Abstract
Infection with a recombinant murine-feline gammaretrovirus, MoFe2, or with the parent virus, Moloney murine leukemia virus, caused significant reduction in B-lymphoid differentiation of bone marrow at 2 to 8 weeks postinfection. The suppression was selective, in that myeloid potential was significantly increased by infection. Analysis of cell surface markers and immunoglobulin H gene rearrangements in an in vitro model demonstrated normal B-lymphoid differentiation after infection but significantly reduced viability of differentiating cells. This reduction in viability may confer a selective advantage on undifferentiated lymphoid progenitors in the bone marrow of gammaretrovirus-infected animals and thereby contribute to the establishment of a premalignant state.
Feline leukemia virus (FeLV) and murine leukemia virus (MuLV) are closely related gammaretroviruses that induce malignant tumors of hematopoietic and lymphoid tissues through a complex, multistep process. Previous studies have demonstrated the significant impact of gammaretrovirus infection on bone marrow hematopoiesis in the early stages of disease induction (1, 8, 11-13, 21, 28). For example, Moloney MuLV (M-MuLV) infection induces compensatory extramedullary hematopoiesis, a result of diminished support of hematopoiesis by bone marrow stromal cells (2, 6, 8, 24). Altered progenitor cell distribution has also been observed at 2 to 4 weeks postinfection with SL3-3 MuLV (21). Virus replication in the bone marrow, and consequent disruption of hematopoiesis, is also a hallmark of FeLV infection (1, 13, 14, 18, 20). In a recent longitudinal study, significant depression in circulating red blood cell counts and segmented neutrophils was observed in infected cats during the first 4 weeks postinoculation (4).
FeLV-945 is a unique isolate of FeLV naturally associated with malignant, degenerative, and proliferative disorders of non-T-cell origin. Experimental infection with a virus bearing the unique genetic hallmarks of FeLV-945 resulted in the rapid induction of a multicentric lymphoma of B-lymphoid origin, in contrast to the long-latency T-cell lymphomas characteristic of natural, horizontally transmissible FeLV (3, 4). The basis for the shift in tumor spectrum remains unknown; however, the long terminal repeat (LTR) element of FeLV-945 contains a unique repeat motif comprised of a 21-bp element triplicated in tandem beginning 25 bp downstream of the canonical transcriptional enhancer (5). To investigate the influence of the FeLV-945 LTR on bone marrow hematopoiesis, a murine-feline recombinant retrovirus was constructed by substituting the triplicate-containing U3 region of FeLV-945 for that of M-MuLV. The recombinant virus, termed MoFe2-MuLV (MoFe2), infects mice and induces T-cell lymphomas with kinetics comparable to those of M-MuLV (10, 23).
To determine whether MoFe2 infection causes extramedullary hematopoiesis and splenomegaly early in disease progression, neonatal NIH/Swiss mice were inoculated intraperitoneally with equivalent amounts of MoFe2 or M-MuLV as normalized by reverse transcriptase activity. Groups of four to six animals were sacrificed at regular intervals 2 to 8 weeks postinoculation, and spleen weight was determined as a percentage of body weight. The results demonstrated significantly greater spleen weights in M-MuLV-infected mice than in MoFe2-infected or mock-infected animals at 2, 4, 6, and 8 weeks postinoculation (P < 0.05 [one-way analysis of variance {ANOVA}]). For example, the average spleen weight in M-MuLV-infected mice was 3.6-fold greater at 2 weeks postinoculation and 2.1-fold greater at 4 weeks postinoculation than in MoFe2-infected or mock-infected animals. Spleen weights were indistinguishable between MoFe2-infected and mock-infected animals (data not shown). Thus, replacement of the M-MuLV LTR with that of FeLV-945 was found to influence pathogenesis in the early, preleukemic stage.
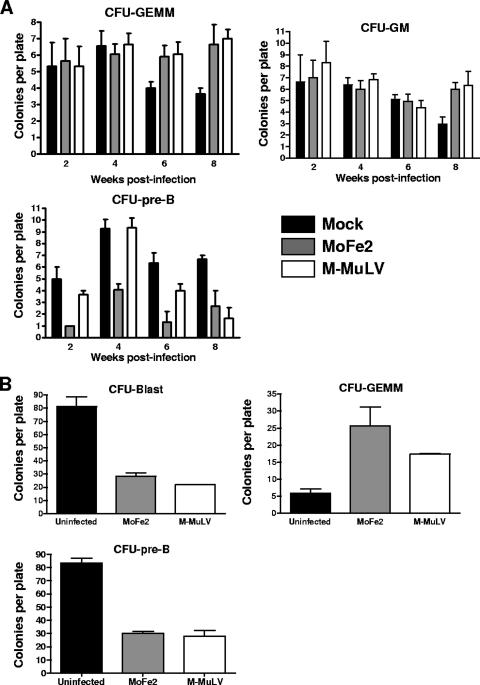
Bone marrow was also collected from each animal at 2, 4, 6, and 8 weeks postinoculation for use in progenitor cell colony-forming assays. For analysis of myeloid progenitors, unfractionated bone marrow from individual animals was deposited at a density of 2 × 104 nucleated cells per ml in triplicate into medium containing 50 ng/ml stem cell factor, 10 ng/ml interleukin 3 (IL-3), 10 ng/ml IL-6, and 3 units/ml erythropoietin in methylcellulose (MethoCult M3434; StemCell Technologies, Inc., Vancouver, Canada). Significantly increased CFU-granulocyte-erythrocyte-monocyte-megakaryocyte (GEMM) clonogenic potential was recovered from the bone marrow of animals infected with either virus at 6 and 8 weeks postinfection; increased CFU-granulocyte-monocyte/macrophage (GM) clonogenic potential was also detected in the bone marrow of infected animals at 8 weeks postinfection (Fig. 1A). Pre-B clonogenic progenitor cells were quantified by depositing unfractionated bone marrow from individual animals at a density of 5 × 104 nucleated cells per ml in triplicate into medium containing 10 ng/ml IL-7 in methylcellulose (MethoCult M3630; StemCell Technologies, Inc., Vancouver, Canada). In contrast to the myeloid hyperplasia, a significant decrease in CFU-pre-B-cell clonogenic potential was observed at 2, 4, and 6 weeks postinfection for MoFe2-infected mice and at 8 weeks postinfection for M-MuLV-infected mice (Fig. 1A). This observation represents the first report of suppression of the B-lymphoid differentiation potential in bone marrow from gammaretrovirus-infected animals during the preleukemic stage.
FIG. 1.
Colony-forming assays of bone marrow and EML cells following infection with MoFe2 or M-MuLV. (A) Unfractionated bone marrow collected at intervals after mock infection or infection with MoFe2 or M-MuLV was deposited in semisolid culture medium optimized for the differentiation and growth of myeloid colony-forming progenitors (CFU-GEMM, CFU-GM) or pre-B-cell colony-forming progenitors (CFU-pre-B). After incubation for 14 days (myeloid) or 7 days (pre-B lymphoid), colonies were typed and enumerated by light microscopy. Statistical analysis using one-way ANOVA showed significantly increased CFU-GEMM (P < 0.05) at 6 and 8 weeks postinfection with either virus, significantly increased CFU-GM (P < 0.05) at 8 weeks postinfection with either virus, significantly decreased CFU-pre-B (P < 0.05) at 2, 4, and 6 weeks postinfection with MoFe2, and significantly decreased CFU-pre-B (P < 0.05) at 8 weeks postinfection with M-MuLV compared to age-matched mock-infected control animals. (B) Infected and uninfected EML cells were stimulated with 10−5 M all-trans retinoic acid and 10 ng/ml IL-3 to remove the block to myeloid differentiation and were then plated in semisolid culture medium optimized for the differentiation and growth of myeloid colony-forming progenitors (CFU-GEMM). Undifferentiated blast colonies form under these conditions as well (CFU-Blast). EML cells were also plated in medium optimized for the differentiation and growth of pre-B-cell colony-forming progenitors (CFU-pre-B). After incubation for 14 days, colonies were typed and enumerated by light microscopy. Statistical analysis using one-way ANOVA showed significantly decreased CFU-Blast (P < 0.05), significantly increased CFU-GEMM (P < 0.05), and significantly decreased CFU-pre-B (P < 0.05) after infection with either virus.
Studies of the impact of gammaretrovirus infection on hematopoiesis have generally analyzed bone marrow removed directly from the animal or from long-term bone marrow cultures, both of which are complex, multicomponent systems. To simplify this analysis, we used the murine lymphohematopoietic progenitor cell line EML, a bone marrow-derived, stem cell factor-dependent progenitor cell line capable of differentiation along erythroid, myeloid, and lymphoid lineages. EML was established from normal mouse bone marrow by transducing a retrovirus vector that expresses a dominant-negative retinoic acid receptor (26). Previous reports reveal that EML cells represent a reliable and authentic model of hematopoietic progenitor lineage commitment and differentiation (7, 15, 19, 27).
To examine myeloid clonogenic potential, EML cells were stimulated with 10−5 M all-trans retinoic acid (Sigma, St. Louis, MO) and 10 ng/ml IL-3 (Peprotech, Rocky Hill, NJ) for 72 h and were then deposited in triplicate at 103 cells per ml into MethoCult M3434 medium. After 14 days of incubation, colonies were typed and enumerated by light microscopy. A significantly decreased number of undifferentiated CFU-blast colonies and a significantly increased number of CFU-GEMM were generated by cells infected with either virus (Fig. 1B). To quantify CFU-pre-B-cell clonogenic potential, EML cells were deposited in triplicate at 4 × 103 cells per ml into MethoCult M3630 medium supplemented with an additional 40 ng/ml IL-7 and 10 ng/ml bone morphogenetic protein 2 (BMP-2) (Peprotech, Rocky Hill, NJ). Colonies were typed and enumerated by light microscopy after 7 days of incubation. Consistent with observations of infected bone marrow, EML cells demonstrated significantly decreased CFU-pre-B-cell clonogenic potential after infection with either MoFe2 or M-MuLV (Fig. 1B).
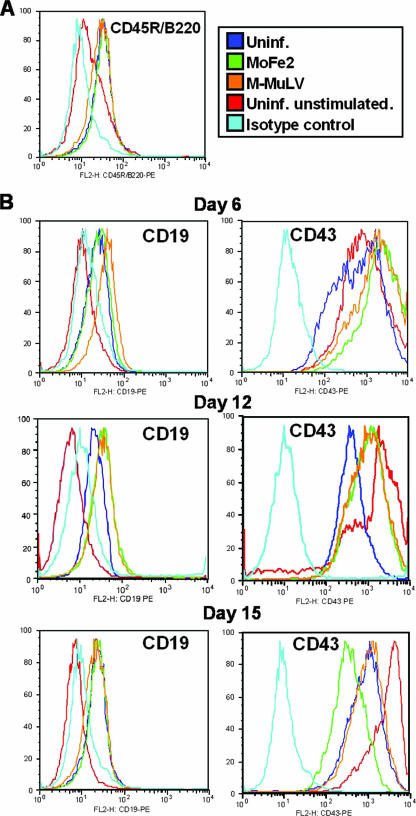
To examine the stage of B-lymphoid differentiation affected by infection, cells were cultured in Iscove's modified Dulbecco medium (Invitrogen, Carlsbad, CA) supplemented with 20% horse serum, 50 ng/ml IL-7, and 10 ng/ml BMP-2, refreshed every 2 to 3 days. B-lymphoid differentiation was monitored by flow cytometry to measure expression of the temporally regulated cell surface markers CD45R/B220, CD43, CD19, and CD25. As described by others, CD45R/B220 is expressed early and throughout differentiation; CD43 is expressed early, persists through immunoglobulin H (IgH) D-J gene rearrangement, and declines at the pro-B-cell stage; CD19 appears at the time of IgH D-J gene arrangement and persists; and CD25 is not expressed until the time of IgH VDJ gene rearrangement at the pre-B cell stage (9, 16, 17, 22). In our study, CD45R/B220 was detectable by day 3 and persisted throughout the study equally in uninfected and infected cells (Fig. 2A). CD19 was first detected at day 6 and was indistinguishable in uninfected and infected cells (Fig. 2B). The predicted down-regulation of CD43 expression at the pro-B-cell stage was observed similarly on uninfected and infected cells beginning at day 12, although some delay was apparent in cells infected with either virus (Fig. 2B). In contrast, CD25 expression was not detected in any population, indicating that the cells do not reach the pre-B-cell stage (data not shown).
FIG. 2.
Temporal expression of the surface markers CD45R/B220, CD19, and CD43 on EML cells stimulated to undergo B-lymphoid differentiation. Expression of surface markers on MoFe2-infected and M-MuLV-infected EML cells was examined by flow cytometry using phycoerythrin-conjugated monoclonal antibodies (BD Biosciences, Palo Alto, CA). Included as controls were uninfected EML cells stimulated in parallel to undergo B-lymphoid differentiation (Uninf.) and uninfected EML cells in the absence of stimulation (Uninf. unstimulated). Uninfected, unstimulated cells examined with an isotype control antibody were included as a negative control. Histograms depict 10,000 events per sample, and results are representative of three independent experiments. The results shown in panel A represent day 6 of differentiation. The results shown in panel B represent day 6, 12, or 15 of differentiation, as indicated.
Rearrangement of the Ig heavy-chain gene (IgH) was then examined with a single-cell PCR procedure described by others (25) and recently modified in a manner to be detailed elsewhere (M. Gunthart and N. Rosenberg, unpublished data). Infected and uninfected EML cells were collected at regular intervals for 15 days during B-lymphoid differentiation, and single cells were placed directly into amplification reactions by limiting dilution. Samples were amplified in two sequential rounds of PCR using multiple primer sets designed to amplify the germ line IgH J region, DJ(H) rearrangements, and VDJ rearrangements involving three of the largest VH families. Loss of germ line configuration was detected between 3 and 6 days in all populations. DJ(H) rearrangements were first apparent by day 3 and were evident at comparable frequencies in all populations thereafter. VDJ rearrangements were first detected at day 6 in uninfected cells but were evident in all populations at comparable frequencies thereafter (Table 1).
TABLE 1.
Ig heavy-chain gene rearrangement in infected and uninfected EML cells as measured by single-cell PCRa
| IgH locus | EML cell sample | % Heavy-chain gene rearrangement
|
||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 12 | Day 15 | ||
| Germ line | Uninfected | 100 | 75 | 25 | 0 | 0 |
| MoFe2 infected | 100 | 100 | 0 | 0 | 0 | |
| M-MuLV infected | 100 | 100 | 38 | 0 | 0 | |
| D-J | Uninfected | 0 | 88 | 50 | ND | 13 |
| MoFe2 infected | 0 | 63 | 13 | ND | 38 | |
| M-MuLV infected | 0 | 75 | 13 | ND | 13 | |
| VHJ558 | Uninfected | ND | 0 | 0 | 50 | 50 |
| MoFe2 infected | ND | 0 | 0 | 25 | 63 | |
| M-MuLV infected | ND | 0 | 0 | 25 | 75 | |
| VHQ52 | Uninfected | ND | ND | 25 | 13 | 25 |
| MoFe2 infected | ND | ND | 0 | 0 | 13 | |
| M-MuLV infected | ND | ND | 0 | 0 | 38 | |
| VH7183 | Uninfected | ND | ND | 25 | 13 | 63 |
| MoFe2 infected | ND | ND | 0 | 25 | 50 | |
| M-MuLV infected | ND | ND | 0 | 25 | 63 | |
Uninfected EML cells or cells infected with MoFe2 or M-MuLV were stimulated to B-lymphoid differentiation and collected at regular intervals for 15 days. At each collection, single cells were deposited directly into amplification reactions by limiting dilution. Single-cell PCR amplification was performed with multiple primer sets to detect the germ line IgH J region, DJ(H) rearrangements, and VDJ rearrangements involving three of the largest VH families (J558, Q52, and 7183) by a procedure described by others (25). Eight independent amplifications were examined with each primer set at each timed collection, and the results are reported as percentages demonstrating the indicated target. ND, not done.
Because a delay or block in differentiation did not account for the reduction in the B-lymphoid potential of infected EML cells, we examined effects on cell viability. EML cells were deposited at 5 × 105 cells per ml and stimulated to undergo B-lymphoid differentiation. Cell viability was measured 3 days later by MTS assay (CellTiter-96AQueous One Solution cell proliferation assay; Promega, Madison, WI). The results demonstrated significantly reduced viability of differentiating EML cells when infected with either MoFe2 or M-MuLV (Fig. 3).
FIG. 3.
Diminished viability of infected EML cells after stimulation to B-lymphoid differentiation. EML cells infected with MoFe2 or M-MuLV and uninfected control cells were stimulated to undergo B-lymphoid differentiation as described in the text. Cell viability was measured at 3 days poststimulation by MTS dye reduction assay (Promega, Madison, WI). Statistical analysis by one-way ANOVA showed significantly decreased viability (P < 0.05) of EML cells infected with either virus. The data shown are averages (± standard errors) from triplicate experiments.
The studies reported here examine the influence of a recombinant murine-feline gammaretrovirus, MoFe2, on hematopoiesis in order to assess the impact of the unique FeLV-945 LTR it contains. A significant and selective reduction in the potential for B-lymphoid differentiation in the bone marrow of mice infected with either MoFe2 or the parent virus, M-MuLV, was observed, while myeloid potential was significantly increased (Fig. 1A). EML cells were shown to recapitulate this effect on progenitor cell differentiation (Fig. 1B) and were therefore used to explore the mechanism of suppression in a relatively simplified system. By two independent measures, infected EML cells appeared to undergo relatively normal B-lymphoid differentiation (Fig. 2 and Table 1). The viability of infected EML cells, however, was significantly diminished within 3 days after the B-lymphoid differentiation stimulus (Fig. 3). These observations suggest that, while B-lymphoid differentiation proceeds normally in cells that survive, the reduction in viability reduces the B-lymphoid differentiation potential overall. This reduction in viability may therefore confer a selective advantage on undifferentiated lymphoid progenitors in the bone marrow of gammaretrovirus-infected animals and thereby contribute to the establishment of a premalignant state.
Acknowledgments
This work was supported by NIH grants CA83823 (L.S.L.) and CA33771 (N.R.) from the National Cancer Institute and by support from the Louisiana Cancer Research Consortium. S.L.F. was partially supported by grants from the Cancer Association of Greater New Orleans and from the Louisiana Board of Regents.
The advice and assistance of Patricia Lobelle-Rich and Mirja Gunthart are gratefully acknowledged.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Abkowitz, J. L., R. D. Holly, and J. W. Adamson. 1987. Retrovirus-induced feline pure red cell aplasia: the kinetics of erythroid marrow failure. J. Cell Physiol. 132:571-577. [DOI] [PubMed] [Google Scholar]
- 2.Brightman, B. K., B. R. Davis, and H. Fan. 1990. Preleukemic hematopoietic hyperplasia induced by Moloney murine leukemia virus is an indirect consequence of viral infection. J. Virol. 64:4582-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandhasin, C., P. N. Coan, and L. S. Levy. 2005. Subtle mutational changes in the SU protein of a natural feline leukemia virus subgroup A isolate alter disease spectrum. J. Virol. 79:1351-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandhasin, C., P. N. Coan, I. Pandrea, C. K. Grant, P. A. Lobelle-Rich, A. Puetter, and L. S. Levy. 2005. Unique long terminal repeat and surface glycoprotein gene sequences of feline leukemia virus as determinants of disease outcome. J. Virol. 79:5278-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandhasin, C., P. A. Lobelle-Rich, and L. S. Levy. 2004. Feline leukemia virus LTR variation and disease association in a geographic and temporal cluster. J Gen. Virol. 85:2937-2942. [DOI] [PubMed] [Google Scholar]
- 6.Davis, B. R., B. K. Brightman, K. G. Chandy, and H. Fan. 1987. Characterization of a preleukemic state induced by Moloney murine leukemia virus: evidence for two infection events during leukemogenesis. Proc. Natl. Acad. Sci. USA 84:4875-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du, Y., J. L. Campbell, D. Nalbant, H. Youn, A. C. Bass, E. Cobos, S. Tsai, J. R. Keller, and S. C. Williams. 2002. Mapping gene expression patterns during myeloid differentiation using the EML hematopoietic progenitor cell line. Exp. Hematol. 30:649-658. [DOI] [PubMed] [Google Scholar]
- 8.Fan, H. 1997. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol. 5:74-82. [DOI] [PubMed] [Google Scholar]
- 9.Fleming, H. E., and C. J. Paige. 2002. Cooperation between IL-7 and the pre-B cell receptor: a key to B cell selection. Semin. Immunol. 14:423-430. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, C., P. A. Lobelle-Rich, A. Puetter, and L. S. Levy. 2005. Substitution of feline leukemia virus long terminal repeat sequences into murine leukemia virus alters the pattern of insertional activation and identifies new common insertion sites. J. Virol. 79:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, Q. X., and H. Fan. 1990. Combined infection by Moloney murine leukemia virus and a mink cell focus-forming virus recombinant induces cytopathic effects in fibroblasts or in long-term bone marrow cultures from preleukemic mice. J. Virol. 64:3701-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linenberger, M. L., and J. L. Abkowitz. 1995. Haematological disorders associated with feline retrovirus infections. Baillières Clin. Haematol. 8:73-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linenberger, M. L., and J. L. Abkowitz. 1992. Studies in feline long-term marrow culture: hematopoiesis on normal and feline leukemia virus infected stromal cells. Blood 80:651-662. [PubMed] [Google Scholar]
- 14.Linenberger, M. L., A. M. Beebe, N. C. Pedersen, J. L. Abkowitz, and S. Dandekar. 1995. Marrow accessory cell infection and alterations in hematopoiesis accompany severe neutropenia during experimental acute infection with feline immunodeficiency virus. Blood 85:941-951. [PubMed] [Google Scholar]
- 15.Ma, X., T. Husain, H. Peng, S. Lin, O. Mironenko, N. Maun, S. Johnson, D. Tuck, N. Berliner, D. S. Krause, and A. S. Perkins. 2002. Development of a murine hematopoietic progenitor complementary DNA microarray using a subtracted complementary DNA library. Blood 100:833-844. [DOI] [PubMed] [Google Scholar]
- 16.Maier, H., and J. Hagman. 2002. Roles of EBF and Pax-5 in B lineage commitment and development. Semin. Immunol. 14:415-422. [DOI] [PubMed] [Google Scholar]
- 17.Medina, K. L., and H. Singh. 2005. Genetic networks that regulate B lymphopoiesis. Curr. Opin. Hematol. 12:203-209. [DOI] [PubMed] [Google Scholar]
- 18.Nagashima, N., M. Hisasue, K. Nishigaki, T. Miyazawa, R. Kano, and A. Hasegawa. 2005. In vitro selective suppression of feline myeloid colony formation is attributable to molecularly cloned strain of feline leukemia virus with unique long terminal repeat. Res. Vet. Sci. 78:151-154. [DOI] [PubMed] [Google Scholar]
- 19.Pawlak, G., M. F. Grasset, S. Arnaud, J. P. Blanchet, and G. Mouchiroud. 2000. Receptor for macrophage colony-stimulating factor transduces a signal decreasing erythroid potential in the multipotent hematopoietic EML cell line. Exp. Hematol. 28:1164-1173. [DOI] [PubMed] [Google Scholar]
- 20.Rojko, J. L., and G. J. Kociba. 1991. Pathogenesis of infection by the feline leukemia virus. J Am. Vet. Med. Assoc. 199:1305-1310. [PubMed] [Google Scholar]
- 21.Rulli, K., J. Lenz, and L. S. Levy. 2002. Disruption of hematopoiesis and thymopoiesis in the early premalignant stages of infection with SL3-3 murine leukemia virus. J. Virol. 76:2363-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, E., and M. Sigvardsson. 2004. The roles of transcription factors in B lymphocyte commitment, development, and transformation. J. Leukoc. Biol. 75:973-981. [DOI] [PubMed] [Google Scholar]
- 23.Starkey, C. R., P. A. Lobelle-Rich, S. Granger, B. K. Brightman, H. Fan, and L. S. Levy. 1998. Tumorigenic potential of a recombinant retrovirus containing sequences from Moloney murine leukemia virus and feline leukemia virus. J. Virol. 72:1078-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storch, T. G., P. Arnstein, V. Manohar, W. M. Leiserson, and T. M. Chused. 1985. Proliferation of infected lymphoid precursors before Moloney murine leukemia virus-induced T-cell lymphoma. J. Natl. Cancer Inst. 74:137-143. [PubMed] [Google Scholar]
- 25.ten Boekel, E., F. Melchers, and A. Rolink. 1995. The status of Ig loci rearrangements in single cells from different stages of B cell development. Int. Immunol. 7:1013-1019. [DOI] [PubMed] [Google Scholar]
- 26.Tsai, S., S. Bartelmez, E. Sitnicka, and S. Collins. 1994. Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant-negative retinoic acid receptor can recapitulate lymphoid, myeloid, and erythroid development. Genes Dev. 8:2831-2841. [DOI] [PubMed] [Google Scholar]
- 27.Tsai, S., and S. J. Collins. 1993. A dominant negative retinoic acid receptor blocks neutrophil differentiation at the promyelocyte stage. Proc. Natl. Acad. Sci. USA 90:7153-7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumas-Brundage, K. M., W. Garret, K. Blank, and M. B. Prystowsky. 1996. Murine leukemia virus infects early bone marrow progenitors in immunocompetent mice. Virology 224:573-575. [DOI] [PubMed] [Google Scholar]