Abstract
A recent clinical trial has suggested that recombinant adeno-associated virus (rAAV) vector transduction in humans induces a cytotoxic T-lymphocyte (CTL) response against the AAV2 capsid. To directly address the ability of AAV capsid-specific CTLs to eliminate rAAV-transduced cells in vitro and in vivo in mice, we first demonstrated that AAV2 capsid-specific CTLs could be induced by dendritic cells with endogenous AAV2 capsid expression or pulsed with AAV2 vectors. These CTLs were able to kill a cell line stable for capsid expression in vitro and also in a mouse tumor xenograft model in vivo. Parent colon carcinoma (CT26) cells transduced with a large amount of AAV2 vectors in vitro were also destroyed by these CTLs. To determine the effect of CTLs on the elimination of target cells transduced by AAV2 vectors in vivo, we carried out adoptive transfer experiments. CTLs eliminated liver cells with endogenous AAV2 capsid expression but not liver cells transduced by AAV2 vectors, regardless of the reporter genes. Similar results were obtained for rAAV2 transduction in muscle. Our data strongly suggest that AAV vector-transduced cells are rarely eliminated by AAV2 capsid-specific CTLs in vivo, even though the AAV capsid can induce a CTL response. In conclusion, AAV capsid-specific CTLs do not appear to play a role in elimination of rAAV-transduced cells in a mouse model. In addition, our data suggest that the mouse model may not mimic the immune response noted in humans and additional modification to AAV vectors may be required for further study in order to elicit a similar cellular immune response.
Adeno-associated virus (AAV) is a single-stranded DNA virus which carries the rep and capsid (cap) genes flanked by 165-bp terminal repeats (TRs). By replacement of endogenous sequence between the TRs, recombinant AAV (rAAV) creates a genetic cassette that can be packaged into intact virions and delivered preferentially to specific cell types. Following virus entry, nuclear trafficking, and uncoating, the released genome becomes double stranded and via intra- or intermolecular recombination of the TRs persists as circular monomers or concatemers (3). This episomal DNA is capable of long-term transgene expression using various transgenes in species: over 1 year in mice, 3 years in dogs, and 5 years in primates (1, 25, 36). This characteristic of rAAV, along with others, has put it at the forefront of gene therapy efforts, as evidenced by its administration to human infants and adults for a spectrum of diseases (over 20 clinical trials). In these trials, rAAV2 has achieved over 6 years of transgene expression and, more importantly, has proven safe (26, 34).
Although the AAV virion induces a strong humoral immune response, it cannot transduce dendritic cells (DCs) efficiently and is thought to be less immunogenic for cytotoxic T-lymphocyte (CTL) induction (38). This is indirectly supported by the observations of long-term transgene expression in transduced cells in vivo. Recently, the ability of rAAV to induce a CTL response resulting in the eradication of transduced hepatocytes was suggested in an rAAV clinical trial for factor 9 (F9) gene addition therapy (15). Therapeutic levels of F9 in the blood were detected from 2 to 6 weeks postinjection of rAAV2/F9 and then decreased to baseline. Concomitantly, transaminases released from liver damage increased. These observations strongly suggest that the AAV2 capsid can initiate a cellular immune response to eliminate rAAV2-transduced liver cells. Further study of this phenomenon in a mouse model demonstrated an rAAV2 capsid-specific CTL response following muscle administration of an adenovirus (Ad) vector carrying the cap gene of AAV2 (28). In addition, injection of rAAV2 into the liver of mice also stimulated a capsid-specific CTL response (2). These results clearly demonstrate the induction of AAV2 capsid-specific CTLs through classical antigen presentation (endogenous antigen) and cross-presentation (exogenous protein) pathways. Although AAV2 capsid-specific CTLs eradicate AAV2 peptide-pulsed spleen cells in vivo, conflicting results exist regarding the effect of these CTLs on the elimination of rAAV2-transduced liver cells (12, 21, 32). In one study, after liver delivery of rAAV2/F9, adoptive transfer of AAV2 capsid-specific CTLs reduced rAAV2 transduction in mouse liver (21). In another conflicting report, AAV2 capsid CTLs did not eradicate AAV2-transduced liver cells in mice immunized with the AAV8 capsid (AAV2 and AAV8 capsids share the same major histocompatibility complex [MHC] class I epitopes) or in mice with adoptive transfer of AAV2 capsid-specific CTLs (12, 32). The aforementioned in vitro or in vivo experiments relied solely on CTL assays using spleen cells pulsed with AAV2 capsid peptides (2, 12, 28, 32). Moreover, these studies did not utilize rAAV2-transduced cells as targets to provide direct evidence of CTL eradication of rAAV2-transduced cells, as opposed to endogenous capsid expression or peptide delivery.
To explore direct killing of target by CTLs induced by endogenous capsid expression or cross-presentation of AAV2 capsid, we first immunized mice with DCs to enhance specific CTL induction. Then the cytotoxic effect was investigated in vitro and in vivo. We demonstrated that target cells, which express endogenous AAV2 capsid, were eliminated by activated AAV2 capsid-specific CTLs in vitro and in vivo. The adoptive transfer of AAV2 capsid-specific CTLs only inhibited reporter transgene expression when the reporter and the AAV2 cap gene were delivered by a single vector. No such decrease was observed when the reporter alone was packaged into the AAV2 virion administrated through portal vein and intramuscular injections. Our results further suggest that AAV2 capsid-specific CTLs are not fully functional in vivo to eliminate AAV2-transduced target cells.
MATERIALS AND METHODS
Plasmids.
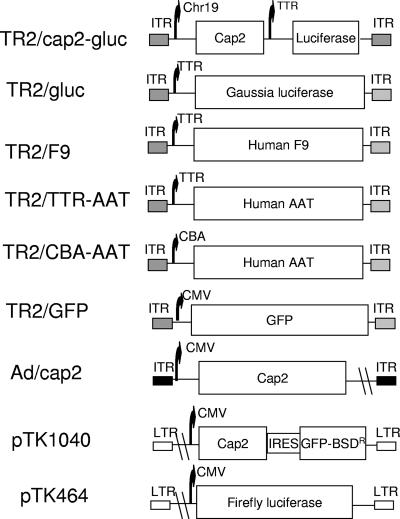
The F9 cDNA in the transthyretin (TTR) expression construct pTR2/TTR-F9 was substituted for by the Gaussia luciferase (gluc) from pCMV-GLUC-1 (NanoLight Technology, AZ) or the human α-antitrypsin cDNA (AAT) from pTR2/CBA-AAT (a generous gift from T. R. Flotte) to generate the infectious constructs pTR2/TTR-gluc and pTR2/TTR/AAT. Chromosome 19 (Ch19) and mini-cytomegalovirus (CMV) promoter fragments were amplified by PCR and used to replace the chicken β-actin (CBA) promoter of pTR2/CBA-AAT to generate pTR2/Ch19-AAT. The Ch19 fragment has demonstrated a strong liver-specific enhancer function (C. Li and R. J. Samulski, unpublished data). The AAV2 cap gene from pXR2 was used to replace the AAT cDNA of pTR2/Ch19-AAT to produce pTR2/Ch19-cap2. Finally, pTR2/Ch19-cap2/TTR-gluc was constructed by insertion of the TTR-gluc cassette from pTR2/TTR-gluc downstream of Ch19-cap2 in pTR2/Chr19-cap2. The green fluorescent protein (GFP) expression construct pTR2/GFP was previously described (11) (Fig. 1). AAV2 cap was subcloned into a shuttle vector, pShuttle-CMV, to generate pAd/cap2 (Fig. 1). To construct pTK464 (human immunodeficiency virus [HIV]-CMV-firefly luc), the firefly luc fragment from pGL3 (Promega Co., WI) was cloned into pTK208 (8). The AAV2 capsid fragment from pXR2 was inserted into the HpaI site of pTK208 to form construct pTK1040 (HIV-CMV-VP1-GFP) (Fig. 1).
FIG. 1.
Schematic diagram of constructs. Details are described in Materials and Methods. ITR, inverted terminal repeats; IRES, internal ribosome entry site; BSDR, blasticidin resistance; LTR, long terminal repeat.
Viruses and cell lines.
rAAV2 vectors were made by a calcium phosphate cotransfection method described previously (37). The lentiviruses HIV-luc and HIV-cap2 were produced from transfection of plasmids pTK464 and pTK1040, respectively, with helper plasmids as described, and the titer was determined by p24 enzyme-linked immunosorbent assay (8).
pAd/cap2 was used to generate adenovirus (Ad) recombinants through homologous recombination with the Ad backbone vector, pAdEasy-1, in BJ5183 bacterial cells. After linearization with Pac I, the Ad recombinants were used to produce Ad/cap2 vectors in 293 cells. Further amplification of Ad vector stocks was achieved by infection in 293 cells. Virus titers were determined by fluorescence-forming units using a monoclonal antibody directed against the Ad hexon protein (Sigma) and fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Sigma) (23). Typical virus titers were 109 to 1010 fluorescence-forming units/ml (9).
Colon carcinoma (CT26) cells were generously provided by M. J. Cho (School of Pharmacy, University of North Carolina at Chapel Hill). A CT26/luc cell line was established by infection of CT26 cells with HIV-luc vectors at a multiplicity of infection (MOI) of 10. Single clones from limited dilutions of transduced CT26 cells were obtained and screened for luc activity. A CT26/cap2-luc cell line was established by infection of CT26/luc cells with HIV-cap2 vectors at an MOI of 10. Blasticidin at 100 μg/ml was added for selection. Single clones were obtained from limited dilutions of HIV-cap2-transduced CT26/luc cells. Clones were confirmed by a Western blot for capsid proteins and by assaying luc activity.
YAC-1 cells (ATCC) were used to assay NK activity. A YAC-1/luc cell line was established by infection of HIV-luc vectors at an MOI of 10. Single clones were obtained by serial limited dilutions and verified by luc activity.
Mice.
Female BALB/c mice aged 5 to 6 weeks (The Jackson Laboratory) were used for this study. All mice were maintained in a specific-pathogen-free facility at the University of North Carolina at Chapel Hill. The use and care of these animals were in accordance with institutional guidelines from the National Institutes of Health.
DC generation from bone marrow cells.
Primary bone marrow DCs were obtained from mouse bone marrow precursors as described previously (10). Briefly, the bone marrow was obtained from mouse tibias and femurs by flushing them with culture medium. Next, red blood cells (RBCs) were lysed by resuspending the cell pellet in red blood lysis buffer (Sigma). Bone marrow cells were cultured at 106 cells/ml in RPMI 1640 medium (supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 10 mM HEPES, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin) containing 20 ng/ml murine granulocyte-macrophage colony-stimulating factor (R&D Systems) and 10 ng/ml murine interleukin-4 (IL-4 [R&D Systems]). Culture medium was replaced on days 2 and 4. On day 6, the nonadherent cells obtained from these cultures were considered to be bone marrow-derived DCs (immature). FACScan analysis confirmed the phenotypic markers of DCs (data not shown).
DCs pulsed with AAV2 vectors.
DCs (1 × 106) were incubated with 1 × 1013 genome-containing particles of rAAV2/GFP in 1 ml for 2 h at 37°C. Maturation of DCs was induced by adding 10 μg/ml of lipopolysaccharide to the culture medium for 24 h.
Transduction of DCs by Ad vectors.
DCs (1 × 106) were incubated with Ad/cap2 at an MOI of 100 in 1 ml for 24 h at 37°C without addition of lipopolysaccharide for maturation, since Ad infection induces DC maturation (18).
Immunohistochemistry by TSA.
Immunohistochemistry was performed by tyramide system amplification (TSA [Perkin-Elmer]) according to the manufacturer's instructions with modifications. DCs were seeded on two-chamber glass slides pretreated with l-lysine at 5 × 104 cells/well. Two hours later at 37°C, cells were fixed and permeabilized in 100% methanol at −20°C for 10 min and then washed three times for 5 min in room-temperature phosphate-buffered saline (PBS). Cells were blocked in TSA blocking buffer for 1 h at room temperature. Cells were then incubated with the monoclonal antibody A20 diluted 1:10 in PBS-0.05% Tween 20 (PBS-T). Cells were washed in PBS-T three times for 5 min and incubated for 1 h with the secondary antibody (horseradish peroxidase [HRP]-conjugated rabbit anti-mouse [Sigma]) diluted 1:5,000 in PBS-T. Cells were again washed three times for 5 min in PBS-T, incubated for 10 min in tyramide-Cy3 labeling solution, and washed three times for 5 min in PBS-T (in a light-protected Coplin jar). Slides were imaged immediately by fluorescence microscopy.
Immunization.
Mature DCs (1 × 106) were injected intravascularly into mice once a week for 3 consecutive weeks as described previously (29). Ten days later, spleens were separated and pulverized against a cell strainer. RBCs were then lysed. After washing, single spleen cells were harvested and incubated with Ad/cap2-infected DCs or AAV2 vector-pulsed DCs for 5 days in the presence of IL-2 (R&D Systems) at 10 ng/ml. Then spleen cells were collected for adoptive transfer experiments or for in vitro cytotoxicity assay.
Detection of neutralizing AAV antibodies.
The following method was previously described with slight modifications (19). Briefly, 293 cells were seeded in a 48-well plate at a density of 105 cells/well in 200 μl Dulbecco's modified Eagle's medium containing 10% fetal calf serum. The cells were cultured for 3 to 4 h at 37°C and allowed to adhere to the well. AAV2-GFP (1 × 108 particles) was incubated with serial dilutions of mouse sera collected at day 7 after the first immunization for 2 h at 4°C in a total volume of 25 μl. The mixture was added to cells (final volume of 200 μl) which contained 4 × 106 particles of Ad dl309 and incubated for 24 or 48 h at 37°C. GFP-expressing cells were counted under a fluorescent microscope. The neutralizing antibody titer was calculated using the dilution at which the percentage of GFP cells was 50% less than that in the control without sera.
In vitro CTL function assay.
The CTL cytotoxicity assay was performed by a modified approach using the same strategy described by Matzinger (16). CT26/luc cells were incubated with rAAV2/gluc (106 particles/cell) for 14 h at 37°C and served as target cells; spleen cells from immunized mice were cocultured with target cells. After the indicated time, cocultured cells were harvested by centrifugation and used to measure luc light units (LU) from the remaining target cells. The number of LU from only target cells without addition of spleen cells was used as a maximum, while the number of LU from only spleen cells served as background. The cytotoxicity was calculated as follows: (LU of experiment − background/maximum) × 100%.
NK activity assay.
Yac-1/luc cells were incubated with in vitro-restimulated spleen cells at different ratios for 4 h. The target cells were harvested and lysed with passive buffer, and the number of LU from target cells was measured (see above). Cytotoxicity was calculated as follows: (LU of experiment − background/maximum) × 100%.
Firefly luc assay.
The cell pellet from the coculture of target and activated spleen cells was lysed with lysis buffer (Stratagene, CA), and then 10 μl of the lysis solution was transferred to a 96-well plate. The number of LU was measured following addition of 100 μl firefly luc substrate (Stratagene, CA) by a Victor 02 instrument (Perkin-Elmer, MA).
In vivo tumor graft experiment.
Ten days after the last vaccination of DCs, mice were challenged subcutaneously with 2 × 105 cells of AAV2-transduced CT26/luc or CT26/cap2-luc in the right or left flanks, respectively. Tumors were measured with calipers in two dimensions every 3 to 4 days, and the size was calculated as length × width2 × 0.52.
gluc assay.
Blood was harvested from mice using heparinized capillary needles at the indicated time points after injection of rAAV2/cap-gluc or rAAV2/gluc vectors. Two and a half microliters of plasma was added to each well of a 96-well plate. gluc substrate (NanoLight Technology, AZ) was reconstituted with methanol to 1 mg/ml and then diluted 24-fold in dilution buffer (0.6 M NaCl in Tris [pH 7.5]) to make a working solution. One hundred microliters of the working solution was added to each sample. The number of LU of gluc was measured by a Victor 02 instrument.
F9 and AAT detection.
F9 and AAT levels in the blood were measured by enzyme-linked immunosorbent assay as described earlier (24).
RESULTS
Uptake of AAV2 vector particles by DCs.
Recently, it was demonstrated that AAV2 capsids induce a CTL response from endogenous AAV2 cap expression or rAAV2 transduction in mouse models by a very sensitive assay, gamma interferon (IFN-γ) intracellular staining or enzyme-linked immunospot (ELISPOT) (2, 28). However, the results from these experiments are indirect, and there is no evidence to confirm that CTLs induced by AAV2 capsids actually kill transduced target cells or those with endogenous AAV2 cap expression. To answer these questions, we used a different strategy to enhance AAV2 capsid-specific CTL induction by DC immunization. DCs are very potent antigen-presenting cells and can be obtained from bone marrow cell culture. Although DCs are not readily transduced by Ad vectors, the utilization of an Ad/cap2 virus at a high MOI of 100 increased the transduction efficiency, but it was still less than 50%, which was consistent with the previous report by Mercier et al. (18). Next, we wanted to deliver AAV particles to immature DCs by transduction but found these cells nonpermissive: less than 1% of DCs demonstrated GFP activity (data not shown). Therefore, we took advantage of the capacity of immature DCs for antigen uptake and pulsed them with rAAV2/GFP. Immature DCs were incubated with rAAV2/GFP at 107 particles/cell, and over 50% of these cells harbored intact AAV2 capsids, as determined by immunostaining (Fig. 2). To further verify DC antigen presentation of the AAV2 capsid in mice, DCs incubated with rAAV2/GFP were infused into mice by tail vein injections. Seven days later, the titers of neutralizing antibodies against the AAV2 virion were determined at 1:1,000. This confirmed that rAAV2-pulsed DCs present AAV2 capsid epitopes on the cell surface to induce a humoral immune response.
FIG. 2.
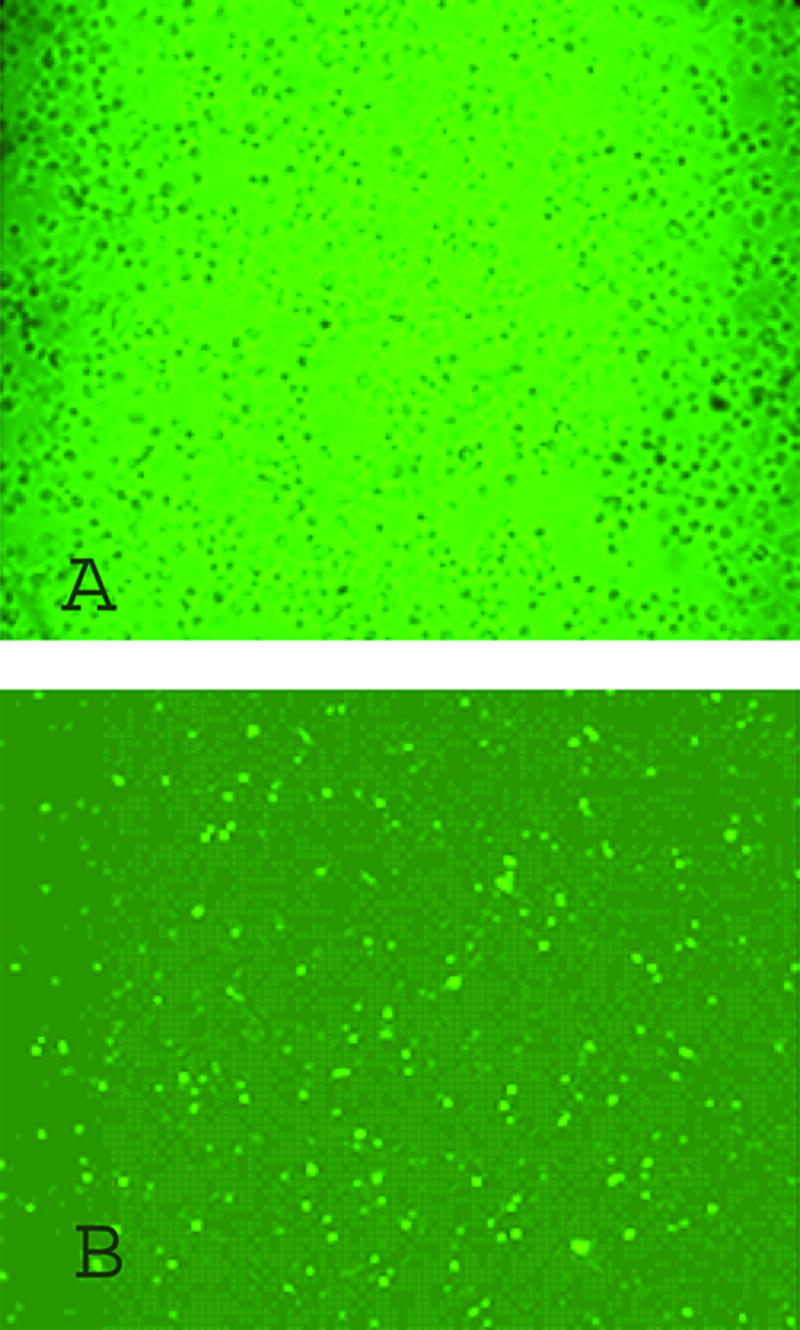
Immunostaining of DCs pulsed by AAV2/GFP vectors. Immature DCs were incubated with rAAV2/GFP vectors at 107 particles/cell for 2 h. The cells were plated onto two-chamber glass slides precoated with l-lysine for 2 h and fixed (Materials and Methods). The cells were then permeabilized, blocked, and incubated with the A20 antibody (intact capsids only) for 1 h. Then DCs were washed and incubated with a HRP-conjugated secondary antibody, and the color was developed by a tyramide-Cy3 labeling solution. The image was obtained with a fluorescence microscope. (A) DCs without AAV pulsing. (B) DCs pulsed with AAV.
AAV2 capsid-specific CTLs were induced from cross-presentation in mice.
After scanning a panel of mouse cell lines for permissivity to AAV2 transduction (data not shown), we chose CT26 colon tumor cells as the optimal targets for an in vitro CTL cytotoxicity assay. This cell line was further modified in two separate steps using lentivirus vectors. First, stable CMV-luc clones were established (CT26/luc) and then used as the parent for isolation of CMV-cap2 integrants (CT26/cap2-luc; Materials and Methods). Clonal populations of the double integrants, luc and cap2, were verified by luc activity and Western blot analysis, respectively (data not shown).
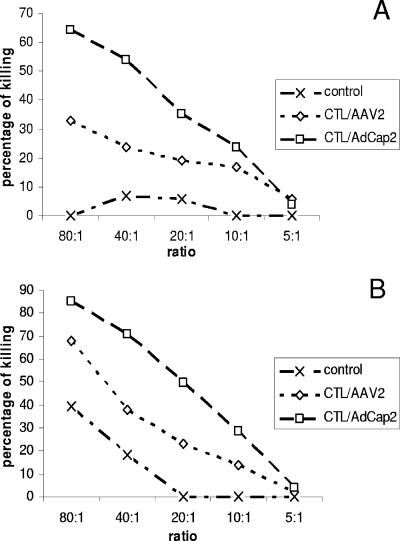
Next, we used DCs to induce an AAV2 capsid-specific CTL response. BALB/c mice were immunized, either with DCs transduced by Ad/cap2 (MOI of 100) or pulsed with rAAV2/GFP (107 particles/DC), by intravascular injection three times at weekly intervals (29). Ten days after the last immunization, spleen cells from mice were collected and used as effectors, while CT26/luc cells transduced by rAAV2/gluc (106 particles/cell for 14 h) served as targets. No cytotoxicity was observed when rAAV2-transduced CT26/luc cells were incubated with spleen cells from immunized mice at the 4-h time point (data not shown). However, after 48 h, obvious target cell killing was inferred by decreased luc activity (Fig. 3A). When the spleen cells from DC-immunized mice were restimulated with DCs, either transduced with Ad/cap2 or pulsed with rAAV2/GFP, and then incubated with AAV2-transduced CT26/luc targets, strong CTL cytotoxicity was obvious after 4 h (Fig. 3B). The CTL cytotoxicity induced by DCs pulsed with rAAV2 was lower than that of Ad/cap2-transduced DCs (Fig. 3A and B). Similar results were observed when CT26 cells with endogenous AAV2 cap and luc expression (CT26/cap2-luc) were used as targets (data not shown). These results further support that the epitopes from AAV2 capsids can be presented on the surface of AAV2-transduced target cells or antigen-presenting cells through the classical antigen presentation and cross-presentation pathways.
FIG. 3.
In vitro cytotoxicity of CTLs against the AAV2 capsid. Mice were immunized with DCs infected with Ad/cap2 or pulsed with rAAV2/GFP or just control medium (Materials and Methods). Ten days after the last vaccination, spleen cells (n = 3) were separated and restimulated with DCs infected with the groups mentioned above for 5 days in the presence of IL-2 at 10 ng/ml. Spleen cells before or after restimulation were used as effectors. CT26/luc cells transduced by rAAV2/gluc at 106 particles/cell were used as targets. The effector/target ratio is depicted as well as cytotoxicity, which is inferred by decreased luc activity. (A) Cytotoxicity of unstimulated spleen cells with an extended coculture period (48 h). (B) Cytotoxicity of restimulated spleen cells after a 4-h incubation with target cells. A representative experiment of three independent experiments is shown.
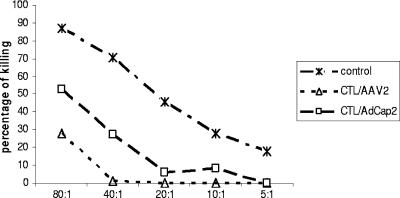
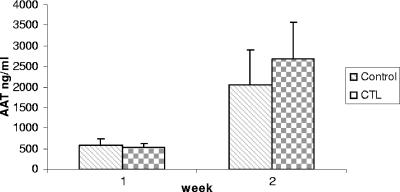
Since the spleen cells incubated with DCs in the presence of IL-2 nonspecifically killed rAAV2/gluc-infected CT26/luc cells (Fig. 3B), we explored whether NK activity was involved. For these experiments, a YAC-1 luc clone (YAC-1/luc) was established by infection with an HIV/luc vector. In vitro-restimulated spleen cells were incubated with YAC-1/luc cells at different ratios for 4 h, and luc activity was then measured. rAAV2/GFP-pulsed DCs did not induce the activation of spleen cells with NK activity compared to the control group (Fig. 4). It was not surprising that spleen cells incubated with control DCs or Ad/cap2-transduced DCs in the presence of recombinant IL-2 (rIL-2) had stronger NK activity since naïve DCs or Ad-infected DCs have been shown to enhance NK potential (6, 7, 27). These results suggest that NK cells were not activated by rAAV2 transduction.
FIG. 4.
NK activity induced by DCs pulsed with AAV2 vectors. Spleen cells from mice (n = 3) immunized with DCs, either infected with Ad/cap2 or pulsed with rAAV2/GFP, were restimulated with the corresponding DCs for 5 days. The spleen cells were then incubated with YAC-1/luc cells at different ratios. After 4 h, luciferase activity was analyzed to represent a cytotoxic effect. Representative data from three independent experiments are shown.
Tumor cells with endogenous AAV2 capsid expression can be eradicated by AAV2 capsid-specific CTLs.
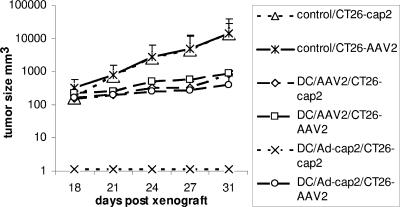
AAV2 capsid-specific CTLs are induced and can eliminate target cells expressing cap of AAV2 or those transduced by rAAV2 in vitro. To confirm the CTL function in vivo, we carried out a tumor xenograft experiment in BALB/c mice. Mice were immunized with DCs, transduced with Ad/cap2, or pulsed with rAAV2/luc (as described above). Ten days after the last vaccination, 2 × 105 CT26/cap2-luc or CT26/luc cells infected with rAAV2/gluc (106 particles/cell for 2 h at 37°C) were subcutaneously injected into the left or right flanks. Tumor mass was monitored every 3 to 4 days after transplantation, and all five control mice developed tumors by day 27 (Table 1 and Fig. 5). In mice immunized with DCs transduced with Ad/cap2, there was no growth of the CT26/cap2-luc cells, while two out of five mice developed tumors from rAAV2/luc-transduced CT26/luc cells, and tumor sizes were smaller than those of control mice (Table 1 and Fig. 5). However, in mice immunized with DCs pulsed with rAAV2, two of five mice were tumor free in both groups: CT26/cap2-luc cells or rAAV2-transduced CT26/luc cells. The other three mice of both groups had tumors with smaller masses than those of mice in the control group (Table 1 and Fig. 5). These results further support that DCs with endogenous cap expression can induce a stronger CTL response than cells simply transduced by the virus. This observation is consistent with our in vitro results demonstrating that classical antigen presentation induces a more potent response than antigen cross-presentation.
TABLE 1.
Number of mice with tumor growth following injection of AAV2 capsid-specific CTLs
| Immunization | No. of mice with tumor growth/total
|
|
|---|---|---|
| CT26/cap2-luc | CT26/luc+AAV2 | |
| DCs | ||
| Ad/cap2 | 0/5 | 2/5 |
| AAV2 | 3/5 | 3/5 |
| Control mice | 5/5 | 5/5 |
FIG. 5.
Protective effect of CTLs on tumor challenge. Mice were immunized with DCs three times as described above. Ten days after the last immunization, 2 × 105 CT26/cap2-luc or CT26/luc cells were transplanted into the right and left flanks subcutaneously. The tumor size was monitored every 2 to 3 days after engraftment. The data represent the average from mice with tumor growth in each group (mouse numbers shown in Table 1).
AAV2 capsid-specific CTLs did not eliminate rAAV2-transduced liver cells.
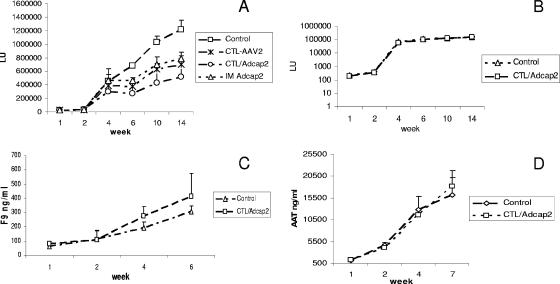
rAAV2 vectors have been used for liver-targeted gene therapy in preclinical and clinical trials (15, 20, 22, 30, 33). The clinical trial with rAAV2/F9 liver delivery suggested that CTLs against the AAV2 capsid eliminated rAAV2-transduced liver cells (15). To interpret this clinical phenomenon, further characterization in animal models yielded contradictory results (12, 21, 32). In these experiments, even though the in vivo cytotoxicity assay was carried out by infusion of peptide-pulsed spleen cells (12, 32), there was no direct positive control for liver cell elimination mediated by AAV2 capsid-specific CTLs. To set up a positive control, we carried out the following experiments. We first made a construct which contained two gene expression cassettes (rAAV2/cap-gluc): the gluc and the AAV2 cap gene driven by the TTR (a liver-specific promoter) and Chr19 promoter, respectively. After delivery of 2 × 1011 particles of rAAV2/cap2-gluc and activated CTLs into the liver, gluc activity in the blood was monitored. Transgene activity was only 25% of that of the control group, with adoptive transfer of in vitro-activated CTLs induced by DCs transduced with Ad/cap2; activities were 50% with CTLs induced by DCs pulsed with AAV2 and 60% with muscular injection of Ad/cap2 (Fig. 6A). This observation indicates that AAV2 capsid-specific CTLs can eliminate AAV2-transduced liver cells with endogenous AAV2 capsid expression, although the detection of CD8+ T-cell infiltration was not performed in the liver. Since more potent CTLs were induced from Ad/cap2-transduced DCs, we adoptively transferred these activated CTLs into mice simultaneous with liver administration of rAAV2 carrying only the gluc gene or a therapeutic gene (F9 or AAT) driven by the TTR promoter (Fig. 1). After delivery of rAAV2/gluc into the liver, no inhibition of transgene expression was demonstrated (Fig. 6B). To test whether the result from gluc gene expression holds true for therapeutic gene expression, we injected rAAV2/F9 into the liver along with the adoptive transfer of capsid-specific CTLs. A slightly higher blood F9 level was obtained in the group with CTL transfer compared to that of the control group (Fig. 6C). A similar result was obtained with rAAV2/AAT delivery into the liver (Fig. 6D). These results suggest that CTLs against the AAV2 capsid do not eliminate rAAV2-transduced liver cells to inhibit transgene synthesis (at least in mice).
FIG. 6.
Transgene expression from liver cells transduced with AAV2 vectors in the presence of AAV2 capsid-specific CTLs. Spleen cells from immunized mice were further stimulated in vitro with DCs for 5 days (Materials and Methods). A total of 2 × 1011 particles of rAAV2 were injected into the liver via the portal vein 30 min prior to tail vein infusion of activated spleen cells. The Control group represents the group without the infusion of AAV2 capsid-specific CTLs, the CTL-AAV2 group represents the group in which the CTLs were induced by DCs pulsed with AAV2 vectors, and the CTL/Adcap2 group represents the group in which CTLs were induced by DCs transduced by Ad/cap2 vectors. Transgene expression was measured at the indicated time points. The data represent the average ± standard deviation from five mice. (A) Liver transduced with AAV2/cap2-gluc. In addition to the infusion of CTLs, Ad/cap2 vectors were injected into the muscle of mice in one group (IM Ad/cap2). (B) Liver transduced with AAV2/gluc. (C) Liver transduced with AAV2/F9. (D) Liver transduced with AAV2/TTR-AAT.
AAV2 capsid-specific CTLs could not eliminate AAV2-transduced muscle cells.
After delivery of rAAV2 into the liver, transgene expression was not inhibited by the simultaneous infusion of AAV2 capsid-specific CTLs. This phenomenon might be liver specific: for example, no F9 inhibitor was demonstrated after liver cells are transduced with rAAV2/F9 in mice, perhaps due to the activation of CD4+ CD25+ regulatory T cells (4, 5, 13, 35). In contrast, previous experiments had shown that inhibitor to F9 was induced when rAAV2/F9 was administered intramuscularly (34). Therefore, we carried out the experiment to determine whether AAV2 capsid-specific CTLs could eliminate the rAAV2-transduced target cells in other tissue (muscle). After 1 × 1011 particles of rAAV2/AAT (AAT is driven by the CBA promoter) were administered into mouse muscle followed by the infusion of AAV2 capsid-specific CTLs, there was no reduction of AAT levels in blood with the CTL application compared to that of the no-CTL control (Fig. 7).
FIG. 7.
AAT expression from muscle cells transduced with AAV2/CBA-AAT vectors in the presence of AAV2 capsid-specific CTLs. A total of 1 × 1011 particles of AAV2/CBA-AAT vectors were injected into muscle of the left hind leg, and 30 min later, in vitro-restimulated spleen cells were injected into mice. The AAT levels in blood were measured at different times postinjection. The data represent the average ± standard deviation from five mice.
DISCUSSION
Our studies demonstrated that the AAV2 capsid can induce a cellular immune response via the classical MHC class I antigen presentation pathway and cross-presentation pathway. The AAV2 capsid-specific CTLs can kill target cells with endogenous AAV2 capsid expression or target cells infected with rAAV2 vectors in vitro. Tumor xenograft experiments demonstrated that CTLs completely inhibit the growth of tumor cells with endogenous cap expression, yet only partially suppress the growth of tumor cells transduced with a high MOI of rAAV2. Activated AAV2 capsid-specific CTLs by adoptive transfer eradicated liver cells with endogenous AAV2 cap expression; however, these CTLs could not eliminate the target cells transduced with rAAV2 regardless of the transgene (gluc, human F9, and AAT) or tissue (liver and muscle).
The AAV2 capsid can induce a CTL response.
The AAV2 capsid can induce a CTL response by endogenous expression of AAV2 capsid in muscle (28). After injection of Ad/cap2 into mouse muscle (BALB/c or C57), spleen cells were stimulated by addition of peptides from AAV2 capsid to enhance IFN-γ secretion. This indicates that the AAV2 capsid has immune epitopes to induce a CTL response in these mice. Bioinformatics tools to predict immune epitopes of AAV2 capsid support the above results from rAAV2 liver injection (2). The CTL response was confirmed by IFN-γ ELISPOT. In vivo experiments also showed AAV2 capsid CTL cytotoxicity by infusion of CFSE (carboxyfluorescein succinimidyl ester) dye-labeled spleen cells pulsed with AAV2 peptides (2, 12, 32). All of the above experiments were performed using peptides to pulse target cells, and no evidence exists to verify CTL cytoxicity to AAV2-transduced cells. Although the utilization of intracellular IFN-γ staining or IFN-γ ELISPOT can detect the CTL frequency, instead we chose to focus on a CTL functional assay that would directly measure CTL activity to eradicate target cells. To accomplish such, we established the cell line CT26/cap2, which expresses AAV2 cap, to test the CTL response in vitro and in vivo. After immunization with DCs (either Ad/cap2 infected or pulsed with rAAV2), fresh spleen cells demonstrated a poor CTL response in vitro. Further activation of these spleen cells in vitro enhanced CTL function (Fig. 3). These results are consistent with prior studies demonstrating that the AAV2 capsid can induce a CTL response through classical MHC class I antigen presentation and cross-presentation pathways (2, 28). To confirm a CTL response in vivo, we performed tumor xenograft experiments. After immunization of mice with Ad/cap2-infected DCs, there was no tumor growth from CT26/cap2 cells (Fig. 5 and Table 1). To further warrant the CTL function against rAAV2-transduced liver cells, we made a construct which carries the AAV2 cap and a reporter gene as a positive control (Fig. 1). After adoptive transfer of in vitro-activated spleen cells, reporter activity decreased (Fig. 6A). All of these experiments provide direct evidence that CTLs against the AAV2 capsid are functional in vitro and in vivo.
CTLs against the AAV2 capsid cannot efficiently eliminate rAAV2-transduced target cells in vivo.
CTLs can be induced to eliminate target cells with endogenous cap expression in vitro and in vivo or pulsed with specific AAV2 peptides. This raises the question of whether AAV2 capsid-specific CTLs eradicate rAAV2-transduced target cells in vivo. Conflicting results have been reported in other studies (12, 21, 32). When spleen cells from rAAV-immunized mice were adoptively transferred to naïve mice previously administered rAAV2/F9 (5 × 1010 particles), F9 levels in the blood were significantly lower than those in control mice (21). This suggests that rAAV transgene expression could be inhibited by CTLs against the AAV2 capsid. Recently, other experiments used a similar approach and demonstrated that AAV2 capsid CTLs could not inhibit rAAV2 transduction of liver cells (12, 32). In our experiments, no inhibition of transgene expression was observed with rAAV2/gluc, while a 75% decrease was observed with rAAV2/cap-gluc after adoptive transfer of AAV2 capsid-specific CTLs (Fig. 6A and B). To see whether this observation holds true for other transgenes, rAAV2/F9 or rAAV2/AAT was delivered into the mouse liver and no inhibition of transgene expression was observed (Fig. 6C and D). rAAV2/F9 administration into liver can induce immune tolerance to F9 due to activation of CD4+ CD25+ regulatory T cells (4, 5, 13, 35), which may contribute to our observation of no inhibition of transgene expression (F9, AAT, or luc [Fig. 6]), although a recent study showed no difference on F9 expression with adoptive transfer of AAV2 capsid-specific CTLs after a depletion of CD4+ CD25+ regulator T cells (12). It should be noted that the CD4+ CD25+ depletion in these experiments was not complete. Therefore, we used muscle as a target for CTL cytotoxicity and observed a similar result: no transgene inhibition (Fig. 7). These results strongly support the finding that CTLs may not eliminate rAAV2-transduced target cells in vivo.
Interpretion of differences between results obtained from the clinical trial and animal models.
The clinical trial for F9 gene therapy suggested that the AAV2 capsid induced a cell immune response which contributed to its therapeutic failure. However, the results from the animal models are inconsistent, and in either case (human or mouse) direct target cell killing by CTLs was not observed (12, 15, 28, 32). It was also suggested that the elimination of rAAV2-transduced liver cells in patients may be mediated by other mechanisms such as the activation of NK cells (12). We tested this possibility, and our data did not support this theory (Fig. 4). Based on our results in mice, rAAV2 induces a CTL response, which can eliminate target cells with endogenous cap expression or transduced with rAAV2 vector in vitro. However, these CTLs could not suppress transgene expression in transduced cells in vivo (Fig. 6 and 7). Explanations of this discrepancy are the weak immunogenicity of the AAV2 capsid in mice along with the low dose of rAAV administered in vivo compared to in vitro. It has been demonstrated that the capacity of antigen cross-presentation is low and dose responsive (14). Perhaps this is why CTLs can kill peptide-pulsed spleen cells or target cells with endogenous cap expression but not rAAV2-transduced liver or muscle cells. Additional factors can also influence these results, such as antigen processing/presentation efficiency and the slow uncoating of AAV2 in vivo (31).
In the clinical setting, the AAV2 capsid may possess strong epitopes leading to CTL induction in these patients. Therefore, an obvious strategy to evade AAV2 capsid-specific CTL induction is to use fewer rAAV particles, while maintaining efficient transgene expression. Perhaps ideal for this purpose is self-complementary rAAV, which initiates faster and higher transgene expression than conventional single-stranded rAAV2 (>10-fold) (17).
In summary, in vitro and in vivo experiments demonstrated that CTLs against the AAV2 capsid eliminated target cells with endogenous AAV2 cap expression but could not eliminate rAAV-transduced target cells in vivo. The data from our studies also indicate the weak immunogenicity of the AAV2 capsid in mice; however, a CTL response may be observed at higher doses of rAAV. It is quite possible that human MHC class I polymorphisms may contribute to the discrepancy between the results of the clinical trial and animal experiments. The AAV2 capsid may induce a very strong cellular immune response in one population but not in others. To mimic the findings from the clinical trial of rAAV2/F9 gene therapy, it is imperative to establish an animal model in which the AAV2 capsid can induce a strong cellular immune response. This will allow a greater understanding of the mechanism of AAV-induced CTL activity in humans and possibly lead to a novel approach to evade the host cellular immune response to rAAV2 in clinical trials.
Acknowledgments
We thank Roland Tisch for comments on the manuscript.
This study was supported by NIH research grants 5P01GM059299, 5P01HL066973, and 2P01HL051818 to R. J. Samulski and R01 DK058702 to T. Kafri.
Footnotes
Published ahead of print on 2 May 2007.
REFERENCES
- 1.Arruda, V. R., H. H. Stedman, T. C. Nichols, M. E. Haskins, M. Nicholson, R. W. Herzog, L. B. Couto, and K. A. High. 2005. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood 105:3458-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, J., Q. Wu, P. Yang, H. C. Hsu, and J. D. Mountz. 2006. Determination of specific CD4 and CD8 T cell epitopes after AAV2- and AAV8-hF.IX gene therapy. Mol. Ther. 13:260-269. [DOI] [PubMed] [Google Scholar]
- 3.Choi, V. W., R. J. Samulski, and D. M. McCarty. 2005. Effects of adeno-associated virus DNA hairpin structure on recombination. J. Virol. 79:6801-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobrzynski, E., J. C. Fitzgerald, O. Cao, F. Mingozzi, L. Wang, and R. W. Herzog. 2006. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc. Natl. Acad. Sci. USA 103:4592-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobrzynski, E., F. Mingozzi, Y. L. Liu, E. Bendo, O. Cao, L. Wang, and R. W. Herzog. 2004. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood 104:969-977. [DOI] [PubMed] [Google Scholar]
- 6.Ferlazzo, G., M. L. Tsang, L. Moretta, G. Melioli, R. M. Steinman, and C. Munz. 2002. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerosa, F., B. Baldani-Guerra, C. Nisii, V. Marchesini, G. Carra, and G. Trinchieri. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haack, K., A. S. Cockrell, H. Ma, D. Israeli, S. N. Ho, T. J. McCown, and T. Kafri. 2004. Transactivator and structurally optimized inducible lentiviral vectors. Mol. Ther. 10:585-596. [DOI] [PubMed] [Google Scholar]
- 9.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, C., and R. J. Samulski. 2005. Serotype-specific replicating AAV helper constructs increase recombinant AAV type 2 vector production. Virology 335:10-21. [DOI] [PubMed] [Google Scholar]
- 12.Li, H., S. L. Murphy, W. Giles-Davis, S. Edmonson, Z. Xiang, Y. Li, M. O. Lasaro, K. A. High, and H. C. Ertl. 2007. Pre-existing AAV capsid-specific CD8(+) T cells are unable to eliminate AAV-transduced hepatocytes. Mol. Ther. 15:792-800. [DOI] [PubMed] [Google Scholar]
- 13.Liang, X., Z. Chen, J. J. Fung, S. Qian, and L. Lu. 2006. Regulatory dendritic cells modulate immune responses via induction of T-cell apoptotic death. Microsurgery 26:21-24. [DOI] [PubMed] [Google Scholar]
- 14.Maecker, H. T., S. A. Ghanekar, M. A. Suni, X. S. He, L. J. Picker, and V. C. Maino. 2001. Factors affecting the efficiency of CD8+ T cell cross-priming with exogenous antigens. J. Immunol. 166:7268-7275. [DOI] [PubMed] [Google Scholar]
- 15.Manno, C. S., G. F. Pierce, V. R. Arruda, B. Glader, M. Ragni, J. J. Rasko, M. C. Ozelo, K. Hoots, P. Blatt, B. Konkle, M. Dake, R. Kaye, M. Razavi, A. Zajko, J. Zehnder, P. K. Rustagi, H. Nakai, A. Chew, D. Leonard, J. F. Wright, R. R. Lessard, J. M. Sommer, M. Tigges, D. Sabatino, A. Luk, H. Jiang, F. Mingozzi, L. Couto, H. C. Ertl, K. A. High, and M. A. Kay. 2006. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 12:342-347. [DOI] [PubMed] [Google Scholar]
- 16.Matzinger, P. 1991. The JAM test. A simple assay for DNA fragmentation and cell death. J. Immunol. Methods 145:185-192. [DOI] [PubMed] [Google Scholar]
- 17.McCarty, D. M., H. Fu, P. E. Monahan, C. E. Toulson, P. Naik, and R. J. Samulski. 2003. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 10:2112-2118. [DOI] [PubMed] [Google Scholar]
- 18.Mercier, S., H. Gahéry-Segard, M. Monteil, R. Lengagne, J.-G. Guillet, M. Eloit, and C. Denesvre. 2002. Distinct roles of adenovirus vector-transduced dendritic cells, myoblasts, and endothelial cells in mediating an immune response against a transgene product. J. Virol. 76:2899-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskalenko, M., L. Chen, M. van Roey, B. A. Donahue, R. O. Snyder, J. G. McArthur, and S. D. Patel. 2000. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J. Virol. 74:1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mount, J. D., R. W. Herzog, D. M. Tillson, S. A. Goodman, N. Robinson, M. L. McCleland, D. Bellinger, T. C. Nichols, V. R. Arruda, C. D. Lothrop, Jr., and K. A. High. 2002. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood 99:2670-2676. [DOI] [PubMed] [Google Scholar]
- 21.Murphy, S., D. Sabatino, F. Mingozzi, S. Edmonson, and K. High. 2006. Cellular immunity to adeno-associated virus capsid attenuates transgene expression in the liver. Mol. Ther. 13(Suppl. 1):S31. [Google Scholar]
- 22.Nathwani, A. C., A. M. Davidoff, H. Hanawa, Y. Hu, F. A. Hoffer, A. Nikanorov, C. Slaughter, C. Y. Ng, J. Zhou, J. N. Lozier, T. D. Mandrell, E. F. Vanin, and A. W. Nienhuis. 2002. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood 100:1662-1669. [DOI] [PubMed] [Google Scholar]
- 23.Philipson, L. 1961. Adenovirus assay by the fluorescent cell-counting procedure. Virology 15:263-268. [DOI] [PubMed] [Google Scholar]
- 24.Rabinowitz, J. E., F. Rolling, C. Li, H. Conrath, W. Xiao, X. Xiao, and R. J. Samulski. 2002. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 76:791-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera, V. M., G. P. Gao, R. L. Grant, M. A. Schnell, P. W. Zoltick, L. W. Rozamus, T. Clackson, and J. M. Wilson. 2005. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 105:1424-1430. [DOI] [PubMed] [Google Scholar]
- 26.Romano, G. 2005. Current development of adeno-associated viral vectors. Drug News Perspect. 18:311-316. [DOI] [PubMed] [Google Scholar]
- 27.Ruzek, M. C., B. F. Kavanagh, A. Scaria, S. M. Richards, and R. D. Garman. 2002. Adenoviral vectors stimulate murine natural killer cell responses and demonstrate antitumor activities in the absence of transgene expression. Mol. Ther. 5:115-124. [DOI] [PubMed] [Google Scholar]
- 28.Sabatino, D. E., F. Mingozzi, D. J. Hui, H. Chen, P. Colosi, H. C. Ertl, and K. A. High. 2005. Identification of mouse AAV capsid-specific CD8+ T cell epitopes. Mol. Ther. 12:1023-1033. [DOI] [PubMed] [Google Scholar]
- 29.Serody, J. S., E. J. Collins, R. M. Tisch, J. J. Kuhns, and J. A. Frelinger. 2000. T cell activity after dendritic cell vaccination is dependent on both the type of antigen and the mode of delivery. J. Immunol. 164:4961-4967. [DOI] [PubMed] [Google Scholar]
- 30.Snyder, R. O., C. H. Miao, G. A. Patijn, S. K. Spratt, O. Danos, D. Nagy, A. M. Gown, B. Winther, L. Meuse, L. K. Cohen, A. R. Thompson, and M. A. Kay. 1997. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet. 16:270-276. [DOI] [PubMed] [Google Scholar]
- 31.Thomas, C. E., T. A. Storm, Z. Huang, and M. A. Kay. 2004. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 78:3110-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, L., J. Figueredo, R. Calcedo, J. Lin, and J. M. Wilson. 2007. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 18:185-194. [DOI] [PubMed] [Google Scholar]
- 33.Wang, L., K. Takabe, S. M. Bidlingmaier, C. R. Ill, and I. M. Verma. 1999. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc. Natl. Acad. Sci. USA 96:3906-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warrington, K. H., Jr., and R. W. Herzog. 2006. Treatment of human disease by adeno-associated viral gene transfer. Hum. Genet. 119:571-603. [DOI] [PubMed] [Google Scholar]
- 35.Wu, W., N. Zheng, Y. Wang, J. J. Fung, L. Lu, and S. Qian. 2006. Immune regulatory activity of liver-derived dendritic cells generated in vivo. Microsurgery 26:17-20. [DOI] [PubMed] [Google Scholar]
- 36.Xiao, X., J. Li, and R. J. Samulski. 1996. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70:8098-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao, X., J. Li, and R. J. Samulski. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72:2224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, Y., N. Chirmule, G.-P. Gao, and J. Wilson. 2000. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. J. Virol. 74:8003-8010. [DOI] [PMC free article] [PubMed] [Google Scholar]