Abstract
Dendritic cells (DCs) are specialized antigen-presenting cells. However, DCs exposed to human immunodeficiency virus type 1 (HIV-1) are also able to transmit a vigorous cytopathic infection to CD4+ T cells, a process that has been frequently related to the ability of DC-SIGN to bind HIV-1 envelope glycoproteins. The maturation of DCs can increase the efficiency of HIV-1 transmission through trans infection. We aimed to comparatively study the effect of maturation in monocyte-derived DCs (MDDCs) and blood-derived myeloid DCs during the HIV-1 capture process. In vitro capture and transmission of envelope-pseudotyped HIV-1 and its homologous replication-competent virus to susceptible target cells were assessed by p24gag detection, luciferase activity, and both confocal and electron microscopy. Maturation of MDDCs or myeloid DCs enhanced the active capture of HIV-1 in a DC-SIGN- and viral envelope glycoprotein-independent manner, increasing the life span of trapped virus. Moreover, higher viral transmission of mature DCs to CD4+ T cells was highly dependent on active viral capture, a process mediated through cholesterol-enriched domains. Mature DCs concentrated captured virus in a single large vesicle staining for CD81 and CD63 tetraspanins, while immature DCs lacked these structures, suggesting different intracellular trafficking processes. These observations help to explain the greater ability of mature DCs to transfer HIV-1 to T lymphocytes, a process that can potentially contribute to the viral dissemination at lymph nodes in vivo, where viral replication takes place and there is a continuous interaction between susceptible T cells and mature DCs.
Dendritic cells (DCs) are specialized antigen-presenting cells derived from CD34+ progenitors in bone marrow. Immature DCs are scattered throughout the peripheral tissues, ready to recognize a wide range of pathogens. When infection takes place, immature DCs capture the pathogen that ends up in the intracellular lytic pathway, allowing the cells to process it into antigens. This way, immature DCs are activated and migrate from the periphery to lymph nodes, where cell activation culminates with the maturation of DCs that are then able to present processed antigens to T cells, promoting specific immune responses (6).
It has been known for years that DCs exposed to human immunodeficiency virus type 1 (HIV-1) transmit a vigorous cytopathic infection to CD4+ T cells (4). However, HIV-1 replication in DCs is generally less productive than that in CD4+ T cells or macrophages, and the frequency of HIV-1-infected DCs found in vivo is also lower (10, 22). Although limited, the small proportion of HIV-infected DCs could spread newly synthesized virus to T cells in a highly efficient way (14). On the other hand, maturation of DCs is known to reduce the ability of these cells to support HIV-1 replication (10). Interestingly, DCs do not need to be productively infected to transmit the virus and spread it in an infectious form, contrasting in this way with other HIV-1 target cells, like CD4+ T lymphocytes or macrophages (9). This particular viral transmission mechanism is known as trans infection and involves the binding and capture of HIV-1, the traffic of internalized virus, and its final release, allowing the transfer to CD4+ T cells (9). The trans infection process has been related to the ability of DC-SIGN (DC-specific ICAM-3-grabbing nonintegrin; formally CD209), a C-type lectin expressed on the surface of monocyte-derived DCs (MDDCs), to tightly bind to the HIV-1 surface envelope (Env) glycoprotein 120 (gp120) (5). However, recent studies also suggest that HIV-1 binding, uptake, and transfer from DCs to CD4+ T lymphocytes may involve alternative DC-SIGN pathways such as mannose binding C-type lectin receptors (24) or other molecules associated with lipid rafts (12). Moreover, the functional relevance of the high-affinity interaction between HIV-1 and DC-SIGN has been hampered in several studies by the use of monomeric gp120 instead of HIV-1 virions. Therefore, different factors have contributed to make the functional role of DC-SIGN in HIV-1 pathogenesis controversial.
It is well documented that the efficiency of HIV-1 transmission through trans infection can be increased upon DC maturation (10, 15, 21). This observation has led to the suggestion that mature MDDC (mMDDC)-enhanced transmission correlates with viral distribution during infectious synapse or with an increase in cell surface expression of the intercellular adhesion molecule ICAM-1, the ligand of T-cell-expressed leukocyte function antigen LFA1, which in turn facilitates viral transfer from mMDDCs to T cells (15, 21). However, the mechanisms that underlie this increase in viral transmission have not been well defined yet, despite the possible implications in augmenting viral dissemination at lymphoid tissue in vivo.
We aimed to comparatively study the effect of maturation in MDDCs and blood-derived myeloid DCs during the HIV-1 capture process. We show how the maturation of MDDCs or myeloid DCs enhances active capture of HIV-1 in a DC-SIGN-independent manner, increasing the life span of trapped virus. We also found that viral envelope glycoprotein is not required to efficiently internalize virus into a CD81- and CD63-positive compartment in mature myeloid DCs. These observations contribute to explain the greater ability of mature DCs to transfer HIV-1 to T lymphocytes, a process that can potentially contribute to the viral dissemination at lymph nodes in vivo, where viral replication takes place and there is a continuous interaction between susceptible T cells and mature DCs.
MATERIALS AND METHODS
Primary cell cultures and cell lines.
PBMCs were obtained from HIV-1-seronegative donors by Ficoll-Hypaque density gradient centrifugation of heparin-treated venous blood. Highly purified monocyte populations (>97% CD14+) were isolated with CD14+ selection magnetic beads (Miltenyi Biotec). The addition of 1,000 U/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (R&D) to 8 × 105 cells/ml cultured in RPMI containing 10% fetal bovine serum (FBS) (Invitrogen) differentiated monocytes into immature MDDCs (iMDDCs) after 7 days. Culture medium containing interleukin-4 and GM-CSF was replaced every 2 to 3 days. mMDDCs were obtained by culturing iMDDCs at day 5 for two more days in the presence of 100 ng/ml of lipopolysaccharides (LPS) (Sigma). After the depletion of CD3+ cells with a RosseteSep CD3 Depletion kit (StemCell), myeloid DCs were isolated from the PBMCs fraction of HIV-1-seronegative donors by employing the CD1c+ (BDCA-1) isolation kit (Miltenyi Biotec). Enriched populations of myeloid DCs were cultured at a final concentration of 8 × 105 cells/ml for 2 days in the presence of 20 U/ml of GM-CSF. After isolation, we obtained an average of 92% CD11c+ cells with traces of CD3 (<1%), where the main contaminants were monocytes and B lymphocytes. However, plastic adherence after 2 days of culture in the presence of GM-CSF reduced the presence of monocytes in suspension to 3% of CD14+ cells. Furthermore, when MDDCs and myeloid blood DC were obtained from the same donor, CD14+ cells were removed before BDCA-1 purification, resulting in reduced levels of contaminating CD14+ cells. We obtained mature myeloid DCs by adding 100 ng/ml of LPS during those 2 days of culture. Myeloid DCs were immunophenotyped at day 2, and MDDCs were stained at day 7 using the following monoclonal antibodies (mAbs): CD3-peridinin chlorophyll protein (PerCP), CD4-PerCP, and HLA-DR-PerCP; CD19-phycoerythrin (PE) (BD); CD11c-fluorescein isothiocyanate (FITC) (Serotec); CD14-FITC, CD83-PE, and CD86-FITC (Pharmingen); and DC-SIGN-PE (R&D). Adequate differentiation from monocytes to iMDDCs was based on the loss of CD14 and the acquisition of DC-SIGN, while maturation upregulated the expression of CD83, CD86, and HLA-DR in MDDCs and myeloid DCs. The review board on biomedical research of the Hospital Germans Trias i Pujol approved this study.
The Raji DC-SIGN B-cell line (kindly provided by Y. van Kooyk) was grown in RPMI with 10% FBS plus 1 mg/ml of geneticin (Invitrogen). The human T-cell line Hut CCR5+ (kindly provided by V. N. KewalRamani) was grown in RPMI with 10% FBS plus geneticin (300 μg/ml) and puromycin (1 μg/ml) (all from Invitrogen). The Ghost CCR5 green fluorescent protein (GFP) indicator cell line (obtained through the NIH AIDS Research and Reference Reagent Program from V. N. KewalRamani and D. R. Littman) was maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS, geneticin (500 μg/ml), hygromycin (100 μg/ml; Invitrogen), and puromycin (1 μg/ml). Hut 78 CCR5-negative and Raji cell lines were grown in RPMI with 10% FBS. HEK-293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS. All media contained 100 U/ml of penicillin and 10 μg/ml of streptomycin (Invitrogen).
Viral stocks and plasmids.
Infectious single-round pseudotyped HIV-1 stocks (HIVJRFL/NL43-Luc) were generated by cotransfecting the envelope-deficient proviral vector pNL4-3.Luc.R−E− containing the firefly luciferase reporter gene (obtained through the NIH AIDS Research and Reference Reagent Program from N. Landau) with plasmid pJRFL expressing the envelope glycoprotein of the CCR5-tropic strain HIV-1JRFL (kindly provided by V. N. KewalRamani). HIVΔenv-NL43, lacking the envelope glycoprotein, was generated by transfecting vector pNL4-3.Luc.R−E− alone. Replication-competent full-length HIV-1 stocks (HIVNFN-SX) were generated by transfecting the proviral construct NFN-SX (19), an HIV-1NL43 provirus that expresses the HIV-1JRFL envelope glycoprotein (kindly provided by W. O'Brien). HIVNL43/eGFP virus was obtained by cotransfecting vector pNL43 with plasmid vpr-eGFP as previously described (29). Briefly, 293T cells were transfected with calcium phosphate (CalPhos; BD), adding up to 20 μg of plasmid DNA. Supernatants containing virus were collected 2 days later, filtered (Millex HV, 0.45 μm; Millipore), and frozen at −80°C until use. Titers of HIVJRFL/NL43-Luc, used for trans infection assays, were determined by using the Ghost CCR5 indicator cell line containing an HIV-2 long terminal repeat linked to a GFP gene (18), resulting in a viral stock of 6.1 × 105 50% tissue culture infective doses/ml. Viral stocks contained 1 to 2 μg of p24gag per ml as measured by enzyme-linked immunosorbent assay (ELISA) (Perkin-Elmer).
Virus binding and capture assays.
A total of 3 × 105 myeloid DCs, MDDCs, Raji DC-SIGN cells, and Raji cells were incubated at 37°C for 2 h with 80 ng of HIVNFN-SX p24gag per 3 × 105 cells at a final concentration of 1 × 106 cells/ml, washed with phosphate-buffered saline (PBS), and lysed with 0.5% Triton X-100 (at a constant concentration of 5 × 105 cells per ml). Lysates were cleared of cell debris by centrifugation (10,000 × g for 5 min) to measure p24gag antigen content by ELISA. Viral capture and subsequent transfer to target cells were compared by pulsing MDDCs, Raji DC-SIGN cells, and Raji cells at 37°C or 4°C for 2 h with 300 ng of HIVJRFL/NL43-Luc p24gag per 3 × 105 cells at a final concentration of 1 × 106 cells/ml. Of note, after 48 h of viral pulsing, neither iMDDCs nor mMDDCs displayed phenotypic changes, as determined by CD83, CD86, and HLA-DR expression levels, excluding the presence of a potential maturation stimulus in the viral stocks, like carryover plasmid DNA.
To potentially inhibit virus capture, cells were preincubated for 30 min at 4°C with mannan (500 μg/ml; Sigma) or the anti-DC-SIGN mAb MR-1 (kindly provided by A. Corbí) at saturating concentrations determined using Raji DC-SIGN cells and then processed as described above. The envelope requirement for viral capture was assessed by pulsing cells with equal amounts of HIVNFN-SX and HIVΔenv-NL43 as described above.
Kinetic analysis was assessed by pulsing 2 × 106 to 4 × 106 MDDCs with 20 ng of p24gag per 5 × 105 cells at a final concentration of 2 × 106 cells/ml. To asses the time course of viral capture, part of the cells were maintained in the presence of the virus at 37°C up to 10 h before they were lysed right after an extensive wash. The rest of the cells were used to monitor viral fate after capture: 2 h after the pulse, cells were washed and kept in culture at 37°C for up to 48 h before lysing them and re-collecting the cell supernatants.
HIVNL43/eGFP was used to monitor viral capture with flow cytometry, pulsing 0.5 × 106 MDDCs with 150 ng of p24gag up to 12 h before fixing them. Analysis was done in the living cell gate determined by forward- and side-scatter light.
To analyze viral degradation in iMDDCs and mMDDCs, cells were preincubated with 250 nM of bafilomycin A1 and 10 μM of clasto-lactacystin β-lactone (both from Sigma) during 30 min at 37°C and then exposed to HIVNFN-SX as previously described. After extensive washing, part of the cells were lysed and analyzed for p24gag. The rest of the cells were left 4 h more at 37°C in the presence of these blocking agents and in the absence of virus before lysing them. Degradation was measured by subtracting cell-associated p24gag values obtained 2 h immediately after the viral pulse to cell-associated p24gag values obtained 4 h later. The percentage of degradation was calculated relative to the viral decay of p24gag values of untreated cells during 4 h, normalized to 100%.
Viral transmission assay.
To characterize viral transmission efficiencies of different DCs, immature and mature cells from the same seronegative donors were pulsed with HIVJRFL/NL43-Luc as described previously (26), with some modifications. Briefly, 3 × 105 to 5 × 105 MDDCs, Raji DC-SIGN cells, and Raji B cells were counted with Perfectcount cytometer beads (Cytognos), pulsed with 180 to 300 ng of HIVJRFL/NL43-Luc p24gag for 3 h at 37°C or 4°C, washed five times with PBS, and counted again with beads on the flow cytometer to ensure viability. In experiments done with myeloid DCs, 2 × 105 cells were pulsed with 120 ng of HIVJRFL/NL43-Luc p24gag for 3 h at 37°C. All pulsed cells were cocultured in duplicate with the target Hut CCR5+ cell line at a ratio of 1:1 (up to 1 × 105 cells per well in a 96-well plate) in the presence of 10 μg/ml of polybrene (Sigma). Cells were then assayed for luciferase activity 48 h later (BrightGLo luciferase system; Promega) in a Fluoroskan Ascent FL luminometer. To detect possible productive infection of pulsed cells, CCR5+ target cells in the cocultures were substituted with CCR5− target cells in all the experiments.
DC-SIGN quantification.
The mean number of DC-SIGN antibody binding sites (ABS) per cell was obtained with a Quantibrite kit (BD) according to the manufacturer's instructions. The mean number of PE-labeled DC-SIGN ABS per cell (antibody-to-PE ratio of 1:1; R&D) was quantified by a standard linear regression curve built with four different precalibrated beads conjugated with fixed amounts of PE molecules per bead. Geometrical mean fluorescence-obtained labeling with goat anti-mouse immunoglobulin G2b (IgG2b) PE was used as an isotype control (BD), and ABS-per-cell values were subtracted from values of corresponding samples. All cells were previously blocked with 1 mg/ml of human IgG (Baxter, Hyland Immuno) to prevent binding through the Fc portion of the antibody. Rainbow calibration particles (BD) were used before quantitation to ensure the consistency of fluorescence intensity measurements throughout all the experiments. All cells were stained at 4°C for 20 min, washed, and resuspended in PBS containing 2% formaldehyde. Samples were analyzed with FACSCalibur (BD) using CellQuest software to evaluate collected data. Forward-angle and side-scatter light gating were used to exclude dead cells and debris from the analysis.
Viral capture and transmission followed by microscopy.
MDDCs and myeloid DCs were pulsed with 50 ng of p24gag of HIVNL43/eGFP during 3 h, washed with PBS, stained with DAPI (4′,6′-diamidino-2-phenylindole) (Sigma), fixed with 2% formaldehyde, and cytospun onto glass slides. Cells were mounted in Dako fluorescent medium and sealed with nail polish to analyze them using a confocal microscope (Laser Optic Leica TCS SP2 AOBS). Colocalization experiments were done by pulsing mature myeloid DCs with equal amounts of HIVNFN-SX and HIVΔenv-NL43 during 4 h as described above. Cells were then fixed, permeabilized (Caltag), and stained with p24gag-RD1 mAb (Beckman Coulter), DAPI, and CD81, CD63, or LAMP-1 (all conjugated with FITC) (BD). Viral kinetics of mMDDCs (n = 3) were analyzed with fluorescence microscopy by comparing HIVNL43/eGFP-pulsed cells with the same cells left in the absence of virus for 4 h more.
Monocytes were negatively selected from PBMCs (Miltenyi Biotec) to avoid magnetic bead interference during electron microcopy observation. Subsequent differentiation to iMDDCs and mMDDCs was performed as stated above. MDDCs were pulsed for 30 min or 24 h with 200 ng of p24gag of HIVJRFL/NL43-Luc per 5 × 105 cells, extensively washed in PBS, and fixed in 2.5% glutaraldehyde for 1 h. Cells were then processed as described elsewhere previously (3) for analysis of ultrathin sections using a Jeol JEM 1010 electron microscope. In order to monitor viral transmission, extensively washed MDDCs pulsed for 24 h were cocultured for an extra hour with Hut CCR5+ cells at a 1:1 ratio before they were fixed.
Macropinocytic characterization and β-methyl-cyclodextrin treatment of MDDCs.
iMDDCs and mMDDCs were incubated with Alexa 488-labeled dextran (Molecular Probes) for 2 h at 4°C or 37°C and then fixed for fluorescence-activated cell sorting analysis. mMDDCs were treated with β-methyl-cyclodextrin (Sigma) at the concentrations indicated for 30 min at 37°C before they were pulsed with HIVNFN-SX and assayed for p24gag as described above. mMDDC viability in the presence of β-methyl-cyclodextrin was assessed with a flow cytometer by labeling cells with propidium iodide and DIOC-6 (Sigma and Molecular Probes, respectively) as previously described (2). Cells were incubated with Alexa 555-conjugated transferrin (25 μg/ml) from Molecular Probes for 1 h at 4°C, washed, and then shifted to 37°C for 30 min. Cells were then stained with DAPI, fixed with 2% formaldehyde, and cytospun onto glass slides for analysis by confocal microscopy.
RESULTS
Mature DCs capture higher amounts of HIVNFN-SX than immature DCs.
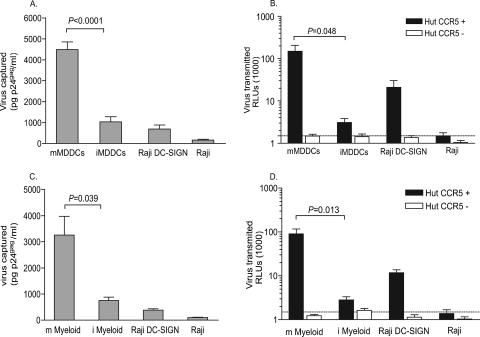
To compare viral capture abilities of mMDDCs and iMDDCs of the same seronegative donors, we pulsed both cell types with equal amounts of HIVNFN-SX at 37°C for 2 h. Raji B cells stably transfected with DC-SIGN were routinely employed to measure DC-SIGN viral capture efficiency, and the parental Raji cell line was used to determine the viral capture background. Interestingly, viral capture determined by p24gag ELISA was much more efficient in mMDDCs than in the rest of the cells tested, suggesting a differential active capture process of HIVNFN-SX in this cell type (P < 0.0001, paired t test) (Fig. 1A). This enhanced mMDDC viral capture correlated with an increased viral transmission efficiency, as observed when setting 48-h cocultures of MDDCs pulsed with pseudotyped HIVJRFL/NL43-Luc and target Hut CCR5+ T cells (P = 0.048, paired t test) (Fig. 1B). Raji DC-SIGN cells were employed to confirm viral transfer efficiency, and parental Raji cells were used as a negative control, showing the same relative light unit (RLU) values as those of nonpulsed cells (Fig. 1B, dotted line). Notably, cocultures with CCR5-negative target cells did not show any infection 48 h postpulse, indicating that neither Raji DC-SIGN cells nor MDDCs were productively infected during this experimental period (Fig. 1B, clear bars). Results were analogous by using HIVNFN-SX to pulse the cells and then measuring viral transmission by intracellular staining of cocultures with an anti-p24 mAb (data not shown). In this case, viral transfer, expressed as a percentage of p24+ cells, was also more efficient when cocultures were done with mMDDCs than when they were done with iMDDCs (P < 0.0001, paired t test).
FIG. 1.
Maturation of MDDCs and myeloid DCs enhances HIVNFN-SX capture and HIVJRFL/NL43-Luc transmission. (A) Comparative capture of HIVNFN-SX by distinct cell types. A total of 3 × 105 MDDCs, Raji DC-SIGN cells, and Raji cells were pulsed for 2 h at 37°C with 80 ng p24gag in 0.3 ml, washed with PBS, and lysed with 0.5% Triton (at a final concentration of 5 × 105 cells per ml) to measure p24gag by ELISA. Results are expressed in pg of p24gag bound per ml of cell lysate. Data show mean values and standard errors of the means (SEM) from six independent experiments including cells from 11 donors. mMDDCs significantly capture higher amounts of virus than iMDDCs (P < 0.0001, paired t test). (B) HIVJRFL/NL43-Luc transmission from MDDCs, Raji DC-SIGN cells, and Raji cells to Hut CCR5+ cells (dark bars). Cocultures were assayed for luciferase activity 48 h postpulse. No productive infection of MDDCs or Raji DC-SIGN cells was detected, as shown when using Hut CCR5-negative target cells (clear bars). The dotted line shows background levels of RLUs observed when nonpulsed cells were lysed. mMDDCs transmit higher amounts of HIVJRFL/NL43-Luc than iMDDCs derived from the same seronegative donors (P = 0.048, paired t test). (C) Comparison of HIVNFN-SX capture by myeloid DCs as described above (A), including cells from four different donors. Mature myeloid DCs (m Myeloid) significantly capture higher amounts of virus than immature myeloid DCs (i Myeloid) (P < 0.039, paired t test). (D) HIVJRFL/NL43-Luc transmission from myeloid DCs, Raji DC-SIGN cells, and Raji cells to Hut CCR5+ cells (dark bars) and to Hut CCR5− cells (clear bars) as described above (B). Mature myeloid DCs transmit higher amounts of HIVJRFL/NL43-Luc than immature myeloid DCs derived from the same seronegative donors (P = 0.013, paired t test).
We next investigated whether myeloid DCs expressing CD1c+ (BDCA-1) antigen would behave similarly to MDDCs in terms of viral capture and trans infection ability. We isolated the myeloid dendritic CD1c+ subset from PBMCs of seronegative donors. A fraction of them were subsequently LPS matured. We performed the same viral capture assay employed with MDDCs, and, as reported above for mMDDCs, viral capture was more efficient in mature myeloid DCs (P = 0.039, paired t test) (Fig. 1C). Regarding viral transmission, we tested the transfer capacity of HIVJRFL/NL43-Luc-pulsed mature and immature myeloid DCs of the same seronegative donors cocultured with Hut CCR5+ cells. As observed for MDDCs, the mean viral transmission ability of mature myeloid DCs was 50 times more efficient than that of immature myeloid DCs (P = 0.013, paired t test) (Fig. 1D). Overall, these data show that mMDDCs and mature CD1c+ myeloid DCs capture and transmit HIV-1 to a higher extent than do iMDDCs and immature myeloid DCs.
Enhanced viral transmission of mMDDCs depends on the amount of virus actively captured.
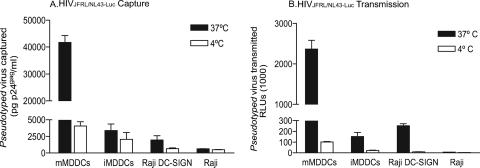
To test whether the higher viral capture of mMDDCs accounted for the increased HIV transmission observed, we pulsed both immature and mature MDDCs with high concentrations of HIVJRFL/NL43-Luc at 4°C and 37°C (Fig. 2). After extensive washing, we lysed part of the cells and determined the cell-associated virus fraction by p24gag ELISA. The rest of the washed cells were cocultured at 37°C with Hut CCR5+ target cells and assayed for luciferase activity. Of note, viral binding at 4°C in mMDDCs was similar to viral capture at 37°C observed in iMDDCs (Fig. 2A). Interestingly, the subsequent viral transmission of these two cell subsets was analogous (Fig. 2B). These results indicate that initial viral capture correlates with final viral transmission, regardless of the maturation status of DCs. It is noteworthy that Raji DC-SIGN viral capture and transmission were also temperature sensitive, showing the same behavior at 4°C as the Raji parental cell line. Again, HIVJRFL/NL43-Luc capture and transmission observed in mMDDCs pulsed at 37°C were the highest of all cell types tested.
FIG. 2.
Enhanced viral transmission of mMDDCs depends on the amount of HIVJRFL/NL43-Luc actively captured. (A) Comparative capture at 37°C and binding at 4°C of HIVJRFL/NL43-Luc by distinct cell types. A total of 3 × 105 MDDCs, Raji DC-SIGN cells, and Raji cells were incubated at 37°C (dark bars) or 4°C (clear bars) with 300 ng of HIVJRFL/NL43-Luc p24gag in a final volume of 0.3 ml, washed five times with PBS, and lysed with 0.5% Triton (at a final concentration of 5 × 105 cells per ml) to measure p24gag by ELISA. (B) HIVJRFL/NL43-Luc transmission to Hut CCR5+ target cells of pseudotyped virus captured at 37°C (dark bars) or bound at 4°C (clear bars). Different cell types pulsed as described above (A) were cocultured with target cells at 37°C and assayed for luciferase activity 48 h postpulse. A and B show a representative experiment including mean values and SEM for three different donors.
In order to exclude the possibility that mMDDCs could better stimulate target cells in the cocultures and thereby lead to a greater readout in RLUs, we also estimated the proliferation capacity of target cells in cultures with mMDDCs or iMDDCs. We found, however, no differences in Hut cell proliferation when cultured with any type of MDDCs (data not shown). These results suggest that higher viral transmission of mMDDCs is highly dependent on active viral capture.
mMDDC viral capture increases over time, and captured virus has a longer life span than in iMDDCs.
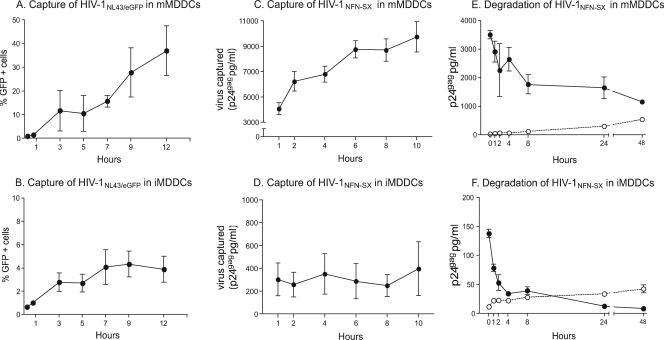
To further characterize the viral capture process, we monitored mMDDCs and iMDDCs exposed to HIVNL43/eGFP fluorescent virus or HIVNFN-SX during 10 to 12 h at 37°C (Fig. 3A to D). After extensive washing, the percentage of GFP-positive cells was analyzed by flow cytometry, and the cell-associated virus fraction was determined by p24gag ELISA at each of the time points indicated. Interestingly, viral capture in mMDDCs increased over time (Fig. 3A and C) while remaining constant in iMDDCs (Fig. 3B and D). These data show that mMDDCs actively capture HIV-1 and that viral capture differences with respect to iMDDCs increase over time.
FIG. 3.
mMDDC viral capture increases over time, and captured virus has a longer life span than in iMDDCs. (A to D) Time course of HIVNL43/eGFP or HIVNFN-SX capture by mMDDCs and iMDDCs showed that mMDDC viral capture increases over time while remaining constant in iMDDCs. (E and F) Fate of HIVNFN-SX captured for 2 h by mMDDCs and iMDDCs, respectively, monitored during 2 days. Cell-associated p24gag measured by ELISA is shown as closed symbols, while p24gag released to the cell culture medium is shown as open symbols. Captured virus showed a prolonged life span in mMDDCs. Data (means and SEM from two independent experiments) include cells from six different donors.
Next, we asked whether captured virus would follow different intracellular pathways in distinct MDDCs. Kinetic analyses of both the cell-associated and the cell-free virus fractions were longitudinally determined by p24gag in cultures of immature and mature MDDCs exposed to HIVNFN-SX and extensively washed to remove unbound virus (Fig. 3E and F). Immediately following viral exposition and washing, cell-associated virus was approximately 25 times more abundant in mMDDCs than in iMDDCs. HIVNFN-SX p24gag antigen associated with iMDDCs became quickly undetectable in the first 4h (Fig. 3F). Although these results suggest that iMDDCs may lead the virus to an intracellular lytic pathway, showing 50% ± 10% viral degradation, the steady increase in soluble p24gag also indicated some degree of virus release to the culture supernatant (22% ± 3% of captured virus). Conversely, mMDDCs degraded 30% ± 10% of captured virus during the first 4 h (Fig. 3E) without a substantial release of trapped virions (7% ± 3%). p24gag associated with mMDDCs decreased 50% ± 12% during the first day of culture without a further increase in soluble antigen, indicating the persistence of detectable cell-associated p24gag.
Finally, to exclude that the greater viral degradation of iMDDCs than of mMDDCs (P = 0.024, paired t test) could account for the viral capture differences observed, we treated cells with bafilomycin A1 (an inhibitor of lysosomic degradation) and clasto-lactacystin β-lactone (an inhibitor of proteasome activity). We found no significant increase in viral capture of drug-treated cells compared to mock-treated cells during 2 h, as previously reported (16). After 4 h in the absence of virus but in the presence of these drugs, mMDDCs degraded a mean of 51% ± 19% of the lost virus throughout lysosomes and 55% ± 15% through the proteasome machinery. Retained viral particles in iMDDCs were processed in a similar way. These data suggest that greater viral degradation in iMDDCs does not account for the viral capture differences observed between both cell types. Overall, these observations support a more efficient capture process and a longer life span of captured HIV-1 in mMDDCs than in iMDDCs.
Higher viral capture in mature DCs than in immature DCs can occur independently of DC-SIGN and does not require viral envelope glycoprotein.
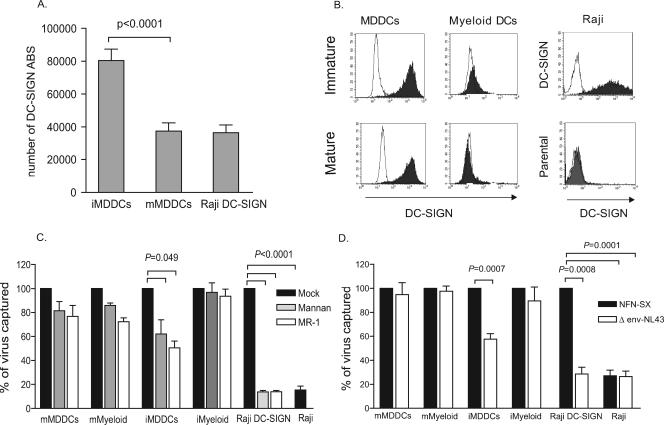
Since the ability of DCs to bind and transmit HIV-1 has been previously related to DC-SIGN expression levels in the 293 transfected cell line (20), we used a fluorescence quantitation method to obtain absolute numbers of DC-SIGN ABS in MDDCs and Raji DC-SIGN cells (Fig. 4A). We focused on MDDCs because the immunophenotype of myeloid DCs showed almost no expression of DC-SIGN (Fig. 4B). Monocytes from 30 HIV-1-seronegative donors were consecutively differentiated into immature and mature MDDCs and assayed in parallel for DC-SIGN expression. The mean number of DC-SIGN ABS per DC-SIGN-positive cell in mMDDCs (4 × 104) was half that of iMDDCs (8 × 104) (P < 0.0001, paired t test) (Fig. 4A). Raji DC-SIGN cells displayed a mean number of DC-SIGN ABS comparable to that of mMDDCs. Thus, we found no correlation between DC-SIGN expression levels and viral capture efficiency of mMDDCs and iMDDCs.
FIG. 4.
Higher viral capture in mature DCs than in immature DCs can occur independently of DC-SIGN and does not require viral envelope glycoprotein. (A) MDDCs of 30 seronegative donors were stained with anti-DC-SIGN mAb labeled with PE (1:1 ratio of mAb to PE) and analyzed for DC-SIGN surface expression using Quantibrite PE standard beads to calculate the number of DC-SIGN ABS displayed per PE-positive cell. Mean values and SEM are represented. iMDDCs had twice the mean number of DC-SIGN ABS per cell compared to those of mMDDCs and Raji DC-SIGN cells (P < 0.0001, paired t test). (B) Expression of DC-SIGN in MDDCs, myeloid DCs, Raji DC-SIGN cells, and Raji cells. Isotype-matched mouse IgG controls are also indicated (empty peaks). (C) Percentage of p24gag HIVNFN-SX captured at 37°C in the presence of different DC-SIGN inhibitors relative to untreated cells normalized to 100% of viral capture. Raji cells were compared to untreated Raji DC-SIGN cells. Viral capture by cells with no inhibitor (dark bars) or preincubated with mannan (light gray bars) or MR-1 (white bars) is depicted. Significant inhibition in the Raji DC-SIGN cell line reached 85% (P < 0.0001, paired t test). Mean p24gag (pg/ml) values of untreated cells used for normalization were 3,960 for mMDDCs, 1,154 for iMDDCs, 1,497 for mature myeloid cells (mMyeloid), 765 for immature myeloid cells (iMyeloid), 566 for Raji DC-SIGN cells, and 105 for Raji cells. Data show mean values and SEM from two independent experiments, including cells from six different donors. (D) Percentage of p24gag HIVΔenv-NL43 virus lacking the envelope glycoprotein captured by MDDCs, myeloid DCs, Raji cells, and Raji DC-SIGN cells relative to HIVNFN-SX capture normalized to 100%. Cells were pulsed with equal amounts of both viruses, and the Raji cell line was compared to Raji DC-SIGN cells. The envelope requirement for mMDDC and myeloid DC viral capture was not significant, while it reached significance in iMDDCs (P = 0.0007, paired t test). Raji DC-SIGN cells captured mainly HIVNFN-SX enveloped virus and bound HIVΔenv-NL43 virus only to levels comparable to the background seen by employing the Raji cell line (P = 0.0001, paired t test). Mean p24gag (pg/ml) values in cells exposed to HIVNFN-SX and used for normalization were 4,880 for mMDDCs, 1,562 for iMDDCs, 4,737 for mature myeloid cells, 1,280 for immature myeloid cells, 895 for Raji DC-SIGN cells, and 243 for Raji cells. Data show mean values and SEM from two independent experiments, including cells from five different donors.
We decided to further address the impact of DC-SIGN on the viral capture process mediated by MDDCs and blood myeloid DCs by testing mAb MR-1 against DC-SIGN or mannan (a C-type lectin competitive inhibitor) to see if they could impair HIVNFN-SX capture at 37°C. Figure 4C shows the percentage of HIVNFN-SX captured in the presence of different inhibitors relative to untreated cells normalized to 100% of viral capture. Pretreatment with these compounds efficiently inhibited Raji DC-SIGN viral capture to levels similar to those displayed by Raji cells (greater than 85% for any of the inhibitors; P < 0.0001, paired t test). We also observed an inhibitory effect in iMDDCs, the DC subset displaying higher amounts of DC-SIGN, but it only reached 50% (P = 0.049, paired t test) (Fig. 4C). However, neither compound had any significant effect on viral capture mediated by mMDDCs (expressing DC-SIGN) or immature and mature myeloid DCs (lacking DC-SIGN expression).
Since DC-SIGN inhibitors had no blocking effect on mature DC viral capture, and this C-type lectin is known to bind with high affinity to the gp120 viral envelope glycoprotein (5), we next analyzed the envelope requirement during the viral capture process. We pulsed MDDCs and myeloid DCs with equal amounts of a viral construct lacking the envelope glycoprotein (HIVΔenv-NL43) and its counterpart expressing the envelope protein (HIVNFN-SX). Figure 4D shows the percentage of HIVΔenv-NL43 captured by each cell type relative to cells pulsed with HIVNFN-SX, normalized to 100% of viral capture. As expected, Raji DC-SIGN cell viral capture was totally dependent on the envelope glycoprotein, as seen by the percentage of delta envelope virus captured compared to that of the wild-type virus (P = 0.0008, paired t test) (Fig. 4D). However, the envelope requirement for viral capture decreased in iMDDCs and was totally absent in myeloid DCs and mMDDCs. Overall, these results suggest that myeloid DC and mMDDC viral capture is independent of viral envelope glycoprotein, data that correlate with the lack of a blocking effect displayed by DC-SIGN inhibitors in these cell types.
Confirmation of viral capture, turnover, and transmission results by microscopy.
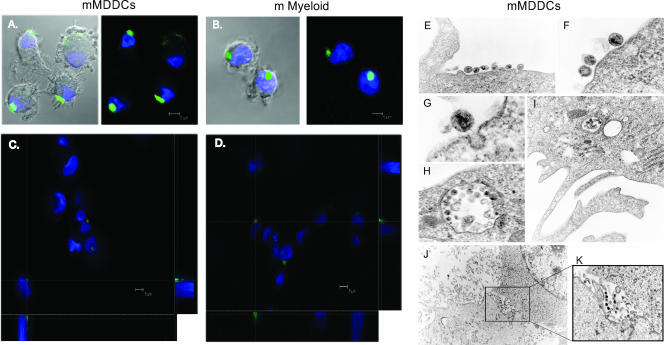
To further address viral capture, HIVNL43/eGFP-pulsed MDDCs and myeloid DCs were monitored by confocal microscopy. In agreement with our capture observations, a considerably greater amount of virions were found in mature DCs than in immature DCs after 4 h of viral exposition (Fig. 5A and B). Many mMDDCs and mature myeloid DCs showed a large single GFP-positive vesicle-like structure, observed by reconstructing a series of x-y sections collected through the nucleus of the cells to project the z-x and z-y planes (Fig. 5C and D). Of note, iMDDCs and immature myeloid DCs did not present any of these large vesicles (data not shown), suggesting differential intracellular viral trafficking in mature DCs.
FIG. 5.
Confocal and electron microscopy of HIV1 capture and transfer mediated by mMDDCs. (A) Confocal images of a section of mMDDCs exposed to HIVNL43/eGFP for 4 h and stained with DAPI. Merged images of the section showing the cells and the green and blue fluorescence are shown. (B) Composite of a series of x-y sections collected through the entire thickness of the cell nucleus of mature myeloid DCs (m Myeloid) exposed to HIVNL43/eGFP and projected onto a two-dimensional plane. A three-dimensional reconstruction of a series of x-y sections collected through part of the cell nucleus can be found as movie S1 in the supplemental material. (C) Composition of a series of x-y sections of mMDDCs collected through part of the cell nucleus and projected onto a two-dimensional plane to show the x-z plane (bottom) and the y-z plane (right) at the points marked with the dotted white axes. (D) Confocal image composition of mature myeloid DCs constructed as described above (C). (E to G) mMDDCs presented large amounts of viral particles associated with the cell membrane outside dendrites (E and F) or initiating endocytosis (G). (H and I) Large vesicles containing numerous viral particles could be found proximal to the plasma membrane surface or deep inside the cellular cytoplasm. (J) Infectious synapse could also be observed in mMDDC and Hut CCR5+ cell cocultures. The marked box on the picture shows the attachment of mMDDCs (with light cytoplasm at the bottom) to a Hut CCR5+ cell (with granulated cytoplasm and a large nucleus at the top). (K) Magnification of the marked box in J is shown on the right, where viral particles are polarized to the cell-to-cell contact area, to form what has been described as a virological synapse.
To better understand viral kinetics, HIVNL43/eGFP-pulsed mMDDCs were analyzed by fluorescence microscopy. After 4 h of viral capture, 97% ± 3% of the pulsed mMDDCs were GFP positive, presenting captured virus in one single vesicle (55% ± 8%), polarized to one side of the cell membrane (25% ± 7%), and randomly distributed throughout the cell surface (14% ± 9%). The same pulsed mMDDCs, washed and analyzed 4 h later, presented a similar viral distribution pattern, although the mean percentage of captured virus in one single vesicle increased up to 75% ± 4%, and randomly distributed virus decreased down to 4% ± 6%. These data indicate that viral vesicles represent a more stable compartment, as they contain most of the captured virus at the time when viral degradation reaches the plateau (Fig. 3E). However, the differences observed were not statistically significant, suggesting that viral turnover was comparable in each of the cell populations found.
To gain further insights into the mechanism of viral capture and transmission, HIVJRFL/NL43-Luc-pulsed MDDCs were monitored by electron microscopy. After 24 h of exposition, viral particles in mMDDCs attached to the extracellular cell membrane proximal to the soma between dendrites (Fig. 5E and F). Initial endocytic events were also observed (Fig. 5G), and vacuoles containing numerous viral particles consistent with our previous confocal microscopy observation were found proximal to the plasma membrane or deep inside the cytoplasm (Fig. 5H and I). Quantitative differences between iMDDCs and mMDDCs confirmed our previous results: among 113 mMDDCs analyzed, 33% of them showed at least one virus in the cell surface, and 28% had endocytosed virus. Conversely, among 90 iMDDCs analyzed, only 9% of them showed at least one virus in the cell surface, and 6% had endocytosed virus. Virions were associated with dendrites in only two cases (one for mMDDCs and one for iMDDCs).
Viral transmission was also monitored by using MDDCs cocultured with Hut CCR5+ cells. mMDDCs filled with HIVJRFL/NL43-Luc established associations with Hut CCR5+ cells, and virus could be found to be restricted to the site of the cell contact area (Fig. 5J and K). Polarization of viral particles to the cell-to-cell interface, where intimate contact between mMDDCs and Hut CCR5+ cells takes place, suggested the formation of an infectious synapse.
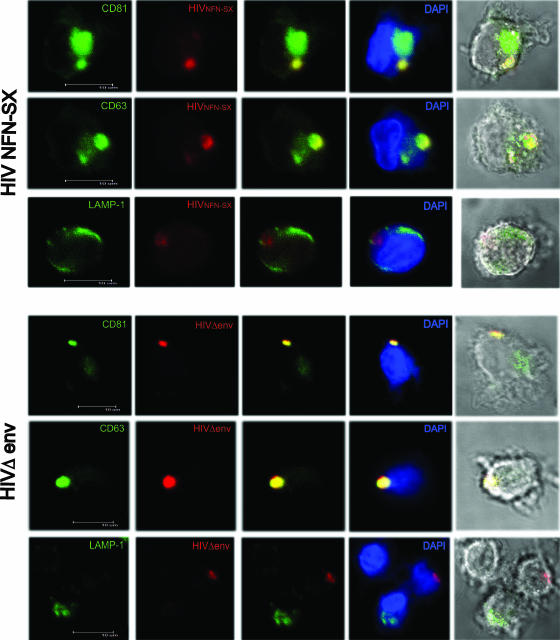
Mature myeloid DCs retain HIVNFN-SX and HIVΔenv-NL43 in a compartment similar to that of mMDDCs.
Previous work has demonstrated that in mMDDCs, HIV1 accumulates in intracellular vacuoles containing CD81 and CD63 tetraspanins (8). We extended these observations to mature myeloid DCs and found that HIVNFN-SX colocalizes with CD81 and CD63 (Fig. 6, top) but not with the LAMP-1 lysosomic marker. Furthermore, by employing HIVΔenv-NL43, we observed accumulation in the same intracellular compartments (Fig. 6, bottom). Hence, in mature myeloid DCs, the process of viral accumulation in vesicles is not directed by the envelope glycoprotein, in agreement with our previous data (Fig. 4D). Furthermore, both types of captured viruses are retained in a single CD81- and CD63-positive compartment.
FIG. 6.
Mature myeloid DCs retain HIVNFN-SX and HIVΔenv-NL43 in a CD81- and CD63-positive compartment. (Top) Confocal images of a section of mature myeloid DCs exposed to HIVNFN-SX for 4 h, fixed, and permeabilized to stain them with DAPI and p24gag (RD1). Cells were labeled in parallel for CD81, CD63, or LAMP-1 (all conjugated with FITC). Images show, from left to right, individual green and red fluorescence and the combination of both either alone, merged with DAPI, or with bright-field cellular shape. (Bottom) Confocal images of a section of mature myeloid DCs exposed to HIVΔenv-NL43 for 4 h, fixed, and permeabilized to stain them with DAPI and p24gag (RD1). Cells were labeled in parallel for CD81, CD63, or LAMP-1 (all conjugated with FITC). Images show, from left to right, individual green and red fluorescence and the combination of both either alone, merged with DAPI, or with bright-field cellular shape.
mMDDC viral capture is not mediated through pinocytosis and requires cholesterol.
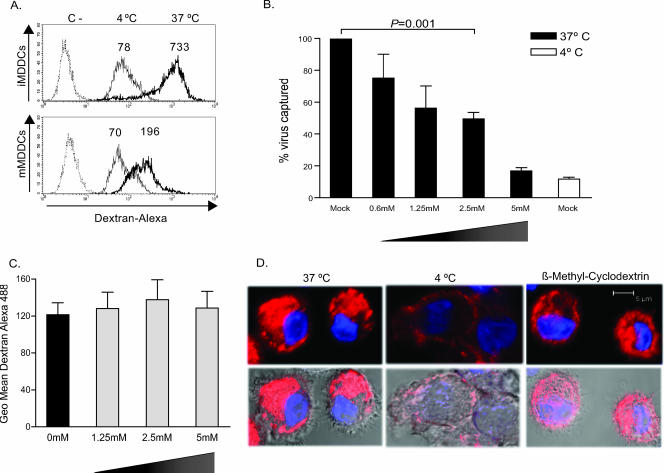
We next asked whether pinocytosis events could determine the enhanced viral capture efficiency of mature DCs. We confirmed a greater macropinocytic capacity of iMDDCs than of mMDDCs by analyzing the capture of dextran particles labeled with Alexa 488 (Fig. 7A). Fluorescence-activated cell sorting analysis revealed that both cell types showed similar capacities to bind to dextran at 4°C. However, active dextran pinocytosis was higher at 37°C in iMDDCs. These preliminary data suggested that pinocytosis does not account for the enhanced viral capture observed in mature DCs.
FIG. 7.
mMDDC viral capture is not mediated through pinocytosis and depends on cholesterol-enriched domains. (A) Increased macropinocytosis of dextran labeled with Alexa 488 in iMDDCs versus mMDDCs. Thin histograms represent dextran binding at 4°C, and thick histograms represent dextran capture at 37°C. Numbers above the peaks of the histograms indicate geometric mean fluorescence intensities. The negative control was done in the absence of dextran (dotted histograms). (B) mMDDCs were preincubated with increasing concentrations of β-methyl-cyclodextrin, a cholesterol-sequestering reagent, below observed toxic concentrations. Viral capture (performed as described in the legend of Fig. 1A) was inhibited in a dose-dependent manner, reaching statistical significance at 2.5 mM (P = 0.001, paired t test). (C) mMDDCs were preincubated with increasing concentrations of β-methyl-cyclodextrin for 30 min at 37°C, and dextran was then added to measure macropinocytosis at 37°C. Geometric mean (Geo mean) fluorescence intensity for each condition is graphed, subtracting negative controls done in the absence of dextran but in the presence of the β-methyl-cyclodextrin concentrations indicated. (D) Confocal images of a single plane of mMDDCs exposed to transferrin Alexa 555 at 4°C for 1 h and then shifted to 37°C (where transferrin bound to its receptor is able to enter through clathrin-mediated endocytosis) or left at 4°C (as a control for transferrin external binding). Previous treatment of mMDDCs with 2.5 mM β-methyl-cyclodextrin did not substantially affect transferrin clathrin-mediated endocytosis.
Previous works have shown that β-methyl-cyclodextrin, a cholesterol-sequestering reagent, efficiently blocks iMDDC viral binding and capture (12). Therefore, we addressed whether cholesterol could also play a role during mMDDC viral capture. We first checked mMDDC viability in the presence of β-methyl-cyclodextrin with a flow cytometer, labeling cells with propidium iodide and DIOC-6 to analyze the drug induction of necrosis and apoptosis. We confirmed that mMDDCs were not affected by β-methyl-cyclodextrin until we reached a concentration of 10 mM (data not shown). We then measured the effect of β-methyl-cyclodextrin on mMDDC viral capture at 37°C by employing nontoxic increasing concentrations of the drug (Fig. 7B). We found a dose-dependent inhibition of mMDDC active viral capture, reaching levels similar to those of viral binding observed at 4°C (Fig. 7B). Thus, mMDDC viral capture is a temperature-sensitive process that can be blocked by β-methyl-cyclodextrin.
To assess the specificity of β-methyl-cyclodextrin and exclude the possibility that it could be affecting other endocytic cellular pathways aside from lipid rafts, we analyzed the effect that this drug had on mMDDC pinocytosis by using dextran labeled with Alexa 488 (Fig. 7C). We found no significant effect of β-methyl-cyclodextrin on mMDDC macropinocytosis when we compared fluorescence intensities of pinocytosed dextran in mMDDCs exposed to increasing concentrations of the drug to those of mock-treated cells. This finding further supported our preliminary data indicating that mMDDC viral capture does not take place through pinocytosis. Finally, to analyze β-methyl-cyclodextrin activity regarding clathrin-mediated endocytosis, we employed transferrin labeled with Alexa 555, a control that is known to enter the cell through a clathrin-mediated pathway. We pulsed mMDDCs previously exposed to β-methyl-cyclodextrin or mock treated with transferrin at 4°C to allow binding to the cellular surface. We then washed away unbound transferrin, leaving part of the cells at 37°C to incorporate labeled ligands for 30 min and keeping the rest of the cells at 4°C to obtain binding controls. Cells were then fixed and analyzed by confocal microscopy (Fig. 7D). mMDDCs exposed to β-methyl-cyclodextrin at 2.5 mM had almost no inhibition of transferrin uptake, being absent in some of the donors tested and never exceeding 20% of inhibition (Fig. 7D). However, cells exposed to β-methyl-cyclodextrin at 5 mM showed an inhibition of transferrin uptake, indicating that this drug has collateral effects on mMDDC clathrin-mediated endocytosis. Thus, we can conclude that β-methyl-cyclodextrin at 2.5 mM is blocking mMDDC viral capture by affecting cholesterol pathways to a higher extent than clathrin-mediated endocytosis.
DISCUSSION
Soon after HIV-1 exposure, both mature and immature DCs are able to transfer the virus preferentially to antigen-specific CD4+ T cells in the absence of productive infection (14, 17). This viral transmission process, known as trans infection, is enhanced when DCs are matured in the presence of CD40 ligand, gamma interferon, poly(I:C), and lipopolysaccharide (21).
The present study compares the effect of LPS maturation on MDDCs and myeloid DCs during HIV-1 viral capture, underscoring new insights into a mechanism that might have relevant implications during pathogenesis, given the interaction of mature DCs with CD4+ T cells at lymph nodes. Previous works have shown that iMDDCs capture virus to a higher or similar extent compared to mMDDCs (7, 21). When we performed binding experiments at 4°C, no major differences between these cell types were observed. Strikingly, when cells were exposed to virus at 37°C, higher amounts of virus were found in mMDDCs, and this difference increased over time. We extended our observations to blood-derived DCs of myeloid lineage and found that mature myeloid DCs also capture higher amounts of virus than immature myeloid DCs. Thus, conversely to previous studies, our cell culture system and the viral isolates that we employed underscore a viral capture mechanism that is dramatically enhanced upon maturation that takes place independently of fusion events and does not require the envelope glycoprotein. Hence, although cell-associated p24gag values provided in Fig. 1 to 3 do not distinguish between viral capture and fusion events, the uptake of virus lacking the envelope (Fig. 4D and 6, bottom) helps to discriminate this nonfusogenic viral entry pathway. This mechanism could explain why mature DCs, with limited antigen capture activity, sequester significantly larger numbers of intact whole viral particles than immature DCs, as previously reported (7).
Early works proposed that iMDDC viral capture protects virions against degradation (9, 13), but we and others have shown that iMDDCs show rapid degradation of captured viral particles (16, 25). It is noteworthy that we found still greater amounts of virus 48 h postpulse in mMDDCs than in iMDDCs immediately after pulsing. Thus, possible explanations for enhanced viral transmission in mMDDCs versus iMDDCs include an increased ability for capturing the virus and a longer life span of trapped virions. Furthermore, our results correlate net viral capture with final viral transmission, regardless of the MDDC maturation status (Fig. 2). Therefore, in our experimental settings, mMDDC differences in ICAM-1 expression or viral distribution during the infectious synapse cannot exclusively account for the enhanced HIV-1 transmission displayed by this cell type (15, 21). In vivo, however, viral capture would need to be considered along with DCs for primary CD4 T-cell contact and any subsequent stimulation arriving from this.
Although DC-SIGN has been proposed to be the most important HIV-1 attachment factor that concentrates virus particles on the surface of DCs, recent studies also suggest that HIV-1 binding, uptake, and transfer from DCs to CD4+ T lymphocytes may involve alternative pathways (11) such as mannose binding C-type lectin receptors (23, 24) or other molecules associated with lipid rafts (12). Overall, our results further confirm these observations. Despite the fact that DC-SIGN expression is almost absent in myeloid DCs and that mMDDCs display half the number of DC-SIGN ABS compared to iMDDCs, HIV-1 capture and transfer to target T cells are augmented upon maturation in both cell types. Even though the efficiency of DC-SIGN-transfected cell lines transmitting HIV-1 has been previously related to DC-SIGN expression levels (1, 20), we confirm that the cellular context in which DC-SIGN is expressed determines capture and transmission efficiency (27, 28). Furthermore, DC-SIGN-blocking agents had minimal effects on mature DC viral capture while completely abrogating Raji DC-SIGN cell viral capture. Regarding HIV-1 envelope requirements during the capture process, we found that this glycoprotein is not necessary for myeloid DC and mMDDC viral uptake. Thus, analyzing the role of adhesion receptors dragged from the membrane of infected cells during viral budding might aid in identifying the molecular determinants that lead to viral capture in mature DCs. These factors might be ubiquitous, because we observed an increased viral uptake pattern in mature DCs compared to immature DCs exposed to viral stocks produced in 293T cells, MOLT-4 cells, and stimulated PBMCs (N. Izquierdo-Useros, unpublished results) (P = 0.006, paired t test).
We have also shown how MDDC maturation induces a rapid decrease in macropinocytic activity of fluid-phase markers such as dextran particles. Therefore, mMDDC-enhanced viral capture through a pinocytic pathway is highly unlikely, since this transportation system is less active in mMDDCs than in iMDDCs. Recent studies showed brief colocalization of HIV with transferrin (13), the archetypical cargo of clathrin-mediated endocytosis. However, virus-containing compartments in mMDDCs colabel with lipid raft-associated tetraspanins CD81, CD82, and CD9 (8). We have extended these observations and found that blood myeloid DCs also accumulate HIV1 in a CD81- and CD63-positive compartment and that this process is not mediated by the viral envelope glycoprotein. In addition, it has been shown previously that depleting cholesterol from membranes with agents such as β-methyl-cyclodextrin inhibits iMDDCs viral capture (12). That is why we have focused on cholesterol-mediated pathways of entry, further confirming their role during viral capture. Although we found β-methyl-cyclodextrin concentrations that blocked mMDDC viral capture without affecting transferrin clathrin-mediated endocytosis, higher concentrations did affect this process. We have seen, however, that β-methyl-cyclodextrin does not affect dextran pinocytosis. It is then more likely that cholesterol-enriched lipid rafts account for mMDDC viral capture, as has been previously suggested for iMDDCs, although further work should address this issue.
In conclusion, our results suggest that the unique capability of mature DCs to increase HIV-1 infection could play a key role in augmenting viral dissemination at the lymphoid tissue, a major site of viral replication and continuous interaction between susceptible T cells and mature DCs. Thus, elucidating the mechanisms underlying mature DC-enhanced HIV-1 transmission is required to design effective strategies for therapeutic intervention aimed at blocking viral spread. Furthermore, this knowledge would indeed increase the success expectations of therapeutic vaccines based on mature DC immune response-priming capacities.
Supplementary Material
Acknowledgments
We thank M. Fernández for technical assistance with flow cytometry quantification, L. Tam for manuscript editing, M. Roldán from the UAB Microscopy Service, and P. Parrales from the Pathology Department of our hospital.
J.M.-P. and J.B. are supported by the Institute for Research on Health Sciences Germans Trias i Pujol in collaboration with the Catalan Health Department and research grants SAF2004-06991, FIS 05/504, and FIPSE 36536/05, the Spanish AIDS Network grant RD06/006, and the HIVACAT program. N.I.-U. is supported by DURSI from the Generalitat de Catalunya.
Footnotes
Published ahead of print on 2 May 2007.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Baribaud, F., S. Pohlmann, G. Leslie, F. Mortari, and R. W. Doms. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135-9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco, J., J. Barretina, B. Clotet, and J. A. Este. 2004. R5 HIV gp120-mediated cellular contacts induce the death of single CCR5 expressing CD4 T cells by a gp41 dependent mechanism. J. Leukoc. Biol. 76:804-811. [DOI] [PubMed] [Google Scholar]
- 3.Bosch, B., J. Blanco, E. Pauls, I. Clotet-Codina, M. Armand-Ugon, B. Grigorov, D. Muriaux, B. Clotet, J. L. Darlix, and J. A. Este. 2005. Inhibition of coreceptor-independent cell-to-cell human immunodeficiency virus type 1 transmission by a CD4-immunoglobulin G2 fusion protein. Antimicrob. Agents Chemother. 49:4296-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 5.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figdor, C. G., Y. van Kooyk, and G. J. Adema. 2002. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2:77-84. [DOI] [PubMed] [Google Scholar]
- 7.Frank, I., M. Piatak, Jr., H. Stoessel, N. Romani, D. Bonnyay, J. D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76:2936-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia, E., M. Pion, A. Pelchen-Matthews, L. Collinson, J. F. Arrighi, G. Blot, F. Leuba, J. M. Escola, N. Demaurex, M. Marsh, and V. Piguet. 2005. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6:488-501. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 10.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granelli-Piperno, A., A. Pritsker, M. Pack, I. Shimeliovich, J. F. Arrighi, C. G. Park, C. Trumpfheller, V. Piguet, T. M. Moran, and R. M. Steinman. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 175:4265-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gummuluru, S., M. Rogel, L. Stamatatos, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 77:12865-12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 14.Lore, K., A. Smed-Sorensen, J. Vasudevan, J. R. Mascola, and R. A. Koup. 2005. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 201:2023-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 16.Moris, A., C. Nobile, F. Buseyne, F. Porrot, J. P. Abastado, and O. Schwartz. 2004. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood 103:2648-2654. [DOI] [PubMed] [Google Scholar]
- 17.Moris, A., A. Pajot, F. Blanchet, F. Guivel-Benhassine, M. Salcedo, and O. Schwartz. 2006. Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood 108:1643-1651. [DOI] [PubMed] [Google Scholar]
- 18.Morner, A., A. Bjorndal, J. Albert, V. N. Kewalramani, D. R. Littman, R. Inoue, R. Thorstensson, E. M. Fenyo, and E. Bjorling. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien, W. A., Y. Koyanagi, A. Namazie, J. Q. Zhao, A. Diagne, K. Idler, J. A. Zack, and I. S. Chen. 1990. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature 348:69-73. [DOI] [PubMed] [Google Scholar]
- 20.Pohlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Munch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76:7812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinman, R. M., A. Granelli-Piperno, M. Pope, C. Trumpfheller, R. Ignatius, G. Arrode, P. Racz, and K. Tenner-Racz. 2003. The interaction of immunodeficiency viruses with dendritic cells. Curr. Top. Microbiol. Immunol. 276:1-30. [DOI] [PubMed] [Google Scholar]
- 23.Turville, S. G., P. U. Cameron, J. Arthos, K. MacDonald, G. Clark, D. Hart, and A. L. Cunningham. 2001. Bitter-sweet symphony: defining the role of dendritic cell gp120 receptors in HIV infection. J. Clin. Virol. 22:229-239. [DOI] [PubMed] [Google Scholar]
- 24.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 25.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 26.Wu, L., A. A. Bashirova, T. D. Martin, L. Villamide, E. Mehlhop, A. O. Chertov, D. Unutmaz, M. Pope, M. Carrington, and V. N. KewalRamani. 2002. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. USA 99:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17-23. [DOI] [PubMed] [Google Scholar]
- 28.Wu, L., T. D. Martin, Y. C. Han, S. K. Breun, and V. N. KewalRamani. 2004. Trans-dominant cellular inhibition of DC-SIGN-mediated HIV-1 transmission. Retrovirology 1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, Y. J., T. Hatziioannou, T. Zang, D. Braaten, J. Luban, S. P. Goff, and P. D. Bieniasz. 2002. Envelope-dependent, cyclophilin-independent effects of glycosaminoglycans on human immunodeficiency virus type 1 attachment and infection. J. Virol. 76:6332-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.