infection of cells at a high multiplicity of infection. We therefore were unable to determine whether the strong attenuation of this virus is due to impaired RNA replication and gene expression or to impaired cell-to-cell spread. Finally, we tried to estimate the extent of attenuation of BDV-LRD-ΔX-X5′ by determining its replication competence in the brains of three newborn Lewis rats. In contrast to parental BDV-LRD or a BDV-LRD variant that expresses GFP (12), both of which propagated efficiently, BDV-LRD-ΔX-X5′ failed to productively infect newborn rats. Immunohistochemical analysis of brain sections of these animals, killed at 28 days postinfection, yielded no evidence of virus spread (data not shown), indicating that BDV-LRD-ΔX-X5′ was more severely attenuated in vivo than predicted from the growth analyses of Vero cells. Although the viral fitness and virulence/attenuation of a virus are not necessarily the same for a persistent virus like BDV, the fact that BDV-LRD-ΔX-X5′ failed to productively infect the highly susceptible newborn rats suggests that unbalanced expression or reduced levels of X protein strongly impair fitness.
Altogether, our study indicates that the X protein of BDV serves essential functions in the viral multiplication cycle. We assume that the apparent strict requirement of this nonstructural protein of BDV is related to the previously noted ability of X to regulate viral polymerase activity (7, 8, 13). In this context, it is of interest to note that the smear-like appearance of RNA derived from BDV-LRD-ΔX-X5′-infected Vero cells (Fig. 2A) was reproducible. This finding raises the possibility that reduced levels of X might result in modified processivity of the polymerase complex of BDV-LRD-ΔX-X5′, which would be in accordance with the postulated regulatory function of this protein. However, our study cannot exclude the possibility that X has additional activities, some of which might serve to modulate the antiviral response of the infected host. Such functions have recently been assigned to several small nonstructural proteins of other RNA viruses, like, for example, NSs of bunyaviruses (1, 16), V of paramyxoviruses (9), or NS1 of influenza A (4) or influenza B (2) virus. These viral proteins represent nonessential virulence factors that mainly seem to antagonize the interferon response of the host (5).
An interesting observation of this study was that insertion of an X expression cassette at an ectopic site in the viral genome yielded a virus that grew reasonably well in Vero cells but not rat brain. A possible explanation of this result might be that X gene expression in the BDV-LRD-ΔX-X5′ mutant is not as tightly linked to expression of P as in the wild-type situation, where the two proteins are translated from a single bicistronic mRNA. For unclear reasons, a balanced expression of these two proteins might be of most critical importance during virus growth in the central nervous system. In this context, it is of interest to note that the mRNA that codes for X and P has an unusually long 5′ nontranslated region, which is suspected to contain unrecognized regulatory elements (11).
Acknowledgments
We thank Rosita Frank for excellent technical assistance and Martin Schwemmle, Georg Kochs, and Otto Haller for comments on the manuscript.
This work was supported by grants SCHN 765/1-5 and STA 338/8-1 from the Deutsche Forschungsgemeinschaft.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Billecocq, A., M. Spiegel, P. Vialat, A. Kohl, F. Weber, M. Bouloy, and O. Haller. 2004. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol. 78:9798-9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dauber, B., G. Heins, and T. Wolff. 2004. The influenza B virus nonstructural NS1 protein is essential for efficient viral growth and antagonizes beta interferon induction. J. Virol. 78:1865-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Torre, J. C. 2006. Reverse-genetic approaches to the study of Borna disease virus. Nat. Rev. Microbiol. 4:777-783. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 5.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin, A., P. Staeheli, and U. Schneider. 2006. RNA polymerase II-controlled expression of antigenomic RNA enhances the rescue efficacies of two different members of the Mononegavirales independently of the site of viral genome replication. J. Virol. 80:5708-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez, M., A. Sanchez, B. Cubitt, D. Rosario, and J. C. de la Torre. 2003. A reverse genetics system for Borna disease virus. J. Gen. Virol. 84:3099-3104. [DOI] [PubMed] [Google Scholar]
- 8.Poenisch, M., G. Unterstab, T. Wolff, P. Staeheli, and U. Schneider. 2004. The X protein of Borna disease virus regulates viral polymerase activity through interaction with the P protein. J. Gen. Virol. 85:1895-1898. [DOI] [PubMed] [Google Scholar]
- 9.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 10.Schneemann, A., P. A. Schneider, R. A. Lamb, and W. I. Lipkin. 1995. The remarkable coding strategy of borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology 210:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Schneider, U. 2005. Novel insights into the regulation of the viral polymerase complex of neurotropic Borna disease virus. Virus Res. 111:148-160. [DOI] [PubMed] [Google Scholar]
- 12.Schneider, U., A. Ackermann, and P. Staeheli. 2007. A Borna disease virus vector for expression of foreign genes in neurons of rodents. J. Virol. 81:7293-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider, U., M. Naegele, P. Staeheli, and M. Schwemmle. 2003. Active Borna disease virus polymerase complex requires a distinct nucleoprotein-to-phosphoprotein ratio but no viral X protein. J. Virol. 77:11781-11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider, U., M. Schwemmle, and P. Staeheli. 2005. Genome trimming: a unique strategy for replication control employed by Borna disease virus. Proc. Natl. Acad. Sci. USA 102:3441-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwardt, M., D. Mayer, R. Frank, U. Schneider, M. Eickmann, O. Planz, T. Wolff, and M. Schwemmle. 2005. The negative regulator of Borna disease virus polymerase is a non-structural protein. J. Gen. Virol. 86:3163-3169. [DOI] [PubMed] [Google Scholar]
- 16.Weber, F., A. Bridgen, J. K. Fazakerley, H. Streitenfeld, N. Kessler, R. E. Randall, and R. M. Elliott. 2002. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 76:7949-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wehner, T., A. Ruppert, C. Herden, K. Frese, H. Becht, and J. A. Richt. 1997. Detection of a novel Borna disease virus-encoded 10 kDa protein in infected cells and tissues. J. Gen. Virol. 78:2459-2466. [DOI] [PubMed] [Google Scholar]
- 18.Wolff, T., R. Pfleger, T. Wehner, J. Reinhardt, and J. A. Richt. 2000. A short leucine-rich sequence in the Borna disease virus p10 protein mediates association with the viral phospho- and nucleoproteins. J. Gen. Virol. 81:939-947. [DOI] [PubMed] [Google Scholar]