Abstract
Foamy viruses (FV) are retroviruses that naturally infect many hosts, including most nonhuman primates (NHPs). Zoonotic infection by primate FV has been documented in people in Asia who reported contact with free-ranging macaques. FV transmission in Asia is a concern, given abundant human-NHP contact, particularly at monkey temples and in urban settings. We have developed three assays capable of detecting the presence of FV in Asian NHP species that are commensal with humans: enzyme-linked immunosorbent assay (ELISA), Western blot assays using recombinant viral Gag protein, and an indicator cell line that can detect macaque FV. The recombinant ELISA correlates very well with the presence of FV sequences detected by PCR. We have used these assays to demonstrate both that FV is highly prevalent among free-ranging NHPs and that seroconversion occurs at a young age in these animals. These assays should also prove useful for large-scale analysis of the prevalence of FV infections in human populations in Asia that are commensal with free-ranging NHPs.
Foamy viruses (FV) comprise a subfamily of retroviruses (22). FV were first identified over 50 years ago (10) as contaminants in monkey tissue culture explants. They are highly cytopathic in tissue culture. Infection of a number of cell types, including fibroblasts and epithelial cells, leads to rapid syncytium formation, vacuolization, and cell death. Despite this, infection in animal hosts does not produce a recognized disease state. Rather, FV establish a persistent asymptomatic infection in both natural and zoonotic hosts (reviewed in reference 23). Although proviral DNA can be found in nearly every tissue, indicating infection, the virus only replicates to a detectable level in the oral mucosa. Replication at this site facilitates transfer to other hosts through saliva (26). Although it is not known how latency is maintained in vivo, an in vitro latency model has been described in which viral replication is controlled at the transcriptional level (24).
FV are widespread and have been isolated from a variety of nonprimate species, including cows, cats, and horses (reviewed in reference 27). All nonhuman primates (NHPs) examined to date, including gorillas, chimpanzees, orangutans, baboons, African green monkeys, and macaques (reviewed in reference 12) also harbor FV, called simian foamy viruses (SFV). Infection among captive populations of NHPs is high. Studies from captive and free-ranging populations show that up to 100% of adult NHPs are infected with SFV (2, 7, 8, 16, 17, 19). Curiously, despite its widespread infection among NHPs, evidence suggests that there is no human-specific FV (reviewed in reference 23). A single report describing HFV (human foamy virus) in a tissue culture that was derived from a Kenyan man (1) is now believed to represent a zoonotic transmission of SFV from chimpanzees (32). There are several reports of zoonotic transmission of SFV from various taxa of NHPs. Many of the infected individuals, such as zoo keepers and animal care workers, had frequent contact with captive primates (5, 9, 15, 28, 32). Zoonotic infection of SFV has also been documented among bushmeat hunters in Africa (34) and in a monkey temple worker in Asia (17). The potential for zoonotic transmission of SFV, especially in Asia, is increasingly recognized. Several Asian and Southeast Asian cultures venerate NHPs and honor centuries-old traditions of human-NHP commensalism (close interactions associated with habitually sharing a space). Human-NHP contact in Asia occurs in a variety of contexts, including urban settings, temples, pet NHPs, monkey performances, ecotourism, and bushmeat hunting. In particular, urban and temple monkeys are found throughout South and Southeast Asia (14), and the sheer number of people who come into contact with monkeys in these contexts is large. Consequently, the amount and intensity of contact that occurs between humans and monkeys in Asia puts large numbers of people at risk for SFV infection (11, 13, 19).
Traditionally, humans have been screened for SFV infection by Western blotting (WB), using viral protein lysate prepared by infecting tissue cell cultures with different SFV strains. Some studies have yielded false positives because of the presence of serum antibodies to cellular proteins (reviewed in reference 23). In many cases, the presence of virus in humans has been confirmed by sequence analysis. However, neither of these assays is particularly convenient for high-throughput screens of large numbers of samples. Several groups have used enzyme-linked immunosorbent assays (ELISAs), using crude tissue culture lysates from uninfected and infected cells as antigens (2, 34). However, it is hard to standardize such assays, as the level of antigen in such lysates can vary between preparations and different cell proteins will cross-react depending on the cell type used. Additionally, recent data (33) show that SFV are genetically heterogeneous, with significant virus variation among NHP taxa. This is an important consideration in areas and contexts where humans come into contact with multiple species of NHPs (17, 18, 29). It is important to take into consideration this viral diversity in the development of immunoassays that are capable of detecting a broad range of SFV infections. In this study, we describe the development of assays for the detection of both SFV and SFV antibodies from six taxa of NHPs in Asia (Macaca assamensis, M. fascicularis, M. arctoides, M. nemestrina, M.mulatta/fascicularis hybrid, and Presbytis obscura). We have created a cell line that can be used to quantitate virus from at least three macaque species. We describe the development of novel methods of testing for SFV antibodies, including ELISA using recombinant viral protein, WB with lysates and recombinant protein, and an assay for neutralizing antibodies using the indicator line. We demonstrate that SFV isolates and antisera from a number of NHP species can be detected with all of the reagents. We have used the ELISA to screen a large number of free-ranging NHPs in Thailand and have determined the seroprevalence of SFV infection in these populations and an estimated age of seroconversion.
MATERIALS AND METHODS
Collection of monkey samples. (i) Oregon and Washington National Primate Research Centers.
Serum, blood, and saliva were collected from M. mulatta from animals housed at the Oregon National Primate Research Center (ONPRC) in Beaverton, OR. These animals and samples are described in reference 26. Serum and blood were collected from M. fascicularis and M. nemestrina from animals housed at the Washington National Primate Research Center (WaNPRC) in Seattle, WA. All studies were conducted in accordance with the standards set by the centers' Animal Care and Use Committees.
(ii) Sample collection from commensal, free-ranging monkeys from Thailand and Singapore.
Since 2004, commensal, free-ranging and pet monkeys (M. fascicularis, M. arctoides, M. mulatta/fascicularis hybrid, M. assamensis, M. nemestrina, and P. obscura) from Thailand and M. fascicularis from Singapore were trapped, sampled, and released using a previously described method (19). Briefly, free-ranging macaques and langurs were trapped in portable cages (measuring approximately 2.5 m by 2.5 m by 1.5 m) fabricated at the WaNPRC. Captured primates were hand injected intramuscularly with 3 mg of Telazol/kg of body weight from outside the cage. All anesthetized NHPs were given a complete physical exam. While under anesthesia, data collected included morphometric measurements and biological samples (blood and buccal swabs). Preserved whole blood and buccal swabs were frozen while in the field. Serum was extracted using Vacutainer separator tubes, followed by centrifugation, and then frozen. Each NHP's weight and dental formula were collected and recorded for age assessment.
Following sample collection, the anesthetized animals were placed in a recovery cage and allowed to recover fully from anesthesia before being released as a group back into their home range. NHPs were given a unique identifier via a subcutaneous Avid microchip to avoid resampling. This data collection protocol was reviewed and approved by the University of Washington's Institutional Animal Care and Use Committee (3143-03).
Tissue culture cells and virus isolation.
Peripheral blood mononuclear cells (PBMC) were separated from whole blood over Ficoll and were cocultured with TF cells, a fibroblast cell line from M. mulatta that had been immortalized by expression of human telomerase (21). TF cells are highly susceptible to SFV infection and produce high-titer virus. Cultures were passaged several times until extensive cytopathic effects (CPE) were noted. At that time, culture supernatants were used to infect fresh cultures of TF cells. After CPE were noted, cells were scraped from plates along with the medium, and the mix was frozen and thawed three times. Cell debris was removed by low-speed centrifugation to produce virus stocks. Viruses were isolated from several different M. mulatta (ONPRC), M. nemestrina (WaNPRC), and M. fascicularis (WaNPRC) animals. All SFV isolates used in these studies were passaged minimally in tissue culture (less than 1 month). TF and SFAB (for “simian foamy activation of β-galactosidase”) cells (see below) were grown in Dulbecco's modified Eagle's minimum essential medium containing 10% fetal calf serum.
SFAB assay.
SFAB cells were derived from BHK-21 cells by transfection of a plasmid carrying the SFV-1 long terminal repeat (LTR) derived from a molecular clone (25) driving β-galactosidase, as previously described for creation of the prototype foamy virus (PFV) indicator FAB cell line (35). Individual clones were picked and infected with SFV-1, the laboratory strain of SFV from M. mulatta (25). A clone yielding the highest viral titer and the lowest background was designated SFAB. The titers of the viruses were determined on SFAB cells as previously described for FAB cells. Cells were infected with virus and fixed and stained 2 days later (35), except that SFAB cells were stained for 1 h at 37°C rather than the 6 h used for FAB cells. At this time, cells were washed with isotonic buffer and left at room temperature overnight, and blue cells were counted the next day. The 1-h staining period is critical for SFAB cells, as staining for longer periods leads to a large number of cells with light-blue background staining in the uninfected samples, something not seen with FAB cells. By definition, 1 infectious unit (IU) of virus leads to one blue cell or colony in the SFAB assay.
Neutralization assay.
The neutralization assay followed the protocol previously described for simian immunodeficiency virus neutralization (36), with some modifications. Dilutions of virus and serum were incubated for 1 h at 37°C in wells of 48-well tissue culture plates. After incubation, 1.5 × 104 SFAB cells in 100 μl of medium were seeded into each well. Blue cells were counted, as described above, 2 days later. For assay of the PFV derived from chimpanzees, FAB cells were added to the wells rather than SFAB cells. Neutralization assays were done in duplicate or triplicate, and the average was calculated.
ELISA.
The ELISA was done as previously described (26) using 50 ng of either glutathione S-transferase (GST)-Gag or GST. GST-Gag contains the N-terminal 193 amino acids from pSFV-1 (25). The optical densities at 450 nm (OD450) from the GST plates at each serum dilution were subtracted from the GST-Gag plates. For monkey sera, the GST values at all serum dilutions were very low. Each assay included a known positive and negative serum.
WB.
TF cells were infected with SFV isolated from different macaque species or were left uninfected. When cytopathic effects appeared (3 to 4 days after infection), cells were lysed in sodium dodecyl sulfate buffer and aliquots were electophoresed on polyacrylamide gels as previously described (31). After transfer, membranes were probed with a 1/1,000 dilution of each serum and a 1/3,000 dilution of goat anti-monkey immunoglobulin G (MP Biomedicals, Inc). Protein was detected using one of two methods. ECL reagents (Amersham) were used for most assays. However, for assays done in the field, protein on individual membrane strips was detected using the TMB (3,3′,5,5′-tetramethylbenzidine) color development-stabilized substrate for localization of horseradish peroxidase-conjugated antibodies (Promega) by incubating washed membranes with reagent until bands appeared (but in all cases less than 10 min), as recommended by the manufacturer. Filters were washed in water and scanned. This reagent is much simpler to use in field situations, as it does not require film or a dark room.
PCR and RT-PCR analysis.
DNA from peripheral blood and RNA from buccal swabs were isolated as previously described (26). Data for the captive ONPRC animals were obtained using quantitative PCR or reverse transcription-PCR (RT-PCR) with SFV gag and c-myc primers and was taken from our prior work (26). PCR was performed on DNA extracted from whole blood obtained from free-ranging M. fascicularis animals from Singapore using the highly conserved pol primers described by Schweizer et al. (30). PCR products were detected on 2% agarose gels using DNA samples from uninfected or infected TF tissue culture cells as positive and negative controls, respectively. Integrity of DNA was confirmed using the c-myc primers described in Murray et al. (26).
RESULTS
A new quantitative infectivity assay for SFV isolated from macaques.
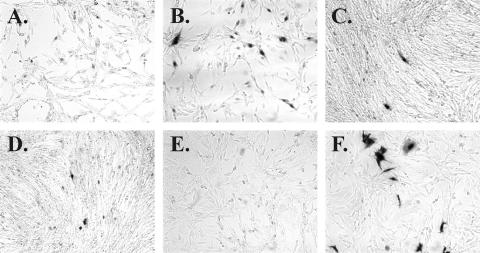
To quantitate the amount of virus present in an SFV sample, we used SFAB cells. SFAB is a clonal line of baby hamster kidney (BHK-21) cells containing an SFV-1 LTR (25) driving expression of a LacZ reporter gene. They are similar to FAB cells, which are used to measure infectivity of chimpanzee-derived FVs, such as PFV (35). FAB or SFAB cells were infected with dilutions of viral stocks and stained 2 days later. Using SFAB cells, we were able to determine the titers of viruses derived from the three captive macaque species tested: M. nemestrina, M. fascicularis, and M. mulatta. As shown in Fig. 1, virus from all three macaque species show distinct staining of SFAB cells (panels B to D, dark cells). The infected foci are generally smaller than those seen after infection of FAB cells with PFV, the chimpanzee virus derived from a zoonotically infected human (Fig. 1F). SFAB cells do exhibit some light background staining with β-galactosidase-detection reagents, probably because of leakiness of the SFV-1 LTR promoter in the absence of the Tas transactivator protein (Fig. 1A; compare to panel E for FAB cells). However, if staining is limited to 1 h, infected cells stain much darker than background and are easily distinguished. The background staining seen using SFAB cells is independent of viral strains or density of seeded cells. The macaque viruses do not induce LacZ in FAB cells, and PFV does not induce LacZ in SFAB cells (data not shown), indicating that the Tas proteins of PFV and SFVmac are specific to their own LTR sequences. We do not currently have available virus from M. assamensis or P. obscura to test in this assay.
FIG. 1.
SFAB assay detects SFV from three species of macaques. Cells were infected with virus and fixed and stained 2 days later as previously described (35), except that SFAB cells were stained for 1 h and FAB cells were stained for 6 h at 37°C, at which time the cells were washed with buffer, left at room temperature overnight, and counted the next day. (A) Uninfected SFAB; (B) SFAB plus SFV from M. fascicularis (housed at WaNPRC); (C) SFAB plus SFV from M. mulatta (housed at ONPRC); (D) SFAB plus SFV from M. nemestrina (housed at WaNPRC); (E) uninfected FAB; (F) FAB plus PFV.
Antibodies from infected NHPs cross-react with SFV from commensal NHPs from Asia.
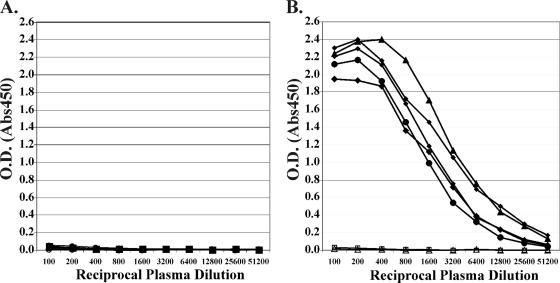
We used an ELISA based on the FV structural protein Gag. Plates were coated with either GST or GST-Gag (amino acids 1 to 193) derived from pSFV-1, which is a molecular clone derived from SFV isolated from M. mulatta (25). Previously we reported that this assay allows quantitation of antibodies from M. mulatta animals housed at ONPRC (26). Macaque plasma showed little or no reactivity to GST-coated ELISA plates (Fig. 2A). However, high levels of reactivity were seen with GST-Gag. Figure 2B shows the results of five representative plasma samples from M. mulatta that were determined to be positive for FV nucleic acid using quantitative PCR assays. Also shown are two representative plasma samples, from animals housed in specific-pathogen-free facilities at ONPRC, that are negative for FV nucleic acid using quantitative PCR assays (26). Plasma from SFV DNA-positive M. mulatta clearly react with GST-Gag (Fig. 2B); the midpoint titers for these representative five animals ranged from ∼1/1,500 to 1/3,200. The ELISA also allows detection of SFV antibodies in sera from other macaque species as well as from the Asian langur P. obscura (data not shown).
FIG. 2.
ELISA for SFV Gag antibodies. Plates were coated with 50 ng of purified GST (A) or GST-Gag (B) protein, as previously described (26). Plasma samples were assayed from five positive (closed symbols) and two negative (open symbols) M. mulatta animals (housed at ONPRC). Results for all seven plasma samples are shown in panel B.
Correlation of ELISA and RT-PCR/PCR assays.
We assayed by ELISA a total of 29 corral-housed animals from ONPRC whose FV status was confirmed by FV-specific quantitative PCR (proviral DNA in blood) or quantitative RT-PCR (viral RNA in saliva). In each instance, there was complete concordance between the ELISA and the presence or absence of FV nucleic acid (Table 1). The positive animals all had midpoint ELISA reciprocal titers of 356 to 8,530 (the serum dilution at which half-maximum signal was obtained).
TABLE 1.
Correlation of Gag ELISA and FV nucleic acid (NA) status in M. mulatta housed at ONPRC
| ELISA statusc | PCRa
|
RT-PCRb
|
No./No. with concordant NA and ELISA statusd | ||
|---|---|---|---|---|---|
| No. positive | No. negative | No. positive | No. negative | ||
| Positive | 17 | 0 | 7 | 0 | 24/24 positive |
| Negative | 0 | 3 | 0 | 2 | 5/5 negative |
Quantitative real-time PCR data are summarized from reference 26 or are from data not shown, using SFV gag primers. Copies range from 13 to 980 copies per 105 PBMC. The limit of detection was 10 DNA copies per 105 PBMC, and negative animals were below this level. Blood samples were used.
Quantitative real-time RT-PCR data summarized from reference 26 or from data not shown, using SFV gag primers. RNA copies range from 1.3 × 102 to 1.6 × 106 per 104 cell equivalents. The limit of detection was 50 RNA copies per 104 cell equivalents, and negative animals were below this level. Saliva samples were used.
No., number of animals.
We also examined the ELISA and PCR status of 21 free-ranging M. fascicularis animals from Singapore, representing different age groups, from which both serum and whole-blood samples were available. Saliva samples were not available for RNA analysis, but DNA was extracted from whole blood and PCR analysis was done using SFV pol and control c-myc primers. Samples which did not yield positive bands with the c-myc primers were not included in the analysis. Of the 21 macaques examined, 19 yielded positive PCR results with control c-myc primers (Table 2). For all of the ELISA-positive samples, the OD450 value was ≥0.400 at serum dilutions of either 1:200 or 1:500. The ELISA-negative samples all had OD450 values of ≥0.09 at serum dilutions of 1:200. In 18 of the macaques, there was a correlation between serum reactivity and the presence of SFV DNA sequences in peripheral blood. One macaque had a positive PCR result but a negative ELISA. This animal (SM57) was estimated to be between 13 and 18 months old based on its dental eruption sequence and morphometrics. It is possible that it had become recently infected and had not yet seroconverted.
TABLE 2.
Correlation of Gag ELISA and FV nucleic acid (NA) status in free-ranging M. fascicularis from Singapore
| ELISA statusa | PCRb
|
No./No. with concordant NA and ELISA statusc | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 13 | 0 | 13/13 positive |
| Negative | 1 | 5 | 5/6 negative |
ELISA data are summarized from data not shown.
PCR products were detected on agarose gels using SFV pol primers. Blood samples were used.
No., number of animals.
WB and neutralization assays.
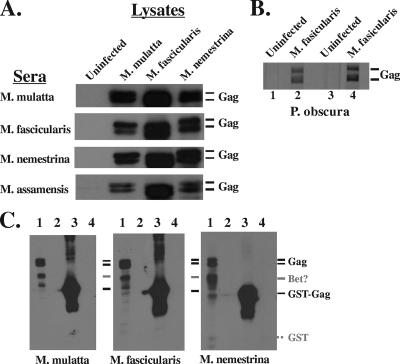
We next tested whether sera from commensal NHPs in Asia (four different macaque species, M. mulatta, M. fascicularis, M. assamensis, and M. nemestrina, and one species of langur, P. obscura) were capable of detecting viral Gag proteins from several different macaque SFV isolates using WB. FV Gag protein is cleaved only once near the carboxyl terminus by the viral protease, and it migrates on polyacrylamide gels as a doublet of ca. 71 and 68 kDa. As shown in Fig. 3A, macaque sera were tested against protein lysates prepared from the infection of tissue culture cells with SFV isolated from three macaque species. Uninfected and infected lysates were prepared from TF cells, a line of telomerase-immortalized M. mulatta neonatal fibroblasts (21). Antisera from all macaque species detected the presence of SFV Gag (as detected by ECL; Amersham) from the three macaque strains from which we have available infectious virus. We also tested sera from a number of individual P. obscura animals with Gag protein prepared from cells infected with SFV isolated from M. fascicularis and got positive reactions (as detected by TMB reagent; Promega). Results with sera from two positive animals are shown in Fig. 3B. Assays of these sera were done in the field. At present, sera are not available from FV-positive P. obscura animals for further testing.
FIG. 3.
WB analysis of NHP sera reactivity with SFV Gag. Cell lysates were made from uninfected TF cells or cells infected with the indicated viruses. Viruses were from M. nemestrina and M. fascicularis, housed at WaNPRC; M. mulatta, housed at ONPRC; and M. assamenis and P. obscura, free ranging in Thailand. (A) The source of the lysates is shown at the top, and the source of the sera is on the left. Protein was detected using ECL reagents (Amersham). The lysates were not normalized for the amount of protein loaded; equal volumes were added to each lane. (B) Protein on individual membrane strips was detected using TMB. Lanes 1 and 2 used serum from one P. obscura animal, and lanes 3 and 4 used serum from a second animal. (C) WB were probed with sera from the indicated animals. Lanes 1, lysate from TF cells infected with SFV from M. fascicularis; lanes 2, lysate from uninfected TF cells; lanes 3, 1 μg of GST-Gag; lanes 4, 1 μg of GST. The dotted line indicates the position of GST not recognized by the sera.
We next reacted sera from M. mulatta, M. nemestrina, and M. fascicularis animals with lysates from SFV (M. fascicularis)-infected or uninfected TF cells as well as purified GST-Gag or GST proteins (Fig. 3C). All three sera again reacted with Gag proteins from the lysates (lanes 1) but showed no reactivity with uninfected lysates (lanes 2). There is an additional band detected in the blots that migrates at a position consistent with the viral Bet protein (about 60 kDa, denoted by a gray line). However, we do not have an antibody that reacts only with SFV Bet. Therefore, we cannot determine whether this band is Bet or a breakdown product of Gag. Macaque sera do react with GST-SFV-Bet protein (data not shown). We do not detect any distinct bands larger than Gag that would correspond to Pol and Env proteins. Using the macaque sera, the major reactivity is to Gag. We also tested reactivity to GST-Gag used in the ELISA (lanes 3) as well as GST (lanes 4). All sera react strongly to the GST-Gag with no background reactivity to GST.
Sera from one randomly chosen M. mulatta, M. fascicularis, or M. nemestrina were tested for the presence of antibodies that can neutralize SFV isolated from the other macaque species. We found that heat-inactivated sera (56° for 1 h) from each macaque species could neutralize virus from all three species. Aliquots of virus were incubated with or without dilutions of serum for 1 h at 37°C. After incubation, virus was plated directly on SFAB cells, and blue cells were counted after 2 days. As a control for nonspecific reactivity, the chimpanzee-derived virus PFV was also tested with the sera and assayed on FAB cells. The data from two to three independent assays are summarized in Table 3. All sera neutralized all viruses with about the same midpoint titers, ranging from about 1:40 to 1:320. None of the sera neutralized PFV at dilutions as low as 1:10. There does not appear to be any preference for neutralization of homologous virus over heterologous macaque FV.
TABLE 3.
Midpoint neutralization serum titers from three macaque species against homologous and heterologous virusesa
| Virus | Midpoint serum titers from NHP species
|
||
|---|---|---|---|
| M. mulatta | M. fascicularis | M. nemestrina | |
| SFV M. mulatta | 160 | 160 | 80 |
| SFV M. fascicularis | 320 | 60 | 40 |
| SFV M. nemestrina | 160 | 160 | 160 |
| PFV | <10* | <10* | <10* |
Titers are given as the reciprocal of the serum dilution that neutralized 50% of viral infectivity. Each experiment was done two or three times, and the average of the results is shown. One random animal from each species was tested. *, no neutralization was detected at the lowest serum dilution tested, 1:10.
Prevalence of foamy virus infection in commensal, free-ranging NHPs in Thailand.
The ELISA and WB assay can detect SFV infection in the most commonly encountered Asian NHPs, including macaques and langurs (Fig. 2 and 3 and data not shown). These assays are suitable for screening large numbers of samples in the field using simple reagents and the most basic laboratory equipment. The ELISA plates and WB strips can be prepared ahead of time. Previous screens of Old World primates used WB with lysates combined with cells infected with two different SFV. Such assays were able to detect a number of SFV strains (16). However, use of an ELISA which correlates with WB would greatly increase the efficiency of screening.
Sera collected from 127 pet and free-ranging NHPs in Thailand were tested at a 1:100 dilution by ELISA for SFV antibodies using both GST and GST-Gag plates. This concentration always yielded high values using sera from FV-positive test animals and no reactivity using sera from FV-negative specific-pathogen-free animals (Fig. 2 and data not shown). The sera fell into two distinct groups when the values for GST were subtracted from the GST-Gag values: 17 of the serum samples gave values ranging from 0.0 to 0.04, while the other 110 samples had values ranging from 0.4 to 2.5. No intermediate values were obtained, as was found for the captive animals (see Fig. 2). We assumed that all the samples below 0.04 were from uninfected animals, and the other samples were from SFV-infected animals. Six of the samples from the group with GST-Gag values of <0.04 were screened by WB using protein lysates from uninfected cells or cells infected with SFV from M. fascicularis. All of these samples were negative (data not shown). Sixty-one of the NHP samples that had a value of >0.4 were also screened, and all samples reacted with Gag from lysates (data not shown). Thus, we found a perfect correlation between the ELISA and WB results, similar to what was seen in other populations (Table 4). Whole-blood or saliva samples from these Thai NHPs are unfortunately not available for PCR or RT-PCR testing.
TABLE 4.
SFV prevalence among several taxa of NHPs in Thailand
| Species | Status | Total no. sampled | % SFV positivea |
|---|---|---|---|
| M. fascicularisb | Free ranging | 48 | 81 |
| M. assamensisb | Free ranging | 30 | 93 |
| M. arctoidesb | Free ranging | 2 | 100 |
| M. mulatta/fascicularis hybridc | Free ranging | 23 | 100 |
| M. nemestrinab | Free ranging | 1 | 100 |
| P. obscurab | Free ranging | 14 | 100 |
| M. nemestrina | Pet | 8 | 25 |
| M. assamensis | Pet | 1 | 0 |
Determined by ELISA.
These populations of NHPs were sampled from six different temple sites in north, central, and south-central Thailand.
This hybrid population of M. mulatta/M. fascicularis was sampled from an urban park in northeast Thailand.
The prevalence of antibodies to SFV among the multiple taxa of commensal NHPs was very high in our sample populations (Table 4). Nine pet monkeys were included in this sample, but only one of these animals had antibodies to SFV (Table 4). Overall, 108/118 free-ranging animals were SFV positive (Table 5). Seroprevalences within age classes (determined by using dental eruption sequences and body weight, where animals <1 year were classified as young juveniles, between 1 and 3 years as older juveniles, 3 and 5 years as subadults, and over 5 years as adults) for all 118 free-ranging NHPs are presented in Table 5. Table 6 presents SFV seroprevalence by age class and species. Regardless of species, most animals seroconverted by 3 years of age (Tables 5 and 6).
TABLE 5.
SFV seroconversion as a function of agea
| Age class | Total | No. positive | % SFV positive |
|---|---|---|---|
| Young juvenileb | 4 | 1 | 25 |
| Older juvenilec | 37 | 30 | 81 |
| Subadultd | 15 | 15 | 100 |
| Adulte | 62 | 62 | 100 |
| Total | 118 | 108 | 92 |
Data are from Table 4, but only free-ranging NHPs are included.
Young juvenile, deciduous dentition only, aged <1 year.
Older juvenile, adult first molar and/or second molars in occlusion, aged 1 to 3 years.
Subadult, third molars and/or canines not fully erupted, aged 3 to 5 years.
Adult, third molars and canines fully erupted, aged >5 years.
TABLE 6.
SFV prevalence among several taxa of NHPs in Thailand by age classa
| Species | No. SFV positive/total no. by age class (%)
|
|||
|---|---|---|---|---|
| Young juvenile | Older juvenile | Subadult | Adult | |
| M. assamensis | 0/1 (0) | 3/4 (75) | 5/5 (100) | 20/20 (100) |
| M. fascicularis | 1/3 (33) | 16/22 (73) | 7/7 (100) | 16/16 (100) |
| M. mulatta/ fascicularis hybrid | 0 | 6/6 (100) | 2/2 (100) | 15/15 (100) |
| P. obscura | 0 | 5/5 (100) | 1/1 (100) | 8/8 (100) |
DISCUSSION
We have developed reagents that have proved useful for detecting FV infection among Asian NHPs. The reagents are both sensitive and specific and are also applicable to the NHP species in Asia that have the greatest interactions with the indigenous and tourist populations. Both the ELISA and WB (using TMB) assay can easily be performed with previously prepared plates or nitrocellulose strips in field situations. Previous FV assays have utilized crude lysates as antigens in both the ELISA and WB assay. The use of such assays has led to false-positive results in humans in the past (reviewed in reference 23). The advantage of cloned proteins is that they minimize variation from assay to assay. The results of the ELISA showed that the cutoff values for negative results (OD450 < 0.04) did not vary between species. All sera tested reacted very strongly with the GST-Gag recombinant proteins (Fig. 3C) with no background with GST.
We found a very high correlation between the presence of SFV Gag or Pol RNA sequences in saliva or DNA sequences in whole-blood cells and a positive ELISA result. Of 48 macaques examined, only 1 yielded the anomalous result of PCR positive and ELISA negative. This animal was estimated to be between 13 and 18 months of age at the time of sampling and may represent a very recent infection. Recent data obtained from one macaque that had been transfused with blood from an SFV-positive animal showed that viral DNA sequences could be detected by PCR about 4 weeks prior to the appearance of antibodies detected by WB (6). There have been no careful studies of the time course of infection in macaques infected by more natural routes. However, the Brooks et al. data would suggest that this juvenile could have become infected shortly before our sampling (6). Controlled infections of macaques and/or examination of more naturally infected older juveniles will have to be examined to confirm this. Overall, our data show that the ELISA using recombinant Gag protein, which can easily be done in field settings without sophisticated laboratory equipment, is an excellent indicator of FV infection.
Our data show that 92% of the free-ranging NHPs we sampled in Thailand were SFV positive and that all animals over the age of 3 years had seroconverted. These and earlier results (17, 19) suggest that SFV transmission in free-ranging primates occurs prior to sexual maturity. This is in sharp contrast to the recently published results of Calattini et al. (8), who sampled M. tonkeana from a large free-breeding, but captive, colony in France. They found that most SFV seroconversion did not occur until after 7 years of age and hypothesized that SFV transmission usually occurs in adulthood as a result of bites. These authors used BHK cells infected with chimpanzee SFV as their antigen, which might have led to lower sensitivity and higher backgrounds than our homologous antigens (macaque SFV in macaque cells) as well as our ELISA using purified bacterially expressed SFV Gag protein.
With the exception of the pet monkeys, we found high levels of SFV infection in all of the commensal NHPs we sampled. Previous research (18, 29) has shown that most pet NHPs in Asia are taken from the wild before 1 year of age and subsequently have little contact with other NHPs. Thus, low prevalence of SFV infection among pet primates is not surprising.
Risk analysis has been used to model the transmission of SFV from NHPs to visitors at monkey temples in Asia (11). The derived model suggests that visitors to monkey temples, which annually may number in the millions, are at risk for SFV infection. Additionally, large numbers of people in Asia are exposed to NHPs in a variety of other contexts and may be similarly at risk for SFV infection (13, 17, 18, 19, 29). Effective education, communication, monitoring, and management strategies in areas where humans and NHPs come into contact are needed to reduce the risk of exposure and transmission. Laboratory workers exposed to macaques at primate laboratories constitute another group at risk for SFV infection (3, 5). Though human-to-human transmission of SFV has yet to be demonstrated, the recent demonstration of SFV transmission between NHPs via blood transfusion (4, 20) suggests the possibility that transfusion-mediated transmission could also occur in human recipients of SFV-infected blood products. These findings indicate the need for accurate and robust assays for screening large numbers of people or blood products for SFV infection.
Acknowledgments
Work in the laboratory of M.L.L. was supported by NIH-NCI grant R01CA81297 and Institutional Pilot Funds from the Fred Hutchinson Cancer Research Center. L.J.-E., G.A.E., and R.G. were supported by the Royalty Research Fund from the University of Washington, Puget Sound Partners for Global Health, and NIH-NCRR grant P51 RR000166. S.M.M. and K. A.S. were partially supported by NIH training grant T32 CA09229.
We thank the National Parks Board, Singapore, and the National Research Council of Thailand, as well as Suvichai Rojanasathien of the Faculty of Veterinary Medicine and Boonraksar Soonthorntham and Narit Sitasuwan Faculty of Science, Chiang Mai University, for their support. We also thank Elizabeth Majors, Robin Watanabe, Hanna Engel, Leah Engel, Ginger Emel, Pawin Padungtod, Pasapong Temeesiw, Prachayarat Daram, Sandy Romain, Carolyn Begg, and William Ng for excellent technical assistance and Alison Yu for careful reading of the manuscript.
Footnotes
Published ahead of print on 2 May 2007.
REFERENCES
- 1.Achong, B. G., W. A. Mansell, M. A. Epstein, and P. Clifford. 1971. An unusual virus in cultures from a human nasopharyngeal carcinoma. J. Natl. Cancer Inst. 46:299-307. [PubMed] [Google Scholar]
- 2.Blewett, E. L., D. H. Black, N. W. Lerche, G. White, and R. Eberle. 2000. Simian foamy virus infections in a baboon breeding colony. Virology 278:183-193. [DOI] [PubMed] [Google Scholar]
- 3.Boneva, R. S., A. J. Grindon, S. L. Orton, W. M. Switzer, V. Shanmugam, A. I. Hussain, V. B. Bhullar, M. E. Chamberland, W. Heneine, T. M. Folks, and L. E. Chapman. 2002. Simian foamy virus infection in a blood donor. Transfusion 42:886-891. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, J. I., H. W. Merks, H. Fournier, R. S. Boneva, and P. A. Sandstrom. 2007. Characterization of blood-borne transmission of simian foamy virus. Transfusion 47:162-170. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, J. I., E. W. Rud, R. G. Pilon, J. M. Smith, W. M. Switzer, and P. A. Sandstrom. 2002. Cross-species retroviral transmission from macaques to human beings. Lancet 360:387-388. [DOI] [PubMed] [Google Scholar]
- 6.Brooks, J. I., H. W. Merks, J. Fournier, R. S. Boneva, and P. A. Sandstrom. 2007. Characterization of blood-borne transmission of simian foamy virus. Transfusion 47:162-170. [DOI] [PubMed] [Google Scholar]
- 7.Broussard, S. R., A. G. Comuzzie, K. L. Leighton, M. M. Leland, E. M. Whitehead, and J. S. Allan. 1997. Characterization of new simian foamy viruses from African nonhuman primates. Virology 237:349-359. [DOI] [PubMed] [Google Scholar]
- 8.Calattini, S., F. Wanert, B. Thierry, C. Schmitt, S. Bassot, A. Saib, N. Herrenschmidt, and A. Gessain. 2006. Modes of transmission and genetic diversity of foamy viruses in a Macaca tonkeana colony. Retrovirology 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callahan, M. E., W. M. Switzer, A. L. Matthews, B. D. Roberts, W. Heneine, T. M. Folks, and P. A. Sandstrom. 1999. Persistent zoonotic infection of a human with simian foamy virus in the absence of an intact orf-2 accessory gene. J. Virol. 73:9619-9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enders, J., and T. Peebles. 1954. Propagation in tissue culture of cytopathogenic agents from patients with measles. Proc. Soc. Exp. Biol. Med. 86:277-287. [DOI] [PubMed] [Google Scholar]
- 11.Engel, G., L. L. Hungerford, L. Jones-Engel, D. Travis, R. Eberle, A. Fuentes, R. Grant, R. Kyes, and M. Schillaci. 2006. Risk assessment: a model for predicting cross-species transmission of simian foamy virus from macaques (M. fascicularis) to humans at a monkey temple in Bali, Indonesia. Am. J. Primatol. 68:934-948. [DOI] [PubMed] [Google Scholar]
- 12.Falcone, V., M. Schweizer, and D. Neumann-Haefelin. 2003. Replication of primate foamy viruses in natural and experimental hosts, p. 161-180. In A. Rethwilm (ed.), Foamy viruses, vol. 277. Springer, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 13.Fuentes, A. 2006. Human culture and monkey behavior: assessing the contexts of potential pathogen transmission between macaques and humans. Am. J. Primatol. 68:880-896. [DOI] [PubMed] [Google Scholar]
- 14.Fuentes, A., and S. Gamerl. 2005. Disproportionate participation by age/sex classes in aggressive interactions between long-tailed macaques (Macaca fascicularis) and human tourists at Padangtegal monkey forest, Bali, Indonesia. Am. J. Primatol. 66:197-204. [DOI] [PubMed] [Google Scholar]
- 15.Heneine, W., W. M. Switzer, P. Sandstrom, J. Brown, S. Vedapuri, C. A. Schable, A. S. Khan, N. W. Lerche, M. Schweizer, D. Neumann-Haefelin, L. E. Chapman, and T. M. Folks. 1998. Identification of a human population infected with simian foamy viruses. Nat. Med. 4:403-407. [DOI] [PubMed] [Google Scholar]
- 16.Hussain, A. I., V. Shanmugam, V. B. Bhullar, B. E. Beer, D. Vallet, A. Gautier-Hion, N. D. Wolfe, W. B. Karesh, A. M. Kilbourn, Z. Tooze, W. Heneine, and W. M. Switzer. 2003. Screening for simian foamy virus infection by using a combined antigen Western blot assay: evidence for a wide distribution among Old World primates and identification of four new divergent viruses. Virology 309:248-257. [DOI] [PubMed] [Google Scholar]
- 17.Jones-Engel, L., G. A. Engel, M. A. Schillaci, A. Rompis, A. Putra, K. G. Suaryana, A. Fuentes, B. Beer, S. Hicks, R. White, B. Wilson, and J. S. Allan. 2005. Primate-to-human retroviral transmission in Asia. Emerg. Infect. Dis. 11:1028-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones-Engel, L., M. Schillaci, G. Engel, P. Paputungan, and J. Froehlich. 2006. Characterizing primate pet ownership in Sulawesi: implications for disease transmission, p. 196-221. In J. D. Paterson and J. Wallis (ed.), Commensalism and conflict: the human-primate interface. American Society of Primatologists, Norman, OK.
- 19.Jones-Engel, L., G. A. Engel, J. Heidrich, M. Chalise, N. Poudel, R. Viscidi, P. A. Barry, J. S. Allan, R. Grant, and R. Kyes. 2006. Temple monkeys and health implications of commensalism, Kathmandu, Nepal. Emerg. Infect. Dis. 12:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan, A. S., and D. Kumar. 2006. Simian foamy virus infection by whole-blood transfer in rhesus macaques: potential for transfusion transmission in humans. Transfusion 46:1352-1359. [DOI] [PubMed] [Google Scholar]
- 21.Kirchoff, V., S. Wong, and G. S. Pari. 2002. Generation of a life-expanded rhesus monkey fibroblast cell line for the growth of rhesus rhadinovirus (RRV). Arch. Virol. 147:321-333. [DOI] [PubMed] [Google Scholar]
- 22.Linial, M. L., H. Fan, B. Hahn, R. Lower, J. Neil, S. L. Quackenbush, A. Rethwilm, P. Sonigo, J. Stoye, and M. Tristem. 2004. Retroviridae, p. 421-440. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy, 8th report of the ICTV. Elsevier/Academic Press, London, United Kingdom.
- 23.Meiering, C. D., and M. L. Linial. 2001. Historical perspective of foamy virus epidemiology and infection. Clin. Microbiol. Rev. 14:165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meiering, C. D., and M. L. Linial. 2002. Reactivation of a complex retrovirus is controlled by a molecular switch and is inhibited by a viral protein. Proc. Natl. Acad. Sci. USA 99:15130-15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mergia, A., and M. Wu. 1998. Characterization of provirus clones of simian foamy virus type 1. J. Virol. 72:817-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, S. M., L. J. Picker, M. K. Axthelm, and M. L. Linial. 2006. Expanded tissue targets for foamy virus replication with simian immunodeficiency virus-induced immunosuppression. J. Virol. 80:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saib, A. 2003. Non-primate foamy viruses. Curr. Topics Microbiol. Immunol. 277:197-212. [DOI] [PubMed] [Google Scholar]
- 28.Sandstrom, P. A., K. O. Phan, W. M. Switzer, T. Fredeking, L. Chapman, W. Heneine, and T. M. Folks. 2000. Simian foamy virus infection among zoo keepers. Lancet 355:551-552. [DOI] [PubMed] [Google Scholar]
- 29.Schillaci, M. A., L. Jones-Engel, G. A. Engel, Y. Paramastri, E. Iskandar, B. Wilson, J. S. Allan, R. C. Kyes, R. Watanabe, and R. Grant. 2005. Prevalence of enzootic simian viruses among urban performance monkeys in Indonesia. Trop. Med. Int. Health 10:1305-1314. [DOI] [PubMed] [Google Scholar]
- 30.Schweizer, M., and D. Neumann-Haefelin. 1995. Phylogenetic analysis of primate foamy viruses by comparison of pol sequences. Virology 207:577-582. [DOI] [PubMed] [Google Scholar]
- 31.Stenbak, C. R., and M. L. Linial. 2004. Role of the C terminus of foamy virus Gag in RNA packaging and Pol expression. J. Virol. 78:9423-9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Switzer, W. M., V. Bhullar, V. Shanmugam, M. E. Cong, B. Parekh, N. W. Lerche, J. L. Yee, J. J. Ely, R. Boneva, L. E. Chapman, T. M. Folks, and W. Heneine. 2004. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J. Virol. 78:2780-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Switzer, W. M., M. Salemi, V. Shanmugam, F. Gao, M. E. Cong, C. Kuiken, V. Bhullar, B. E. Beer, D. Vallet, A. Gautier-Hion, Z. Tooze, F. Villinger, E. C. Holmes, and W. Heneine. 2005. Ancient co-speciation of simian foamy viruses and primates. Nature 434:376-380. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe, N. D., W. M. Switzer, J. K. Carr, V. B. Bhullar, V. Shanmugam, U. Tamoufe, A. T. Prosser, J. N. Torimiro, A. Wright, E. Mpoudi-Ngole, F. E. McCutchan, D. L. Birx, T. M. Folks, D. S. Burke, and W. Heneine. 2004. Naturally acquired simian retrovirus infections in central African hunters. Lancet 363:932-937. [DOI] [PubMed] [Google Scholar]
- 35.Yu, S. F., and M. L. Linial. 1993. Analysis of the role of the bel and bet open reading frames of human foamy virus by using a new quantitative assay. J. Virol. 67:6618-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuste, E., W. Johnson, G. N. Pavlakis, and R. C. Desrosiers. 2005. Virion envelope content, infectivity, and neutralization sensitivity of simian immunodeficiency virus. J. Virol. 79:12455-12463. [DOI] [PMC free article] [PubMed] [Google Scholar]