Abstract
Electron microscopy revealed that the entry of Rice dwarf virus (RDV) into insect vector cells involved endocytosis via coated pits. The treatment of cells with drugs that block receptor-mediated or clathrin-mediated endocytosis significantly reduced RDV infectivity. However, the drug that blocks caveola-mediated endocytosis had a negligible effect on such infection. Infection was also inhibited when cells had been pretreated with bafilomycin A1, which interferes with acidification of endosomes. Moreover, immunofluorescence staining indicated that the virus is internalized into early endosomes. Together, our data indicate that RDV enters insect vector cells through receptor-mediated, clathrin-dependent endocytosis and is sequestered in early endosomes.
The initiation of a successful virus infection cycle requires the attachment of virions to specific molecules on the surfaces of host cells, with subsequent penetration of and entry into the host cells for the release of viral genomes for replication. Many viruses, including enveloped and nonenveloped viruses, require endocytosis for entry into cells, and they gain access to the cytoplasm via endocytotic vesicles. The endocytotic pathways exploited by viruses include clathrin-mediated endocytosis, caveola-mediated endocytosis, macropinocytosis, and phagocytosis (8, 13, 20, 21). Plant viruses are believed to enter plant cells through a wound. Since it is difficult to culture host cells, the mechanisms of entry of plant viruses into the cells of their respective insect vector hosts remain poorly understood. Some electron microscopic observations have suggested that plant viruses enter their insect vector cells via receptor-mediated endocytosis; for example, luteoviruses were believed to move across the hemocoel or salivary glands of aphid vectors by endocytosis (9, 10), but no direct evidence has been reported, to our knowledge, to confirm this hypothesis.
Rice dwarf virus (RDV) is a nonenveloped virus that is a member of the genus Phytoreovirus in the family Reoviridae (17). RDV has an icosahedral capsid composed of seven proteins organized in two concentric layers that surround a genome of 12 segmented double-stranded RNAs (16). The outer layer consists of three proteins, namely, P2, P8, and P9 (14, 15, 16, 26, 27). The minor outer capsid protein P2 is essential for the infection of insect vectors by RDV (18, 22, 25). It has been proposed that P2 interacts with receptors on insect vector cells and that this interaction is required for the recognition of viral particles by the insect cells (18). The major outer capsid protein P8 also appears to play an important role during the initial stage of infection of insect vector cells (16). To our knowledge, little else is known about the mechanism of entry of plant virus into insect vector cells. The development of a technique for culturing insect vector cells in monolayers (VCMs) allows the efficient propagation of plant viruses in their respective vectors (11, 24). RDV is unusual in that this virus is able to multiply both in a plant and in an insect vector (2). Therefore, the entry of RDV into cells can now be investigated in the context of a cycle of infection using VCMs. In this study, NC-24 cells, originally established from embryonic fragments dissected from eggs of Nephotettix cincticeps, were maintained in monolayer culture at 25°C in growth medium that had been prepared as described previously (11).
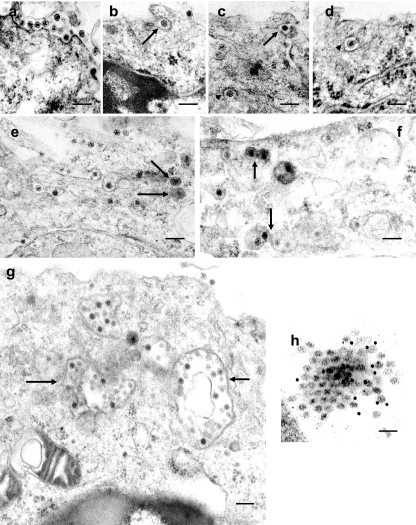
In order to study and dissect the process of entry of RDV into insect cells, VCMs on coverslips were inoculated with RDV at a multiplicity of infection (MOI) of 1, fixed at different times postinoculation (p.i.), and examined by electron microscopy, as described previously (18). At 30 min p.i., we observed the attachment of RDV particles of approximately 70 nm in diameter to the surfaces of VCMs (Fig. 1a). At 1 h p.i., RDV particles were seen within invaginations of the plasma membrane (Fig. 1b and c). These invaginations showed that viral entry into insect vector cells resembled that of clathrin-coated pits. Figure 1c and d show the uptake of RDV via a pit that has detached from the cell surface. After 2 h p.i., most of the viral particles were found within endocytotic vesicles (Fig. 1e through g). A single viral particle was evident within each vesicle (Fig. 1e) at 2 h p.i. Vesicles that contained a viral particle(s) appeared to fuse with one another to form large vesicles (Fig. 1f and g) at 4 h p.i. We never observed similar phenomena in uninfected cells (data not shown).
FIG. 1.
Electron micrographs showing the entry of RDV into insect vector cells. (a) Attachment of RDV particles to the plasma membranes of VCMs 30 min p.i. (b and c) Uptake of RDV particles in coated pits (arrows) 1 h p.i. (c and d) RDV particles internalized within coated pits (arrowheads) 1 h p.i. (e) Single RDV particle enclosed in vesicles (arrows) 2 h p.i. (f) Vesicles with an RDV particle(s) are in close proximity to other vesicles (arrows), possibly prior to fusion, 4 h p.i. (g) RDV particles in electron-lucent compartment (arrow) 4 h p.i. (h) Immunodetection of RDV particles in multivirus-containing compartments using gold particles conjugated to P2-specific IgG 4 h p.i. Bars, 200 nm.
Our observations also revealed that RDV particles within endocytotic vesicles were always in the double-layered form. This observation was supported by immunoelectron microscopic observations of VCMs that had been infected with RDV at an early stage of viral infection. Cells on coverslips were fixed 4 h p.i. and immunostained, as described previously, with the P2-specific immunoglobulin G (IgG) and 15-nm gold particle-conjugated goat antibodies against rabbit IgG (GAR15; British Bifocals International, Cardiff, United Kingdom) (24). The particles in large vesicles reacted specifically with the IgG and were confirmed to be double-layered RDV particles (Fig. 1h). Therefore, it seems that the uncoating of RDV by losing the outer capsid proteins did not occur after endocytosis.
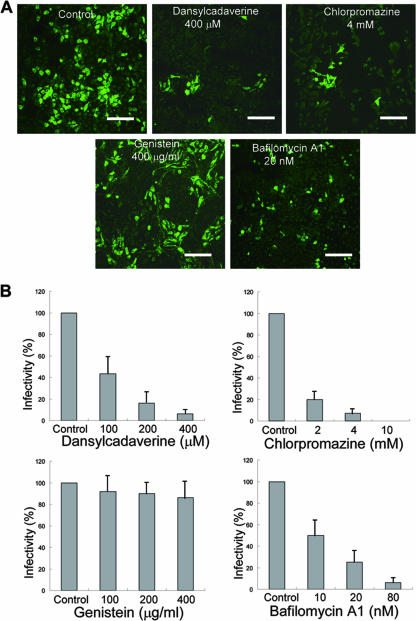
In order to determine whether RDV enters cells via receptor-mediated, clathrin-mediated, or caveola-mediated endocytosis, we examined the effects of dansylcadaverine (Sigma), chlorpromazine (Sigma), and genistein (Sigma) on infection. Dansylcadaverine is a pharmacological inhibitor of receptor-mediated endocytosis that has been extensively used by others to demonstrate receptor-mediated endocytosis of several viruses (4, 5, 6). Chlorpromazine prevents the assembly and disassembly of clathrin lattices at cell surfaces and on endosomes, thereby inhibiting clathrin-mediated endocytosis (23). Genistein interferes with caveola-mediated endocytosis by inhibiting the internalization of viruses through caveolae; the drug acts by blocking the phosphorylation of tyrosine kinase, which is involved in the formation of caveosomes (7, 19). After treating VCMs for 30 min with each drug separately, VCMs were inoculated at an MOI of 0.4 with RDV for 2 h, washed three times, and cultured in medium for 12 h. Cells were fixed for 30 min in 2% paraformaldehyde, stained with virus-specific IgG (raised against intact viruses), conjugated to fluorescein isothiocyanate (FITC), and visualized by confocal fluorescence microscopy, as described previously (24). Infection was quantitated as the ratio of infected cells to total cells. The viral inoculum was diluted to a concentration that resulted in the infection of 40 to 50% of cells. The values in Fig. 2 represent the averages of the results of at least three independent experiments. In preliminary experiments, we tested a range of concentrations for each drug to determine its effective concentrations, thereby avoiding the effects due to their toxicity (data not shown). As shown in Fig. 2, pretreatment of VCMs with dansylcadaverine for 30 min before RDV inoculation significantly reduced the number of infected cells in a dose-dependent manner. Chlorpromazine also inhibited RDV infection in a dose-dependent manner. Cells infected after treatment with dansylcadaverine and chlorpromazine fluoresced, as did the controls, suggesting that the virus infected once multiplied in the same level as that of nontreated controls. Inhibition was almost complete at a drug concentration of 10 mM. By contrast, RDV infection was not inhibited by genistein, even at very high concentrations of this drug (Fig. 2). Taken together, these data indicate that the infection of insect vector cells in monolayers by RDV is sensitive to dansylcadaverine and that RDV enters such cells by receptor-mediated endocytosis. Furthermore, the results obtained in this set of experiments supported the hypothesis that RDV entry mechanisms occur primarily by clathrin-mediated endocytosis.
FIG. 2.
Effects of various drugs on infection of VCMs by RDV. (A) VCMs were incubated for 30 min with 400 μM dansylcadaverine, 4 mM chlorpromazine, 400 μg/ml genistein, and 20 nM bafilomycin A1, as indicated, or without drugs (control) and then inoculated with RDV. Cells were fixed and processed for immunofluorescence staining with virus-specific IgG conjugated to FITC. Bars, 35 μm. (B) VCMs were treated with various concentrations of dansylcadaverine, chlorpromazine, genistein, and bafilomycin A1, as indicated. Viral infection was quantified as the percentage of FITC-stained, drug-treated cells relative to the number of untreated control cells. The data shown are the means and standard deviations of the results from three independent experiments.
To investigate the effects of pH, we examined whether the infection of VCMs by RDV required a low-endosomal pH using bafilomycin A1 (Sigma). This drug is a potent and specific inhibitor of ATPases that inhibits the acidification of endosomes and lysosomes (1, 3). In preliminary experiments, we tested a range of concentrations of the drug to determine effective concentrations and to avoid effects due to the toxicity of the drug (data not shown). After treatment for 30 min with bafilomycin A1, VCMs were incubated for 2 h with RDV at an MOI of 0.4. The infection was quantitated as noted above. As shown in Fig. 2, RDV infection was inhibited by bafilomycin A1, indicating that infection was apparently sensitive to the low pH of endosomes. The pH-dependent entry of RDV into VCMs further suggests that the virus is internalized at the cell surface by endocytosis and is sequestered in the endosomal compartment.
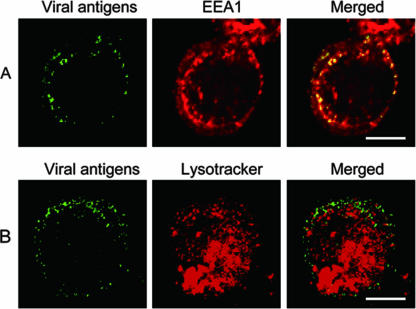
To determine whether internalized RDV particles associate with early or late endosomes, VCMs on coverslips were inoculated with RDV at an MOI of 1, fixed at different times p.i., and processed for immunofluorescence analysis with mouse monoclonal antibodies against early endosomal antigen 1 marker protein (EEA1; Abcam), followed by an Alexa Fluor 594 donkey anti-mouse IgG (Invitrogen) and virus-specific IgG conjugated to FITC. Alternatively, RDV-inoculated VCMs were pretreated with LysoTracker (specific staining dye for late endosomes and lysosomes; Invitrogen) for 30 min before fixation, stained with virus-specific IgG conjugated to FITC, and examined by confocal fluorescence microscopy, as described previously (24). From 2 h to 6 h p.i., a punctate staining pattern with a strong colocalization of RDV antigens and EEA1 was found (Fig. 3 and data not shown), thus suggesting that the viral particles were translocated to the early endosomes after endocytosis. By contrast, samples harvested at the same time points and stained with LysoTracker failed to show any localization of RDV in the late endosomes (Fig. 3 and data not shown). These results suggest that RDV virions do not migrate to the late endosomes. The proteolytic cleavage of the major outer capsid protein P8 of RDV occurs specifically at the residues Asp362 and Pro363, which are considered to be hydrophilic (12). Our data indicated that the low pH present in the early endosome might be sufficient to trigger the outer capsid protein cleavage or the fusion of RDV particles with the membranes of endocytotic vesicles.
FIG. 3.
RDV localization in early endosomal compartments. Early and later endosomes were detected using (A) monoclonal anti-EEA1 and (B) LysoTracker. Alexa Fluor 594 donkey anti-mouse IgG was used for secondary antibodies. Viral antigen was monitored by virus-specific IgG conjugated to FITC. The displayed pictures represent cells in which the infection has been carried out for 4 h p.i. Bars, 5 μm.
In conclusion, electron microscopy, immunofluorescence studies, and experiments with various inhibitors support the hypothesis that RDV enters insect vector cells through receptor-mediated, clathrin-dependent endocytosis and is sequestered in a low-pH-dependent endosomal compartment. To our knowledge, this is the first report of the entry of a plant virus into insect vector cells by clathrin-mediated endocytosis. This mechanism might also be applicable to other viruses in the genera Fijivirus and Oryzavirus (2, 17), which can multiply both in plants and in vector insects.
Acknowledgments
This project was supported by a Postdoctoral Fellowship for Foreign Researchers (15.03567) from the Japan Society for the Promotion of Science; by a Grant-in-Aid for Scientific Research (B; 15380038) from the Japan Society for the Promotion of Science; and by a Grant-in-Aid for Scientific Research on Priority Areas (Structures of Biological Macromolecular Assemblies) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Published ahead of print on 2 May 2007.
REFERENCES
- 1.Aniento, F., F. Gu, R. G. Parton, and J. Gruenberg. 1996. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 133:29-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boccardo, G., and R. G. Milne. 1984. Plant reovirus group. In A. F. Morant and B. D. Harrison (ed.), CM/AAB descriptions of plant viruses, no. 294. Commonwealth Mycological Institute and Association of Applied Biologists, Unwin Brothers Ltd., The Gresham Press, Old Woking, England.
- 3.Brindley, M. A., and W. Maury. 2005. Endocytosis and a low-pH step are required for productive entry of equine infectious anemia virus. J. Virol. 79:14482-14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassell, S., J. Edwards, and D. T. Brown. 1984. Effects of lysosomotropic weak bases on infection of BHK-21 cells by Sindbis virus. J. Virol. 52:857-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu, J. J. H., and M. L. Ng. 2004. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 78:10543-10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuadras, M. A., C. F. Arias, and S. López. 1997. Rotaviruses induce an early membrane permeabilization of MA104 cells and do not require a low intracellular Ca2+ concentration to initiate their replication cycle. J. Virol. 71:9065-9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damm, E. M., L. Pelkmans, J. Kartenbeck, A. Mezzacasa, T. Kurzchalia, and A. Helenius. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitrov, D. S. 2004. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2:109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garret, A., C. Kerlan, and D. Thomas. 1993. The intestine is a site of passage for potato leafroll virus from the gut lumen into the haemocoel in the aphid vector, Myzus persicae Sulz. Arch. Virol. 131:377-392. [DOI] [PubMed] [Google Scholar]
- 10.Gray, S., and F. E. Gildow. 2003. Luteovirus-aphid interactions. Annu. Rev. Phytopathol. 41:539-566. [DOI] [PubMed] [Google Scholar]
- 11.Kimura, I. 1986. A study of rice dwarf virus in vector cell monolayers by fluorescent antibody focus counting. J. Gen. Virol. 67:2119-2124. [Google Scholar]
- 12.Mao, Z. J., Y. Li, H. Xu, H. H. Zheng, J. Schiemann, R. Caspe, and Z. L. Chen. 1998. The 42K protein of rice dwarf virus is a post-translational cleavage product of the 46K outer capsid protein. Arch. Virol. 143:1831-1838. [DOI] [PubMed] [Google Scholar]
- 13.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki, N., K. Hagiwara, H. Naitow, T. Higashi, R. H. Cheng, T. Tsukihara, A. Nakagawa, and T. Omura. 2005. Transcapsidation and the conserved interactions of two major structural proteins of a pair of phytoreoviruses confirm the mechanism of assembly of the outer capsid layer. J. Mol. Biol. 345:229-237. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa, A., N. Miyazaki, J. Taka, H. Naitow, A. Ogawa, Z. Fujimoto, H. Mizuno, T. Higashi, Y. Watanabe, T. Omura, R. H. Cheng, and T. Tsukihara. 2003. The atomic structure of Rice dwarf virus reveals the self-assembly mechanism of component proteins. Structure 11:1227-1238. [DOI] [PubMed] [Google Scholar]
- 16.Omura, T., and J. Yan. 1999. Role of outer capsid proteins in transmission of Phytoreovirus by insect vectors. Adv. Virus Res. 54:15-43. [DOI] [PubMed] [Google Scholar]
- 17.Omura, T., and P. P. C. Mertens. 2005. Phytoreovirus, p. 543-549. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and A. L. Ball (ed.), Virus taxonomy: 8th report of the International Committee on Taxonomy of Viruses. Academic Press, London, United Kingdom.
- 18.Omura, T., J. Yan, B. Zhong, M. Wada, Y. Zhu, M. Tomaru, W. Maruyama, A. Kikuchi, Y. Watanabe, I. Kimura, and H. Hibino. 1998. The P2 protein of rice dwarf phytoreovirus is required for adsorption of the virus to cells of the insect vector. J. Virol. 72:9370-9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parton, R. G., B. Joggerst, and K. Simons. 1994. Regulated internalization of caveolae. J. Cell Biol. 127:1199-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 21.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 22.Tomaru, M., W. Maruyama, A. Kikuchi, J. Yan, Y. Zhu, N. Suzuki, M. Isogai, Y. Oguma, I. Kimura, and T. Omura. 1997. The loss of outer capsid protein P2 results in nontransmissibility by the insect vector of rice dwarf phytoreovirus. J. Virol. 71:8019-8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei, T., T. Shimizu, K. Hagiwara, A. Kikuchi, Y. Moriyasu, N. Suzuki, H. Chen, and T. Omura. 2006. Pns12 protein of Rice dwarf virus is essential for formation of viroplasms and nucleation of viral-assembly complexes. J. Gen. Virol. 87:429-438. [DOI] [PubMed] [Google Scholar]
- 25.Yan, J., M. Tomaru, A. Takahashi, I. Kimura, H. Hibino, and T. Omura. 1996. P2 protein encoded by genome segment S2 of rice dwarf phytoreovirus is essential for virus infection. Virology 224:539-541. [DOI] [PubMed] [Google Scholar]
- 26.Zheng, H. H., L. Yu, C. H. Wei, D. W. Hu, Y. P. Shen, Z. L. Chen, and Y. Li. 2000. Assembly of double-shelled, virus-like particles in transgenic rice plants expressing two major structural proteins of rice dwarf virus. J. Virol. 74:9808-9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong, B., A. Kikuchi, Y. Moriyasu, T. Higashi, K. Hagiwara, and T. Omura. 2003. A minor outer capsid protein, P9, of Rice dwarf virus. Arch. Virol. 148:2275-2280. [DOI] [PubMed] [Google Scholar]