Abstract
Here we show that cells expressing genes inserted into Semliki Forest virus (SFV) vectors generate a large fraction of defective ribosomal products (DRiPs) due to frequent initiation on downstream Met residues. In monopolizing the host cell translational machinery, SFV reduces levels of translation eukaryotic initiation factor 4E (eIF4E), diminishes phosphorylation of ribosome subunit S6, and phosphorylates translation initiation factor eIF2α. We show that the last event is required for SFV mistranslation of inserted genes. Downstream initiation is suppressed by fusing inserted genes with the open reading frame encoding the SFV capsid, demonstrating that one function of the capsid element is to enable ribosomes to initiate translation in the proper location. These results show that in modifying translation, viral vectors can unpredictably increase the generation of truncated polypeptides and thereby the DRiP fraction of inserted gene products, which can potentially affect their yield, therapeutic efficacy, and immunogenicity.
Recombinant viral systems are widely used for heterologous gene expression in mammalian cells and organisms. Typical ex vivo applications include protein production and functional studies of recombinant proteins in the cellular context. Recombinant viruses are also commonly used in vivo as gene therapy and vaccine vectors. Each recombinant virus system offers unique advantages (and disadvantages), and recombinant alphaviruses (rAlphs) are no exception (reviewed in reference 9).
rAlphs, which to date include Semliki Forest virus (SFV), Venezuelan encephalitis virus, and Sindbis virus, can be used as replicating or nonreplicating in vitro-transcribed or virion-packaged RNA (5, 16, 35) or as plasmid-based DNA-launched replicons (4, 5, 27). Protein expression is generally robust in mice and susceptible cells and can be enhanced by the use of a viral translation-enhancing element (11, 30). rAlphs are promising vaccine candidates, able to elicit robust antibody (Ab) and CD8+ T (TCD8+) response and provide protection against lethal viral challenge in animal model systems (1, 14, 20, 37) (reviewed in references 17 and 22).
In maximizing their transmission between hosts, viruses evolve unique strategies for modulating host gene expression to their advantage. SFV, like many acute viruses, severely inhibits host gene product translation in infected cells. Little is known about how SFV accomplishes this, but other viruses are known to alter various defined elements of the translational machinery (4, 27). An important question is the extent to which these alterations modulate the fidelity of protein synthesis. Approximately 25% of newly synthesized proteins are degraded by proteasomes within 30 min of their synthesis (28, 34). An uncertain fraction of these rapidly degraded proteins represent defective ribosomal products (DRiPs), nascent proteins that fail to attain a stable conformation due to imperfections in the process of converting genomic information into polypeptides. DRiPs appear to represent the primary source of peptides presented by major histocompatibility complex class I (MHC-I) molecules to TCD8+ (24).
In the present study, we show that expression of inserted genes by recombinant SFV (rSFV) results in the generation of an extremely high fraction of DRiPs and demonstrate that this is due to a combination of factors related to the virus-induced inhibition of host mRNA translation.
MATERIALS AND METHODS
Cells and viruses.
BHK-21 and HeLa cells obtained from ATCC were cultured in Dulbecco's minimal essential medium supplemented with 5% fetal bovine serum (FBS) and 10 mM HEPES buffer. Mouse embryo fibroblasts (MEF) from wild-type (WT) mice and from Ser51-to-Ala (Ser51A) eukaryotic initiation factor 2α (eIF2α) mutant SV129 mice (26) (kindly provided by Randall Kaufman, University of Michigan, MI) were cultured in Dulbecco's minimal essential medium supplemented with 15% FBS, minimal essential medium essential amino acids, and nonessential amino acids. Influenza A/PR/8 virus (IAV) was grown and prepared as described previously (6). rSFV stocks were prepared as previously described (31). SFV1-NP, SFVC-NP, and SFV1-HA were described previously (references 37, 30, and 3, respectively). SFV-NP-SIINFEKL-EGFP was constructed by ligating a SalI/NotI-cut pSCII-NP-S-EGFP fragment and an XhoI/NotI-cut pSFV4.2 vector (kindly provided by P. Liljeström, Karolinska Institutet, Sweden).
Immunochemistry.
Abs used for detection of IAV NP were generated in the lab and included mouse anti-NP (IC5-3A8), rabbit anti-NP-COOH and anti-NP-NH2 polyclonals raised against peptides corresponding to NP2-13 and NP483-497, respectively. IC5-3A8 had previously been determined by RIA to react specifically to folded NP, while the polyclonal Abs (pAbs) have been found to react also to denatured nucleoprotein (NP). As anti-IAV hemagglutinin (HA) carboxy terminus, hybridoma supernatants from four pooled HA2-specific monoclonal Abs (MAbs) (RA7-53, H18L10-5R1, RA4-44, RA6-30) were used. Abs to eIF2α (9722), phospho-eIF2α (9721), and phospho-eIF4E (9918) were obtained from Cell Signaling (Danvers, MA), and human anti-mitochondria autoimmune serum was from Immunovision (HMS-0100; Springdale, AR). Confocal microscopy experiments were carried out on cell culture monolayers grown in 24-well dishes on coverslips. Cells were fixed for 20 min at room temperature (RT) in phosphate-buffered saline (PBS) containing 3.2% paraformaldehyde (15710; Electron Microscopy Sciences, Hatfield, PA), washed with PBS, permeabilized using 1% NP-40 (U.S. Biochemical Corporation, Cleveland, OH) for 5 min, washed again with PBS, blocked for 30 min in blocking buffer (5% normal donkey serum [017-000-121; Jackson ImmunoResearch], 0.1% Brij 58 detergent [28336; Pierce, Rockford, IL] in PBS), incubated in primary Abs diluted in blocking buffer for 30 min at RT or +4°C overnight, washed with PBS, overlaid with secondary Abs (donkey antiserum conjugated with fluorescein isothiocyanate, Cy3, or Cy5; Jackson ImmunoResearch), washed, mounted on slides by use of Fluoromount-G (Southern Biotech, Birmingham, AL), sequentially imaged using a Bio-Rad M1024 microscope and Lasersharp software, and analyzed by in-house-built software.
For Western blotting, sample pellets were lysed in 95°C laurel dodecyl sulfate sample buffer (NP0007; Invitrogen, Carlsbad, CA) supplemented with 50 mM dithiothreitol and protease inhibitor cocktail (Complete Mini tablets, 1836170; Roche), heated for 10 min, subjected to reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretic transfer (NuPAGE Novex system, Invitrogen) to 0.45-μm polyvinylidene difluoride membrane (LC2005), blocked in 1% bovine serum albumin in PBS, incubated with primary Abs as indicated for 30 min at RT or +4°C overnight, washed thoroughly, and developed with horseradish peroxidase-coupled anti-mouse/anti-rabbit Abs by use of a chemiluminescent peroxide substrate (1520709; Roche). Luminescence was recorded on Biomax MR film (Eastman Kodak, Rochester, NY), digitized, and analyzed using ImageQuant software (Molecular Dynamics Inc., Sunnyvale, CA).
For immunoelectron microscopy, BHK-21 cells were fixed in 4% paraformaldehyde-0.05% glutaraldehyde (Electron Microscopy Sciences) in 0.1 M phosphate buffer, washed in 0.1 M phosphate buffer, and then incubated at 37°C in 10% gelatin. A pellet was formed by centrifugation at 12,000 rpm using a Microfuge. The sample was placed in ice to solidify. The pellet was cut at 4°C into small cubes infiltrated with 2.3 M sucrose in 0.1 M phosphate buffer and frozen on pins in liquid nitrogen. The pins were stored in liquid nitrogen. Ultracryosections were cut on a Leica Ultracut FCS microtome, picked up by a solution of 2.3 M sucrose and 2% methyl cellulose (50:50) on a loop, and dropped on Formvar/carbon-coated grids and then placed on 2% gelatin on ice. After the gelatin was melted, the sections were quenched in 0.02 M glycine in 0.1 M phosphate buffer, blocked in 0.1% fish skin gelatin in 0.01 M phosphate buffer (Sigma, St. Louis, MO), and incubated with the rabbit anti-NP-COOH pAb (1:100 dilution) (see above). Sections were washed in 0.01% fish skin gelatin in 0.1 M phosphate buffer, incubated with protein A conjugated to 10-nm colloidal gold (Department of Cell Biology, Utrecht University School of Medicine, Utrecht, The Netherlands), and washed in 0.1% fish skin gelatin in 0.1 M phosphate buffer, then in 0.1 M phosphate buffer, and finally with deionized, filtered water. Sections were then stained in 9:1 2% methyl cellulose (Sigma) to 2% uranyl acetate (EMS) and picked up by loops to air dry.
Metabolic labeling and IP.
For metabolic labeling, 106 cells were labeled at 37°C for 5 min (unless otherwise indicated) with 100 μCi per 0.4 ml [35S]Met and subsequently incubated for 20 s in the excess of unlabeled Met. Samples were lysed for 10 min in ice-cold 50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 2 mM EDTA, 1% NP-40, protease inhibitor cocktail (Complete Mini tablets, 1836170; Roche), 1 μg/ml phenylmethylsulfonyl fluoride. The lysates were separated into soluble (supernatant) and insoluble (pellet) fractions by centrifugation at 6,000 rpm for 6 min and stored at −80°C until use. For sequential immunoprecipitation (IP), lysates were precleared using PAG slurry (Ultralink Immobilized Protein A/G, 53133; Pierce, Rockford IL, diluted to 50% in lysis buffer), and low-speed supernatants were incubated for at least 30 min at +4°C in PAG/IC5-3A8 (PAG slurry that had been previously incubated with Ab IC5-3A8 undiluted hybridoma culture medium). Low-speed supernatants were transferred to fresh PAG/IC5-3A8, incubated, and centrifuged twice more as described above. Finally, the supernatants were transferred to PAG/NP-COOH (PAG slurry that had been previously incubated with the NP-COOH pAb) and incubated overnight. All slurry pellets were collected and washed once in buffer A (0.2% NP-40, 10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA), once in buffer B (0.2% NP-40, 10 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 2 mM EDTA), and once in 10 mM Tris-HCl, pH 7.5. Pellets were boiled for 10 min in SDS-PAGE sample buffer, prepared, and subjected to SDS-PAGE, and gels were visualized using fluorography, quantitated using a Typhoon 8600 PhosphorImager (Molecular Dynamics Inc., Sunnyvale, CA), and analyzed using ImageQuant software (Molecular Dynamics).
Quantitation of intracellular viral RNA by reverse transcription-PCR.
For RNA isolation, 107 HeLa cells were infected with SFV1-NP, SFVC-NP, or IAV. Seven (SFV) or 12 h postinfection (p.i.), cells were lysed in 3.5 ml TRIzol (Invitrogen) for 5 min at RT. RNA was isolated from 1 ml lysate following the manufacturer's protocol, precipitated using 2-propanol, washed with 75% ethanol, air dried, resuspended in diethyl pyrocarbonate-treated water by pipetting and incubation at 58°C for 10 min, and stored at −80°C. For cDNA synthesis, either 0.5 or 0.001 μl (experimental repeats; lower amount to verify unsaturating conditions) of isolated RNA was reverse transcribed using SuperScript III (Invitrogen) following the manufacturer's protocol by use of 10 ng/μl random hexamers. For quantitative real-time PCR, the following primers were used: for amplicon 1 (nucleotides [nt] NP55 to NP177), 5′-GGTAGATAATCACTCACTGAGTGACATCAG-3′ and 5′-CCGACGGATGCTCTGATTTC-3′; for amplicon 2 (nt NP107 to NP229), 5′-AAACGGTCTTACGAACAGATGGA-3′ and 5′-GAGTTCGGTGCACATTTGGAT-3′; for amplicon 4 (NP244 to NP345), 5′-TGAGGGACGGTTGATCCAA-3′ and 5′-CCCGCACTGGGATGTTCTT-3′; and for amplicon 8 (NP974 to NP1084), 5′-TACTCTCTAGTCGGAATAGACCCTTTCA-3′ and 5′-TGCCATCCACACCAGTTGAC-3′. Thermal cycling was performed with an ABI PRISM 7900HT sequence detection system (Applied Biosystems) in the presence of SYBR green (QuantiTect SYBR green PCR kit, 204143; QIAGEN, Hilden, Germany) under the following conditions: 95°C for 15 s (2% ramp) followed by 40 cycles of 95°C for 15 s (50% ramp), 60°C for 30 s (100% ramp), and 72°C for 30 s (100% ramp) followed in turn by 95°C for 15 s (100% ramp), 60°C for 15 s (100% ramp), and 72°C for 15 s (2% ramp). For absolute quantitation, we used a standard series of NP-encoding plasmid, ranging from 1.0 fg to 100 ng at 100-fold dilution steps.
Quantitation of EGFP and peptide presentation by cytofluorography.
Detection of enhanced green fluorescent protein (EGFP) as well as Kb-SIINFEKL levels was done by flow cytometry using purified 25-D1.16 MAb (23) conjugated to Alexa Fluor 647 as previously described (24).
RESULTS
rSFV generates large amounts of IAV nucleoprotein DRiPs that localize to mitochondria.
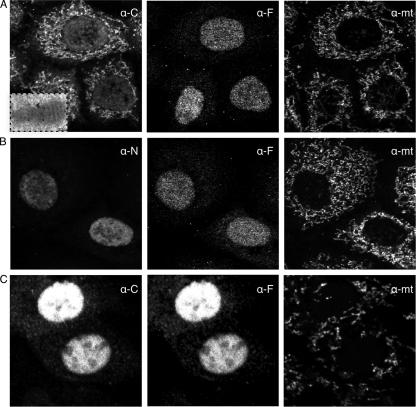
In the course of studying the generation of IAV NP DRiPs, we infected BHK or HeLa cells with a previously described rSFV that expresses NP. NP was localized in fixed and permeabilized cells by indirect immunofluorescence using rabbit pAbs raised to a synthetic peptide corresponding to the COOH terminus of NP. This serendipitously revealed that most NP localized to cytoplasmic structures that proved to be mitochondria, as identified by human anti-mitochondria autoantisera (Fig. 1A). Analysis of SFV-NP-infected cells by immunoelectron microscopy (Fig. 1A, left panel inset) using gold-labeled anti-rabbit immunoglobulin Abs confirmed the localization of NP to mitochondria and further demonstrated that NP is imported into the mitochondria and does not simply decorate the mitochondrial surface.
FIG. 1.
NP localizes partially to mitochondria when expressed by an SFV vector. Confocal microscopy was used to study the subcellular localization of NP. HeLa cells were infected for 7 h with SFV-NP (A and B) or SFVC-NP (C) and fixed and processed for indirect immunofluorescence staining using the MAb IC5-3A8 specific for folded NP (α-F), human α-mitochondria auto-Abs (α-mt), or rabbit sera raised against either the NP COOH terminus (α-C) (panels A and C) or the NH2 terminus (α-N) (panel B). (A inset) Cryoelectron microscopy image of a mitochondrion in a HeLa cell infected with SFV-NP and stained with gold-conjugated α-rabbit immunoglobulin Abs to identify binding of α-C Abs.
By contrast, in the same experiment, NP expressed by IAV-infected cells localized nearly exclusively or predominantly (depending on the cell examined) to the nucleus, as expected. Moreover, cells infected with recombinant vaccinia virus-NP (VV-NP) or adenovirus-NP or cells transfected with plasmids expressing NP under the cytomegalovirus immediate early promoter exhibited nearly exclusive nuclear localization using the same anti-COOH pAbs (not shown). These pAbs (and anti-NH2 pAbs described below) bind to both native and unfolded NP, as determined by their binding to NP in mild detergent extracts in IP and to NP immobilized to polyvinylidene difluoride or nitrocellulose following boiling in Laemmli sample buffer under reducing conditions, respectively (see below).
To examine the conformation of NP expressed from rSFV and other vectors, we used the IC5-3A8 MAb, which is largely if not exclusively specific for folded NP (as determined by its inability to interact with NP in immunoblots or in IP/immunofluorescence using NP that misfolds due to genetic manipulation of its coding sequence [unpublished findings]). IC5-3A8 colocalized extensively with anti-COOH pAbs in cells infected with IAV, adenovirus-NP, or VV-NP (not shown). By contrast, in rSFV-NP infected cells, IC5-3A8 failed to stain mitochondria and instead weakly localized to the nucleus (Fig. 1A, middle panel). A similar staining pattern was exhibited by pAbs raised to a synthetic peptide corresponding to the NP NH2 terminus (Fig. 1B).
These findings indicated that SFV-NP expresses a form of NP that localizes to the mitochondria in an unfolded form in which the NH2 terminus is missing, modified, or otherwise inaccessible to Ab. The most trivial possibility is that SFV-NP possesses sequence alterations that account for its aberrant behavior. Sequencing of the NP gene revealed one alteration between the SFV-NP gene and the GenBank sequence (resulting in an Asp-to-Glu substitution at position 494). Two findings indicate that this does not account for the aberrant trafficking of the gene product. First, an identical alteration was present in rVV-NP, which localizes to the nucleus. Second, converting the NP sequence in rSFV to WT by site-directed mutagenesis did not alter the subcellular localization pattern (data not shown).
Overexpressed proteins frequently demonstrate aberrant trafficking, but this does not account for the mitochondrial localization of SFV-encoded NP. rSFV-NP-infected cells express less NP than IAV-infected cells or recombinant adenovirus-infected cells, as determined either by the intensity of the immunofluorescence signal or by biochemical analysis (not shown). Even at the earliest times following SFV-NP infection when NP can be detected, it localizes predominantly to mitochondria (data not shown).
Generation of NP mitochondrial DRiPs occurs independently of SFV viral penetration and can be abrogated by fusion with the capsid gene.
SFV infects cells via receptor-mediated endocytosis and acid-mediated fusion of viral and endosomal membranes (32). Virion cores localize to the cytosolic surface of endosomal membranes, where viral RNA and protein synthesis is believed to occur (25). Indeed, our study was initiated to determine whether such localized protein synthesis affected the folding efficiency of NP.
To determine whether mitochondrial localization of NP required viral penetration, we took advantage of features of the SFV expression system that enable the delivery of SFV replicons to host cells by transfection with positive-stranded replicon RNA or by replicon-encoding cDNA. Replicon RNA is the same RNA used for the packaging of rSFV stocks (16). DNA replicons are generated by cloning cDNA corresponding to replicon RNA into a plasmid under the control of the cytomegalovirus early promoter (3). When rSFV-NP was expressed following either of these methods, NP again localized predominantly to mitochondria (not shown). This indicates that SFV infection per se is not required for the altered trafficking of NP and therefore that localized synthesis of NP by ribosomes at sites of SFV penetration is not required for the phenomenon of SFV-induced NP mitochondrial DRiPs.
One explanation for the mitochondrial localization of rSFV-NP is that SFV mRNAs are somehow imported in mitochondria and translated by mitochondrial ribosomes with the resulting NP remaining trapped in mitochondria. While this seemed unlikely given that the altered codon usage in mitochondria would introduce over 30 stop codons, we eliminated this possibility by treating cells with chloramphenicol, which selectively inhibits translation on mitochondrial ribosomes, or cycloheximide, which selectively affects cytoplasmic ribosomes. Chloramphenicol had no effect on NP expression or localization, while cycloheximide completely inhibited NP expression (data not shown). This experiment established that mitochondrial NP derives from import from translation products of cytoplasmic ribosomes.
In all of the experiments described to this point, we used NP expressed from vectors in which NP replaces the gene encoding the SFV structural polyprotein (2). The initial portion of this gene, which comprises the SF virion capsid protein, is an intriguing element of SFV. Capsid autocatalytically cleaves itself from the SFV structural polyprotein in a cotranslational manner (32). The capsid-encoding sequence greatly enhances the translation of downstream genes (either the SFV structural polyprotein itself or inserted gene products [30]), probably at the level of translation initiation (11).
We examined the effect of the capsid element on NP DRiP formation by infecting cells with rSFVC-NP, a recombinant with the capsid element fused directly to the NP gene. NP was detected in fixed and permeabilized cells via indirect immunofluorescence using anti-COOH pAbs. As expected, the capsid element enhanced NP expression relative to what was seen for cells infected with rSFV-NP in the same experiment. Surprisingly, however, in rSFVC-NP-infected cells, NP now localized to the nucleus in the complete absence of mitochondrial localization (Fig. 1C).
These findings further support our conclusion that mere overexpression was not the cause of the mitochondrial localization in rSFV-infected cells. Moreover, the findings indicate that the high rate of NP DRiP formation in SFV-infected cells is not an inevitable outcome of NP synthesis by SFV infection. As seen, DRiP formation was reduced for SFVC-NP in a manner related to the precise way that proteins are synthesized in the context of the SFV replication strategy where the capsid is cotranslationally cleaved off and the nascent polypeptide is translocated to another subcellular compartment. In the case of the WT SFV, the nascent polypeptide is the endoplasmic reticulum (ER)-targeted spike polyprotein, and in the case of SFVC-NP the nascent polypeptide is NP with its amino-terminal nuclear localization signal.
rSFV-NP is synthesized from downstream Met.
To better understand the nature of SFV-NP DRiPs, we took advantage of a panel of Abs specific for NP at different folding stages. Cells were infected with SFV-NP, SFVC-NP, or IAV and pulse (5 min) labeled with [35S]Met, and NP from TX-100-soluble material was recovered by incubation with Ab-coated beads. Three sequential incubations with IC5-3A8 beads to quantitatively remove conformed (believed to be completely folded) NP were followed by followed by a single incubation with anti-COOH pAb beads to recover nonconformed NP. Reactive material eluted from beads by boiling in sample buffer was analyzed by SDS-PAGE and analyzed by quantitative fluorography using a PhosphorImager (Fig. 2).
FIG. 2.
A significant fraction of NP expressed from SFV vector is synthesized as truncated forms. BHK cells were infected for 7 h with SFV-NP, SFVC-NP, IAV (Flu), or mock control, as indicated. Cells were metabolically labeled with [35S]Met for 5 min and NP-40 lysates were incubated three times sequentially with beads loaded with IC5-3A8 specific for folded NP (I, II, and III; lanes F) followed by beads loaded with α-COOH pAbs (IV; lanes C). Samples were analyzed by SDS-PAGE followed by quantitative autoradiography. Numbers beside the upper bands indicate the percentage fractions of NP that reacts with the anti-COOH pAbs (IP IV) out of all full-length NP (sums of IPs I to IV, upper area). Numbers beside the lower regions indicate the fractions of shorter fragments reactive to the anti-COOH antiserum (IP IV, lower area) as percentages of all material immunoprecipitated with this antiserum (IP IV, sum of lower and upper regions).
NP recovered from SFVC-NP comigrated with authentic NP, which indicates that capsid is rapidly cleaved from NP, as expected. Fusion of NP with the capsid element enhanced translation of NP by ∼3- to 10-fold in SFV-infected cells, depending on the experiment. The ratio of conformed to nonconformed full-length NP in SFV-NP was similar to that for SFVC-NP (if anything, SFVC-NP demonstrated a higher proportion of nonconformed full-length NP). Interestingly, the ratio of nonconformed NP was decreased three- to fourfold in SFV-infected cells relative to that for IAV-infected cells, which suggests that the optimal folding efficiency of bona fide full-length NP depends on its translation in the context of an IAV infection.
In addition to a band migrating with the expected Mr of NP, we also detected a ladder of smaller species. In the case of IAV-NP, nearly all of the truncated species were reactive with IC5-3A8. By contrast, many of the smaller fragments generated in SFVC-NP-infected cells were nonconformed. Strikingly, most of the truncated species recovered from SFV-NP-infected cells were nonconformed, amounting to more than 30% of the total amount of NP gene products recovered.
Using α-NH2 pAbs, we failed to detect significant amounts of short fragments in lysates from cells infected with IAV, SFV-NP, or SFVC-NP (data not shown), consistent with the idea that the COOH-terminal fragments are not generated by posttranslational cleavage. In support of this conclusion, we found that even after very short pulse radiolabeling (30 s), truncated forms of NP were the predominant form recovered from SFV-NP-infected cells by use of α-COOH pAbs (data not shown). By contrast, >90% of material recovered from IAV- or SFVC-NP-infected cells was full-length NP.
These data suggest that the predominant form of NP synthesized in SFV-NP-infected cells results from initiation on downstream AUG codons. Moreover, the failure of α-NH2 pAbs to bind to mitochondrial NP in these cells suggested the following hypothesis: one or more of the COOH-terminal fragments are targeted to mitochondria by cryptic sequences that function only in the absence of NH2-terminal sequences.
Truncated forms of NP localize to mitochondria.
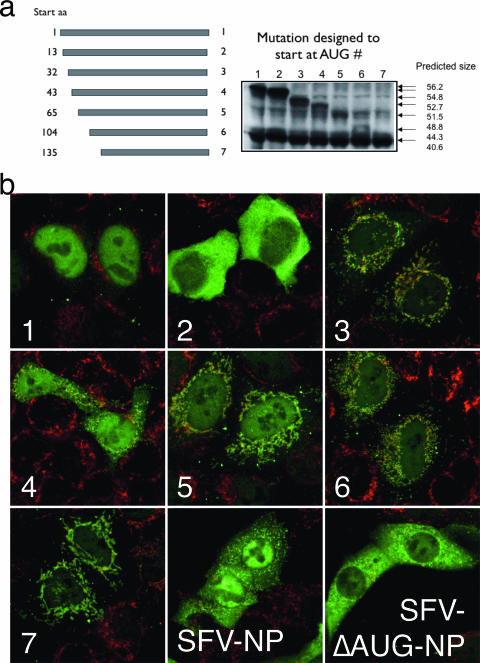
Support for the downstream initiation hypothesis of SFV-NP synthesis came from polypeptide sequence analysis using online tools (http://hc.ims.u-tokyo.ac.jp/iPSORT/; http://ihg.gsf.de/ihg/mitoprot.html). The latter suggested NH2-terminal mitochondrial localization sequences (MLS) in residues 105 to 136 (data not shown). To test this hypothesis, we generated seven truncated versions of NP in which we deleted sequences upstream from the second and third, etc., natural Met residues to the seventh. The constructs were cloned into standard (non-SFV) eukaryotic expression plasmids under the control of the immediate early cytomegalovirus promoter.
NP was localized in HeLa cells transfected with each plasmid by indirect immunofluorescence using α-COOH pAbs (Fig. 3a). Full-length NP localized to nucleus, and NP initiating on Met13 localized to the cytosol, in accord with the findings of Golovina et al. (12). As expected, full-length NP expressed from SFV localized to mitochondria, cytosol, and nuclei, whereas SFV-expressed NP engineered to initiate on Met13 was detected in cytosol and mitochondria but not in the nucleus. Importantly, each of constructs 3 to 7 clearly generated mitochondrial forms of protein (Fig. 3b). Even gene products not predicted to possess an amino-terminal MLS were found to localize to mitochondria, suggesting that NP contains a cryptic internal MLS.
FIG. 3.
NH2-terminal truncated NP localizes to mitochondria. (a) BHK cells were transfected with plasmid DNA encoding truncated NP fragments initiating at the Met residue indicated, corresponding to the first through seventh AUGs in the NP sequence. Total cell lysates were subjected to SDS-PAGE, and NP fragments were visualized by immunoblotting with α-COOH pAbs. aa, amino acid. Predicted sizes are given in kilodaltons. (b) Immunofluorescence analysis of cells transfected with indicated NP variant constructs (1 to 7) or infected with SFV-NP or SFVΔAUG-NP. NP was identified using α-COOH pAbs (green) and mitochondria with human auto-Abs (red).
Downstream initiation is not a result of translation from truncated mRNA.
There are three explanations for the downstream translational start: (i) translation from cleaved transcripts, (ii) translation from mRNA digested from the 5′ end, and (iii) downstream initiation of translation from otherwise intact mRNA.
To distinguish between these possibilities, we employed quantitative real-time reverse transcription-PCR. Using random hexamer primers, cDNA was generated from RNA isolated from cells infected with SFV-NP, SFVC-NP, or IAV and then subjected to real-time PCR using primer pairs amplifying different regions of the coding region. In the case of mechanisms (i) and (ii) described above, there would be proportionally significantly more amplicon templates within regions towards the 3′ end of the transcripts than towards the 5′ end. However, quantitative real-time PCR analyses showed that the amounts of RNA sequences corresponding to nucleotides 974 to 1084 or 244 to 345 were similar to sequences corresponding to the amplicon located most towards the 5′ end (Fig. 4).
FIG. 4.
NP is not translated from truncated mRNA in the context of SFV expression vectors. (Top) Design of primer sets used for the amplification of upstream and downstream regions of the NP gene; (bottom) quantitative real-time PCR analysis of cDNA reverse transcribed from RNA isolated from cells infected with IAV (Flu), SFV-NP, or SFVC-NP and analyzed by three primer sets as indicated. A series of standards was used to determine the absolute concentrations of DNA detected. The amount of cDNA corresponding to amplicons 107 to 229 and 244 to 345 were not lower than the amounts measured for amplicons 974 to 1084. Similar results were obtained in three independent experiments.
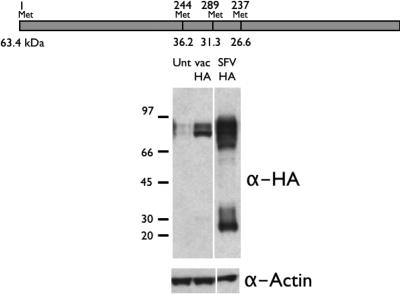
We generalized these findings to another gene product by examining the translation of IAV HA from the HA gene inserted into the SFV in the absence of the capsid element. Unlike NP, which has numerous (25) Met residues, HA has only 4 Met residues. Cells were infected with either rSFV-HA or rVV-HA. HA was detected in immunoblots by use of pooled MAbs specific for COOH-terminal regions of HA (the HA2 chain). This revealed that SFV-HA-infected cells generated considerable amounts of a truncated form of HA, consistent with initiation at one of the downstream Met residues (Fig. 5). Interestingly, the most abundant of the three downstream putative HA fragments was the shortest one, expected to initiate at the fourth AUG codon. We did not investigate this further, but it is noteworthy that of the ribosome binding sequences surrounding the three downstream Met residues, the fourth most closely resembles the consensus Kozak sequence (see Fig. 5 legend).
FIG. 5.
Downstream translation initiation by SFV vector extends to other gene products. Cells were infected with SFV (SFV HA) or vaccinia virus (Unt vac HA) vectors encoding IAV HA. HA species present in cell lysates were resolved using SDS-PAGE and identified by immunoblotting using MAbs specific for the unfolded HA2 domain of HA. α-Actin Abs were also used after stripping of immunoblots to demonstrate the loading of equal amounts cellular material (bottom). (Top) Positions of AUG codons (Met) and putative molecular masses for translation products generated by initiation at those positions. Note that the main portion of full-length HA is glycosylated and migrates with an apparent size greater than 63.4 kDa. Interestingly, the sequences flanking the second, third, and fourth AUG codons were AGGATGA, TCAATGC, and AGGATGG, respectively, with the latter being the most optimal Kozak ribosome binding site.
SFV-induced alteration in host translation factors is required for enhanced DRiP synthesis.
The NP encoding mRNA generated by SFV-NP-infected cells should be highly similar if not identical to that generated by IAV infection or transfection with cytomegalovirus plasmids. How then may the high frequency of the proposed downstream initiation be explained? A logical explanation is that altered initiation results from alterations in the translation machinery associated with SFV shutdown of host protein synthesis.
The SFV subgenomic RNA is capped at the 5′ end (32). Initiation from downstream AUGs would presumably be a result of impaired ribosomal scanning, cap-independent initiation, or both. It was previously suggested that dephosphorylation of translational eIF4E is associated with cap-independent initiation in both nonviral (8) and viral (33) systems. We therefore examined the effect of SFV infection on the generation of phospho-eIF4E. As seen in Fig. 6a, SFV infection resulted in a very large decrease in phosphorylated eIF4E. We also found a decrease in phosphorylation of ribosomal subunit S6, a modification previously linked to translation inhibition (19).
FIG. 6.
Translational initiation from downstream AUG codons requires phosphorylation of eIF2α. (a) SFV induces reduction in phospho-eIF4E levels. BHK-21 cells were infected with SFV-NP, SFVC-NP, or IAV (flu) as indicated. As controls, cells were either incubated in medium lacking bovine serum (0%) or incubated in medium containing elevated levels (20%). Lysates harvested at 12 h p.i. were subjected to SDS-PAGE and processed for Western blotting using antiserum specific for phospho-eIF4E or actin. (b) SFV induces phosphorylation of eIF2α. MEF obtained either from WT mice or from mice expressing a mutant variant of eIF2α unable to become phosphorylated (Ser51A) were infected with SFV-NP or SFVC-NP as indicated. Lysates harvested at 12 h p.i. were subjected to SDS-PAGE and processed for Western blotting using antiserum specific for phospho-Ser51-eIF2α. (c) Both virus-induced protein synthesis inhibition and the ability of the SFV capsid gene to serve as a translation enhancer require phosphorylation of eIF2α. Lysates from WT cells (MEF) and mutant cells unable to phosphorylatable eIF2α (MEF Ser51A) were infected with SFV-NP or SFVC-NP and analyzed by Western blotting for the expression of NP. The expression from the SFV vector does not suffer from host cell translational shutdown in mutant cells (rightmost lane). (d) Mitochondrial targeting of SFV-expressed NP requires phosphorylation of eIF2α. WT (MEF) and mutant cells unable to phosphorylate eIF2α (MEF Ser51A) were infected with SFV-NP and SFVC-NP as indicated and processed for immunofluorescence using Abs to the carboxy-terminal NP epitope (α-C), fully folded NP (α-F), or mitochondria (α-mt). Confocal images were obtained using identical settings for exposure and laser intensity between the samples illustrating the various expression levels. Mitochondrial NP was detectable only in WT cells infected with SFV-NP (upper left image). (e) Mitochondrial targeting of SFV1-NP requires phosphorylation of eIF2α. Immunolabeled samples of WT (MEF) and mutant cells (MEF Ser51A) from the same immunolabeled SFV1-NP specimens shown in Fig. 6d, with the exception that the settings during the confocal imaging were adjusted in order to acquire next-to-saturated images, thus illustrating the mitochondrial localization of SFV1-NP in WT cells stained with the α-C pAb.
eIF2α is another key factor in controlling translation initiation. Phosphorylation at eIF2 Ser51 greatly reduces initiation in uninfected cells and is frequently used by viruses to inhibit translation of host mRNA. We found that SFV infection greatly increases phosphorylated eIF2α as detected by immunoblotting (Fig. 6b), which confirms the findings of McInerney et al. (18).
To test the involvement of eIF2α in enhanced NP DRiP synthesis, we utilized MEF generated from knock-in mice genetically altered to express eIF2α with a Ser51-to-Ala substitution, rendering eIF2α nonphosphorylatable at this critical residue (26). Using these cells (MEF S51A) in comparison with MEF generated from a WT mouse, we could demonstrate the specificity of anti-eIF2 phospho-Ser51 immunoblotting in the SFV-induced phosphorylation of eIF2α (Fig. 6b) following infection with rSFVC-NP or rSFV1-NP. Most importantly, we found that in MEF S51A cells, NP produced by rSFV1-NP no longer localized to mitochondria as described above, and for WT MEF cells, but instead localized to the nucleus (Fig. 6e). Immunofluorescence also revealed that the Ser51A substitution abrogated capsid-induced enhancement of NP translation, since when mutant MEF were used, NP stainings were of similar intensities for rSFVC-NP- and rSRV1-NP-infected cells (Fig. 6d). This finding was confirmed by immunoblotting for NP (Fig. 6c). There are four known protein kinases that mediate phosphorylation of eIF2α and subsequent translational regulation. They share a common kinase domain but are equipped with different regulatory domains. Thus, the first, GCN2, is activated during starvation for many amino acids (7). The second, the protein kinase R (PKR)-like ER-localized eIF2α kinase (PERK) is typically activated during ER stress (36). The third, heme-regulated eIF2α kinase (HRI), is activated in response to heme deficiency, and the fourth, PKR, is activated during viral infections. The most obvious pathway for eIF2α phosphorylation is through activation of PKR. In order to rule out at least the first two pathways, we obtained fibroblasts from mice that were deficient in activated GCN2 and PERK, respectively. As expected, infection of those cells with SFV-NP did not lead to rescued expression levels, and NP was still localized to mitochondria. Since heme deficiency can be ruled out, these experiments suggest that eIF2α is phosphorylated by PKR in SFV-infected cells.
Based on these findings, we conclude that SFV induces alterations in eIF4E and eIF2α and that the latter is required for the increase in NP DRiPs resulting from downstream translation initiation.
Relevance of SFV NP DRiPs to MHC-I antigen presentation.
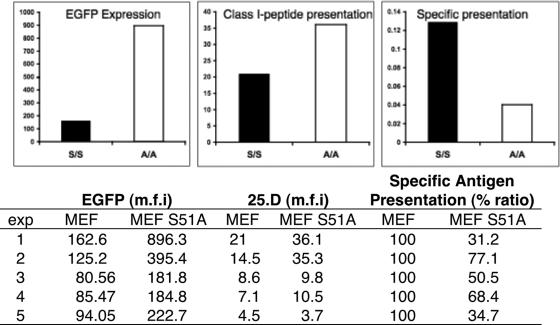
rAlphs have been widely used for vaccination studies, most of which have utilized basic “SFV-like” vectors that are likely to generate truncated polypeptides due to downstream initiation. These truncated forms are by definition DRiPs and potential substrates for degradation, processing, and presentation as MHC-I-peptide antigenic complexes. To directly examine the effect on the generation of antigenic peptides from SFV-encoded NP, we generated rSFV-NP-S-GFP, which produces a chimeric protein consisting of NP with green fluorescent protein (GFP) appended to its COOH terminus and an H-2Kb-binding reporter peptide (SIINFEKL) sandwiched in between. This allows for quantitative analysis of NP-S-GFP accumulation and Kb-SIINFEKL via flow cytometry, measuring autofluorescence from EGFP and binding of Alexa Fluor 647 conjugated to the 25-D1.16 MAb, which is highly specific for Kb-SIINFEKL complexes (23).
Relative to WT MEF (H-2b), MEF S51A cells infected with rSFV-NP-S-EGFP expressed high amounts of fluorescent NP-S-GFP, as expected (Fig. 7, left panel). However, the generation of Kb-SIINFEKL complexes was enhanced to a much lower extent by the S51A genotype (Fig. 7, middle panel). Indeed, in this experiment, the efficiency of generating Kb-SIINFEKL complexes per GFP synthesized was three times higher in S51A MEF than in WT MEF (Fig. 7, right panel). Over a total of five experiments, the efficiency of complex generation per unit fluorescence averaged twofold higher in WT MEF. Thus, the high fraction of DRiPs associated with the phospho-eIF2α-dependent downstream initiation of NP is associated with enhanced generation of peptides by the class I processing pathway.
FIG. 7.
eIF2α phosphorylation-dependent changes in antigen presentation efficiency. WT and Ser51 MEF were infected with SFV-NP-S-EGFP. Quantitative cytofluorography was used to measure transgene expression as determined by EGFP autofluorescence (left). Antigen presentation was measured simultaneously using Alexa Fluor 647-25-D1.16 (right). Specific presentation was computed as arbitrary units dividing the mean fluorescence intensity levels (background subtracted) corresponding to the 25.D staining with the EGFP fluorescence. (Bottom) The experiment was repeated four additional times with similar results, as indicated in the table.
DISCUSSION
Virus infections cause widespread alterations to the intracellular environment. Each virus species evolves its own unique replication strategy for maximizing its transmission in nature. Although it is expected that many of the virus induced-alterations that occur in natural infections will be recapitulated in ex vivo systems, it is inevitable that the recapitulation will be imperfect. Therefore, caution should be exercised in extrapolating ex vivo results to in vivo circumstances. Still, the road to understanding viral replication requires detailed characterization of viral replication in cultured cells.
Here we study the translation of gene product encoded by SFV vectors. In the most commonly used form of these vectors, the open reading frame encoding viral structural components is replaced with the gene of interest (16). This generates replicons that induce inserted gene expression without the formation of progeny virion (2). Due to the expression of early SFV gene products, such replicons induce a lytic cycle accompanied by moderate to high levels of expression of the inserted gene despite an intracellular environment where the host cell gene product translation is severely compromised (16).
We show that SFV replicons lacking the capsid element suffer from their own inhibition of host protein synthesis in the quantity of the inserted gene product translated. It was previously shown that the capsid's ability to serve as a translational enhancer required the intracellular environment of a virus infection (29), and a recent study narrows this observation down by demonstrating that phosphorylation of eIF2α is required for the enhancing effect (18).
We demonstrate that the lack of the capsid element reduces the fidelity of translation itself, since most translation is initiated at downstream Met residues. The precise mechanism for downstream initiation requires further studies. We show that eIF2α phosphorylation is required for both of these effects, but it is likely that other alterations in the translational machinery, such as dephosphorylation of eIF4E and S6, contribute to the phenomenon. Our working hypothesis is that ribosomal scanning fails to initiate translation at the normally functional AUG codon due to reduced levels of the required initiation factors, resulting in ribosomal scanning and erratic initiation reflected in the wide variety of COOH-terminal truncations we detect.
The high rate of DRiP synthesis is clearly a result of the interactions between the SFV expression vector and its host cell. Indeed, unmanipulated SFV avoids the translation inhibition it imposes on host mRNA. Its structural proteins initiate on the proper AUG and are synthesized at high levels. The key is the viral capsid element, which relieves the NP translation inhibition block and restores the fidelity of initiation. Frolov and Schlesinger (11) showed that a predicted hairpin structure 27 nt downstream of the Sindbis virus capsid AUG initiation codon is required for the capsid element to function as a translational enhancer. They proposed that resolving the hairpin structure results in a pause in scanning that enhances initiation (10).
Interestingly, although reinsertion of the capsid element largely restored the initiation of NP to the proper AUG, it still resulted in approximately twice as much NP that was nonreactive with a conformation-sensitive MAb relative to NP produced by IAV-infected cells. It will be of interest to determine the extent to which this is due to virus-specific alterations in translation or posttranslational factors (e.g., molecular chaperones) or modifications that impact NP folding. In any event, the practical point is that expression of a protein outside of its normal context is liable to result in an increase in mistranslation and misfolding, with a concomitant increase in DRiPs and antigen processing. Obviously, this is not what is wanted for gene therapy vectors. Here DRiPs will be worse than useless, since they might interfere with the function of the corresponding native translation products and will increase the chance of TCD8+ recognition and destruction of expressing cells.
The news is not entirely bad, however, as induction of TCD8+ by vaccines that are presented via direct priming (i.e., viral proteins are synthesized by the professional antigen-presenting cells that activate naïve TCD8+) is probably based largely on DRiP generation. A high DRiP rate might contribute to the impressive immunogenicity reported for first-generation SFV vectors, an effect that has previously been attributed strictly to enhanced induction of innate immune mechanisms (13, 15). There is a potential drawback, however, to a high DRiP rate for the induction of TCD8+, since the immunogenicity of vaccines that depend on cross-priming is inversely proportional to the DRiP rate, inasmuch as cross-priming is based on the acquisition of proteasome substrates (and not proteasome products) by professional antigen-presenting cells (21).
Though the two- to threefold increase in antigen presentation efficiency associated with mistranslation of NP-S-GFP may seem modest, it is consistent with our previous findings in which targeting NP-S-GFP for complete and rapid degradation by the N-end rule pathway resulted in only a threefold increase in antigen-processing efficiency (24). Presumably, this relatively modest increase reflects the high fraction of DRiPs (20 to 30%) associated with translation under the best of circumstances.
Finally, we note that the present study is based on a serendipitous discovery using a construct that we and others have studied for well over 10 years without any suspicion that upwards of 50% of the translation products are mistranslated. This particular state of ignorance would not have terminated if not for the fortunate use of conformation-insensitive Abs and the propensity of truncated NP to reside in mitochondria. It is likely that there are other examples of high levels of mistranslation awaiting less-serendipitous discovery through the use of conformation-independent Abs in conjunction with proteasome inhibitors and examination of total cellular material and not just proteins soluble in mild detergents.
Acknowledgments
We thank D. Malide, D. Tokarchick, and A. Weisberg for technical assistance and F. Hornung for thoughtful discussions.
This project was supported by the NIAID intramural research program.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Berglund, P., M. N. Fleeton, C. Smerdou, and P. Liljestrom. 1999. Immunization with recombinant Semliki Forest virus induces protection against influenza challenge in mice. Vaccine 17:497-507. [DOI] [PubMed] [Google Scholar]
- 2.Berglund, P., M. Sjoberg, H. Garoff, G. J. Atkins, B. J. Sheahan, and P. Liljestrom. 1993. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Bio/Technology 11:916-920. [DOI] [PubMed] [Google Scholar]
- 3.Berglund, P., C. Smerdou, M. N. Fleeton, I. Tubulekas, and P. Liljestrom. 1998. Enhancing immune responses using suicidal DNA vaccines. Nat. Biotechnol. 16:562-565. [DOI] [PubMed] [Google Scholar]
- 4.Bushell, M., and P. Sarnow. 2002. Hijacking the translation apparatus by RNA viruses. J. Cell Biol. 158:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, N. L., K. W. Brown, and R. E. Johnston. 1996. A viral vaccine vector that expresses foreign genes in lymph nodes and protects against mucosal challenge. J. Virol. 70:3781-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng, Y., J. Gibbs, I. Bacik, A. Porgador, J. Copeman, P. Lehner, B. Ortmann, P. Cresswell, J. R. Bennink, and J. W. Yewdell. 1998. Assembly of MHC class I molecules with biosynthesized endoplasmic reticulum-targeted peptides is inefficient in insect cells and can be enhanced by protease inhibitors. J. Immunol. 161:1677-1685. [PubMed] [Google Scholar]
- 7.Dever, T. E., and A. G. Hinnebusch. 2005. GCN2 whets the appetite for amino acids. Mol. Cell 18:141-142. [DOI] [PubMed] [Google Scholar]
- 8.Dyer, J. R., S. Michel, W. Lee, V. F. Castellucci, N. L. Wayne, and W. S. Sossin. 2003. An activity-dependent switch to cap-independent translation triggered by eIF4E dephosphorylation. Nat. Neurosci. 6:219-220. [DOI] [PubMed] [Google Scholar]
- 9.Frolov, I., T. A. Hoffman, B. M. Pragai, S. A. Dryga, H. V. Huang, S. Schlesinger, and C. M. Rice. 1996. Alphavirus-based expression vectors: strategies and applications. Proc. Natl. Acad. Sci. USA 93:11371-11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frolov, I., and S. Schlesinger. 1996. Translation of Sindbis virus mRNA: analysis of sequences downstream of the initiating AUG codon that enhance translation. J. Virol. 70:1182-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frolov, I., and S. Schlesinger. 1994. Translation of Sindbis virus mRNA: effects of sequences downstream of the initiating codon. J. Virol. 68:8111-8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golovina, T. N., E. J. Wherry, T. N. Bullock, and L. C. Eisenlohr. 2002. Efficient and qualitatively distinct MHC class I-restricted presentation of antigen targeted to the endoplasmic reticulum. J. Immunol. 168:2667-2675. [DOI] [PubMed] [Google Scholar]
- 13.Hidmark, A. S., G. M. McInerney, E. K. Nordstrom, I. Douagi, K. M. Werner, P. Liljestrom, and G. B. Karlsson Hedestam. 2005. Early alpha/beta interferon production by myeloid dendritic cells in response to UV-inactivated virus requires viral entry and interferon regulatory factor 3 but not MyD88. J. Virol. 79:10376-10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamrud, K. I., J. W. Hooper, F. Elgh, and C. S. Schmaljohn. 1999. Comparison of the protective efficacy of naked DNA, DNA-based Sindbis replicon, and packaged Sindbis replicon vectors expressing hantavirus structural genes in hamsters. Virology 263:209-219. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson, G. B., and P. Liljestrom. 2003. Live viral vectors: Semliki Forest virus. Methods Mol. Med. 87:69-82. [DOI] [PubMed] [Google Scholar]
- 16.Liljestrom, P., and H. Garoff. 1991. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology 9:1356-1361. [DOI] [PubMed] [Google Scholar]
- 17.Lundstrom, K. 2002. Alphavirus-based vaccines. Curr. Opin. Mol. Ther. 4:28-34. [PubMed] [Google Scholar]
- 18.McInerney, G. M., N. L. Kedersha, R. J. Kaufman, P. Anderson, and P. Liljestrom. 2005. Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation. Mol. Biol. Cell 16:3753-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery, S. A., P. Berglund, C. W. Beard, and R. E. Johnston. 2006. Ribosomal protein S6 associates with alphavirus nonstructural protein 2 and mediates expression from alphavirus messages. J. Virol. 80:7729-7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mossman, S. P., F. Bex, P. Berglund, J. Arthos, S. P. O'Neil, D. Riley, D. H. Maul, C. Bruck, P. Momin, A. Burny, P. N. Fultz, J. I. Mullins, P. Liljestrom, and E. A. Hoover. 1996. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J. Virol. 70:1953-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norbury, C. C., S. Basta, K. B. Donohue, D. C. Tscharke, M. F. Princiotta, P. Berglund, J. Gibbs, J. R. Bennink, and J. W. Yewdell. 2004. CD8+ T cell cross-priming via transfer of proteasome substrates. Science 304:1318-1321. [DOI] [PubMed] [Google Scholar]
- 22.Polo, J. M., J. P. Gardner, Y. Ji, B. A. Belli, D. A. Driver, S. Sherrill, S. Perri, M. A. Liu, and T. W. Dubensky, Jr. 2000. Alphavirus DNA and particle replicons for vaccines and gene therapy. Dev. Biol. 104:181-185. [PubMed] [Google Scholar]
- 23.Porgador, A., J. W. Yewdell, Y. Deng, J. R. Bennink, and R. N. Germain. 1997. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6:715-726. [DOI] [PubMed] [Google Scholar]
- 24.Princiotta, M. F., D. Finzi, S. B. Qian, J. Gibbs, S. Schuchmann, F. Buttgereit, J. R. Bennink, and J. W. Yewdell. 2003. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity 18:343-354. [DOI] [PubMed] [Google Scholar]
- 25.Salonen, A., L. Vasiljeva, A. Merits, J. Magden, E. Jokitalo, and L. Kaariainen. 2003. Properly folded nonstructural polyprotein directs the Semliki Forest virus replication complex to the endosomal compartment. J. Virol. 77:1691-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheuner, D., B. Song, E. McEwen, C. Liu, R. Laybutt, P. Gillespie, T. Saunders, S. Bonner-Weir, and R. J. Kaufman. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7:1165-1176. [DOI] [PubMed] [Google Scholar]
- 27.Schneider, R. J., and I. Mohr. 2003. Translation initiation and viral tricks. Trends Biochem. Sci. 28:130-136. [DOI] [PubMed] [Google Scholar]
- 28.Schubert, U., L. C. Anton, J. Gibbs, C. C. Norbury, J. W. Yewdell, and J. R. Bennink. 2000. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404:770-774. [DOI] [PubMed] [Google Scholar]
- 29.Sjoberg, E. M., and H. Garoff. 1996. The translation-enhancing region of the Semliki Forest virus subgenome is only functional in the virus-infected cell. J. Gen. Virol. 77:1323-1327. [DOI] [PubMed] [Google Scholar]
- 30.Sjoberg, E. M., M. Suomalainen, and H. Garoff. 1994. A significantly improved Semliki Forest virus expression system based on translation enhancer segments from the viral capsid gene. Bio/Technology 12:1127-1131. [DOI] [PubMed] [Google Scholar]
- 31.Smerdou, C., and P. Liljestrom. 1999. Two-helper RNA system for production of recombinant Semliki Forest virus particles. J. Virol. 73:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svitkin, Y. V., B. Herdy, M. Costa-Mattioli, A. C. Gingras, B. Raught, and N. Sonenberg. 2005. Eukaryotic translation initiation factor 4E availability controls the switch between cap-dependent and internal ribosomal entry site-mediated translation. Mol. Cell. Biol. 25:10556-10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheatley, D. N. 1989. Protein turnover in relation to growth status and the cell cycle in cultured mammalian cells. Rev. Biol. Celular 21:377-400. [PubMed] [Google Scholar]
- 35.Xiong, C., R. Levis, P. Shen, S. Schlesinger, C. M. Rice, and H. V. Huang. 1989. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science 243:1188-1191. [DOI] [PubMed] [Google Scholar]
- 36.Yan, W., C. L. Frank, M. J. Korth, B. L. Sopher, I. Novoa, D. Ron, and M. G. Katze. 2002. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc. Natl. Acad. Sci. USA 99:15920-15925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou, X., P. Berglund, H. Zhao, P. Liljestrom, and M. Jondal. 1995. Generation of cytotoxic and humoral immune responses by nonreplicative recombinant Semliki Forest virus. Proc. Natl. Acad. Sci. USA 92:3009-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]