Abstract
Epstein-Barr virus (EBV) establishes a latent form of infection in memory B cells, while antibody-secreting plasma cells often harbor the lytic form of infection. The switch between latent and lytic EBV infection is mediated by the two viral immediate-early proteins BZLF1 (Z) and BRLF1 (R), which are not expressed in latently infected B cells. Here we demonstrate that a cellular transcription factor that plays an essential role in plasma cell differentiation, X-box-binding protein 1 (XBP-1), also activates the transcription of the two EBV immediate-early gene promoters. In reporter gene assays, XBP-1 alone was sufficient to activate the R promoter, whereas the combination of XBP-1 and protein kinase D (PKD) was required for efficient activation of the Z promoter. Most importantly, the expression of XBP-1 and activated PKD was sufficient to induce lytic viral gene expression in EBV-positive nasopharyngeal carcinoma cells and lymphoblastoid cells, while an XBP-1 small interfering RNA inhibited constitutive lytic EBV gene expression in lymphoblastoid cells. These results suggest that the plasma cell differentiation factor XBP-1, in combination with activated PKD, can mediate the reactivation of EBV, thereby allowing the viral life cycle to be intimately linked to plasma cell differentiation.
Epstein-Barr virus (EBV) is the causative agent of infectious mononucleosis and is associated with B-cell lymphomas, nasopharyngeal carcinomas, gastric carcinomas, and other malignancies (26, 45). EBV causes lytic infection in normal oral epithelial cells (32, 51) while usually establishing one of the latent forms of infection in circulating memory B cells. In contrast, tonsillar B cells that express antigens specific for plasma markers commonly harbor the lytic form of EBV infection, which results in the production of infectious viral particles (10, 29, 30).
The switch from latent to lytic EBV infection is mediated by the immediate-early (IE) protein BZLF1 (Z) and the immediate-early/early protein BRLF1 (R) (1, 16, 57). Z and R are transcription factors that activate each other's transcription and together are sufficient to activate the entire lytic viral gene expression cascade (17, 49). In latently infected cells, the promoters driving Z and R expression (Zp and Rp) are inactive. Therefore, the activation of Zp and Rp by cellular transcription factors is the crucial initial step required for lytic viral gene expression. B-cell receptor engagement activates lytic EBV gene expression in some B-cell lines in vitro and activates both EBV IE promoters in reporter gene assays (23). Although several different individual cellular transcription factors can activate one or both of the two EBV IE promoters in reporter gene assays (23), to date these factors have not been shown to be sufficient for the efficient reactivation of lytic viral gene expression from the endogenous viral genome in latently infected cells.
While there is a strong correlation between plasma cell differentiation and lytic EBV gene expression in human tonsils, it is not presently understood why this association occurs. Possible explanations include the ability of one or more lytic viral proteins to induce plasma cell differentiation in B cells. Alternatively, plasma cell differentiation may result in the expression of one or more cellular transcription factors that activate lytic viral gene expression. The human protein X-box-binding protein 1 (XBP-1) is a basic-region leucine zipper transcriptional activator protein belonging to the cyclic AMP response element-binding protein/activating transcription factor (CREB/ATF) family that is activated early in the process of plasma cell differentiation and plays an essential role in this process (33, 43). We therefore hypothesized that XBP-1 may also play a role in mediating the reactivation of lytic EBV gene expression during plasma cell differentiation, possibly in conjunction with one or more cellular factors activated by B-cell receptor engagement.
In this work, we demonstrate that the combination of the active (spliced) form of XBP-1 (XBP-1s) with activated protein kinase D (PKD, a histone deacetylase [HDAC] inhibitor that is activated following antigen receptor engagement in B cells) (37, 52) is sufficient to induce lytic EBV gene expression in lymphoblastoid B cells, as well as nasopharyngeal carcinoma epithelial cells. We show that XBP-1s alone efficiently activates Rp in reporter gene assays, while the combination of XBP-1s and PKD is required for the activation of the other EBV IE promoter, Zp. Furthermore, we demonstrate that a small interfering RNA (siRNA) directed against XBP-1 inhibits the low-level constitutive lytic EBV gene expression that occurs in lymphoblastoid cells (LCLs). These results suggest that XBP-1s, in conjunction with activated PKD, induces the switch from latent to lytic EBV infection in plasma cells.
MATERIALS AND METHODS
Cell lines.
HONE-1/EBV is a human nasopharyngeal carcinoma cell line that stably maintains the EBV (Akata strain) genome under G418 selection in a latent form (47). HeLa is a malignant human epithelial cervical cancer cell line. HONE-1/EBV cells were grown in RPMI 1640 medium (Sigma). HeLa cells were grown in Dulbecco's modified Eagle's medium (Sigma). Both media were supplemented with 10% fetal bovine serum and penicillin-streptomycin. Early-passage LCLs (primary human B cells transformed with the B95-8 strain of EBV) were a gift from Bill Sugden at McArdle Laboratory, University of Wisconsin, Madison, and were grown in RPMI 1640 medium with 10% fetal bovine serum.
Plasmids.
Plasmid DNA was purified through columns as described by the manufacturer (QIAGEN). Rp-LUC contains the Rp sequence from +37 to −981 relative to the R transcription start site inserted 5′ of the luciferase gene in the pGL3-basic vector (Promega). A series of 5′ deletion mutants of the Rp-LUC construct (containing the promoter sequence from +37 to −30, +37 to −100, +37 to −197, +37 to −634, or +37 to −750) were also constructed. Zp-LUC contains the IE Zp sequence from +28 to −495 relative to the Z transcription start site inserted upstream of the luciferase gene in pGL3-basic. The Zp-CAT constructs, containing Zp sequences from −221 to +12 inserted upstream of the CAT gene or mutations in these sequences removing either the ZII (cis-acting replication element [CRE]) or ZI (myocyte enhancer factor 2D [MEF2D]) motifs, were a gift from Erik Flemington (Tulane University) (15). Plasmids expressing the FLAG-tagged versions of the XBP-1s or unspliced XBP-1 forms of mouse XBP-1 were a generous gift from Laurie Glimcher (Harvard University). A vector expressing the constitutively active form of calmodulin-dependent calcium kinase IV (CaMKIV) was a generous gift from Xiang-Jiao Yang (McGill University). A vector expressing constitutively active PKD in which the serine residues at positions 738 and 742 have been changed to aspartate was constructed by Alex Toker (Harvard University) and acquired from Addgene.
Luciferase assays.
HeLa cells were transfected using FuGENE 6 (Roche). Luciferase assays were performed 48 to 72 h after transfection by using extracts prepared by freeze-thawing the cell pellet in reporter lysis buffer according to the instructions of the manufacturer (Promega). The luciferase activity in an assay buffer containing 12.5 mM glycylglycine, 2 mM EGTA, 7.5 mM MgSO4, 7.5 mM K2HPO4, 0.5 mM dithiothreitol, 1 mM ATP, 100 μM luciferin, and 50 mM Tris was determined with an Auto Lumat LB953 luminometer (EG&G Berthold).
CAT assays.
HeLa cells were transfected using FuGene 6 (Roche). Cell extracts were prepared 2 days posttransfection and incubated at 37°C with [14C]chloramphenicol (Amersham) in the presence of acetyl coenzyme A (Roche). The percent acetylation was determined by phosphorimager screening (Molecular Dynamics).
Immunoblot analysis.
HONE-1/EBV cells were transfected using FuGene 6 (Roche); LCLs were transfected with 400 nM DNA by using a nucleofector device (Amaxa) in buffer V with program A30. Cells were harvested 2 days postinfection, washed twice with 1× phosphate-buffered saline, and resuspended in a 1:3 mixture of SUMO buffer I (5% sodium dodecyl sulfate [SDS], 0.15 M Tris-HCl [pH 6.8], 30% glycerol) and SUMO buffer II (25 mM Tris-HCl [pH 8.3], 50 mM NaCl, 0.5% NP-40, 0.5% deoxycholate, 0.1% SDS) and 1× complete protease inhibitors (Roche). The cells were briefly sonicated and centrifuged. The resulting supernatants were electrophoresed on a 7% SDS-polyacrylamide gel electrophoresis denaturing gel. The proteins were transferred onto a nitrocellulose membrane (Protran), blocked in 1× phosphate-buffered saline-5% milk-0.1% Tween 20, and incubated with anti-XBP-1 (rabbit; 1:500 [Santa Cruz]), anti-R (mouse; 1:100 [Argene]), anti-Z (mouse; 1:100 [Argene]), anti-EBV diffuse early antigen (BMRF1; mouse; 1:250 [Vector Laboratories]), or anti-β-actin (1:5,000 [Sigma]) antibody for 1 h at room temperature. Vector controls used in these experiments were either the SG5 vector or a vector expressing an unspliceable form of XBP-1.
siRNA experiments.
siRNA against the human XBP-1 message (sense, 5′ ACAGCAAGUGGUAGAUUUATT, and antisense, 5′ UAAAUCUACCACUUGCUGUTT) was purchased from Ambion. Negative control siRNA was purchased from Santa Cruz (SC37007). LCLs (5 × 106) were transfected with 400 nM XBP-1 siRNA (or control siRNA) by using a nucleofector device (Amaxa) in buffer V with program A30. These conditions allowed at least 75% of the cells to be transfected, with approximately 50% viability. Two days postdelivery of the siRNA, immunoblot analysis was performed to determine the expression levels of the early lytic EBV protein BMRF1 and β-actin, whereas reverse transcriptase (RT)-PCR analysis was performed to determine the amounts of XBP-1, Z, R, and β2-microglobulin transcripts. PCR primers used to detect the XBP-1 transcript were 5′ CCTTGTAGTTGAGAACCAGG and 5′ GGGGCTTGGTATATATGTGG. RT-PCR analysis of the β2-microglobulin, Z, and R transcripts was performed as previously described (20).
EMSA.
In vitro-translated XBP-1s was made using a TNT T7 quick-coupled transcription-translation system according to the instructions of the manufacturer (Promega). Electrophoretic mobility shift assay (EMSA) binding reactions were performed with a buffer consisting of 1 mM dithiothreitol, 10 mM HEPES (pH 7.9), 1 mM EDTA, 50 mM KCl, 2 mM MgCl2, and 5% glycerol (5, 9). Two microliters of in vitro-translated XBP-1s from reticulocyte lysate (or the untranslated reticulocyte lysate), 1 μg of poly(dI-dC)/poly(dI-dC) (Pharmacia), and 1 mg of bovine serum albumin/ml were added to the binding reaction mixture, and the mixture was incubated for 5 min at room temperature before the addition of a labeled probe (10,000 cpm) and 20 min at room temperature after the addition of the labeled probe. Anti-XBP-1 antibody was added to the reaction mixture, and the mixture was further incubated at room temperature for 10 min. The reaction mixture was then loaded onto a 4% polyacrylamide gel and run in 0.5% Tris-borate-EDTA buffer at room temperature. The gel was dried on Whatman 3MM chromatography paper and exposed to autoradiography film (Amersham). The film was developed using Kodak developer.
The sequences of the oligonucleotide probes used in EMSAs were as follows: positive control, 5′ CTCGAGATGGATGACGTGTACAATAAAACGTCAAGCTT 3′ (9); Zp CRE motif (−54 to −73), 5′ CCTCTGTGATGTCATGGTTT 3′. Complementary oligonucleotides were annealed, end labeled with 32P, and used in EMSAs.
RESULTS
The combination of XBP-1s and activated PKD is sufficient to induce the reactivation of lytic EBV gene expression in latently infected epithelial cells and lymphoblastoid B cells.
To determine if XBP-1 induces lytic EBV gene expression, latently infected EBV-positive cell lines were transfected with the construct expressing XBP-1s, the spliced (active) form of the XBP-1 gene product, and lytic viral gene expression was monitored by immunoblot analysis of two different lytic viral proteins (BMRF1 and Z) 2 days after transfection. In addition, the effect of the activated form of PKD was also examined because antigen receptor engagement in B cells, which stimulates lytic EBV gene expression in certain cell lines, has been shown to convert PKD into its active form (36, 50, 60). PKD directly phosphorylates class II HDAC proteins, causing them to be sequestered in an inactive form with the 14-3-3 protein in the cytoplasm (52).
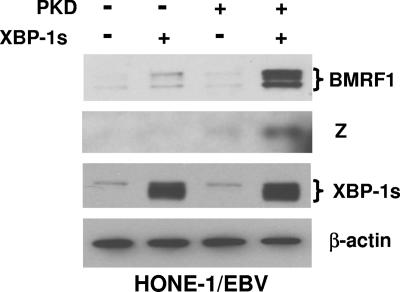
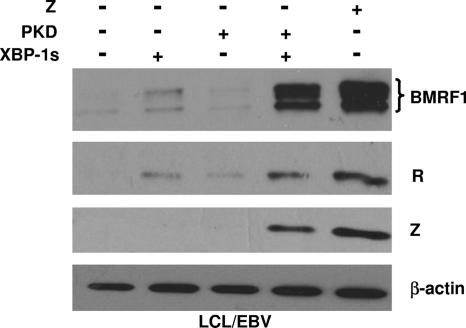
As shown in Fig. 1, the transfection of cells of a latently infected, EBV-positive nasopharyngeal carcinoma cell line (HONE-1/EBV) (47) with the XBP-1s vector alone resulted in low-level expression of the early lytic viral protein BMRF1, while the PKD vector alone had no effect. However, the combination of XBP-1s with activated PKD induced much more expression of the BMRF1 and Z lytic viral proteins than XBP-1s alone. Similar to that in nasopharyngeal carcinoma cells, XBP-1s alone activated low-level expression of the early viral protein BMRF1 in early-passage EBV-immortalized B-cell line LCLs, although the XBP-1s-PKD combination was clearly more effective (Fig. 2). There was no detectable level of Z in cells transfected with XBP-1s alone, while XBP-1s and PKD together significantly activated Z expression. In addition, XBP-1s in combination with PKD significantly activated the expression of the immediate-early/early R protein compared with the low level of R induced by XBP-1s alone. These results indicate that the combination of XBP-1 and activated PKD is sufficient to reactivate lytic EBV gene expression in either a B-cell or epithelial-cell environment.
FIG. 1.
XBP-1s and PKD synergistically activate lytic EBV gene expression in HONE-1/EBV cells. HONE-1/EBV cells were transfected with empty vector (−), XBP-1s, constitutively active PKD (PKD), or the combination of XBP-1s and PKD. Immunoblot analysis of cellular extracts was performed 2 days later to quantitate the expression of two different lytic EBV proteins (Z and BMRF1), a cellular protein (β-actin), and transfected XBP-1s protein. +, present.
FIG. 2.
XBP-1s and PKD synergistically activate lytic EBV gene expression in early-passage LCLs. LCLs were transfected with empty vector (−), the XBP-1s expression vector, constitutively active PKD (PKD), the combination of XBP-1s and PKD, or a Z expression vector. Immunoblot analysis of cellular extracts was performed 2 days later to quantitate the expression of the lytic EBV proteins (BMRF1, R, and Z) and cellular β-actin. +, present; LCL/EBV, EBV-infected LCLs.
XBP-1s alone is sufficient to activate the EBV IE promoter Rp in reporter gene assays, while the combination of XBP-1 and PKD activates Zp.
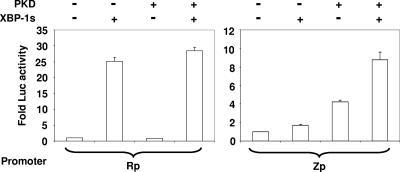
To determine if XBP-1s or activated PKD (alone or in combination) can activate either of the two EBV IE promoters, HeLa cells were transfected with reporter gene constructs containing Rp or Zp driving the luciferase gene and a vector control, the XBP-1s expression vector alone, the PKD expression vector alone, or the combination of both XBP-1s and PKD (Fig. 3). XBP-1s by itself strongly increased the activity of the Rp IE promoter, and this effect was not further enhanced by the addition of PKD. In contrast, the combination of XBP-1s and the constitutively active form of PKD produced more Zp activity than that induced by PKD or XBP-1s alone. A vector expressing the unspliced (inactive) form of the mouse XBP-1 gene product did not enhance Rp or Zp activity (data not shown). These results indicate that XBP-1 alone efficiently activates Rp but not Zp and that PKD augments the XBP-1s-mediated activation of the Zp.
FIG. 3.
XBP-1s and PKD activation of Rp versus Zp. HeLa cells were transfected with vectors containing the luciferase (Luc) gene linked to either the R (Rp-LUC) or Z (Zp-LUC) promoter in combination with either a control plasmid (−), an XBP-1s expression vector, a constitutively active form of PKD (PKD), or both XBP-1s and PKD, as indicated. Luciferase activity was measured 2 days later. Results are presented as the increase (n-fold) in the amount of luciferase activity after transfection with XBP-1s, PKD, or both compared with the amount of activity after transfection with the control vector, as indicated. +, present.
XBP-1s activates Rp indirectly.
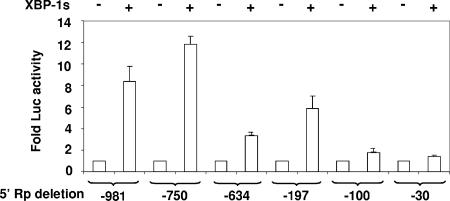
Neither Rp nor Zp contains a consensus XBP-1-binding motif. To map the XBP-1s-responsive region(s) in Rp, a series of 5′ deletion mutations in the Rp-LUC vector were constructed. Rp sequences located between −750 and −634 and between −197 and −100 were both important for XBP-1s activation of the promoter (Fig. 4). In vitro-translated XBP-1s did not bind to any of a series of overlapping probes spanning the two XBP-1s-responsive Rp elements in EMSAs, although it clearly bound to a positive control probe containing the consensus XBP-1-binding motif (data not shown and Fig. 5B). These data suggest that XBP-1s activates Rp via an as-yet-unknown indirect mechanism.
FIG. 4.
XBP-1-responsive regions in Rp. Various 5′ deletions in Rp-LUC were constructed as indicated, and HeLa cells were transfected with the constructs in combination with either empty vector (−) or the vector expressing XBP-1s (+). Results are presented as the increase (n-fold) in the amount of luciferase activity after transfection with XBP-1s versus the vector control for each promoter construct.
FIG. 5.
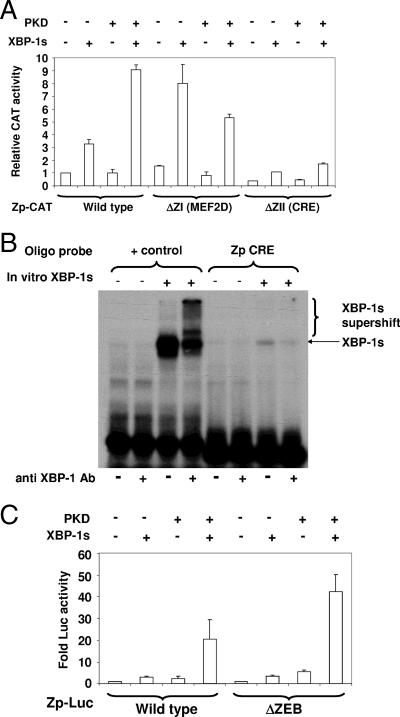
MEF2D and CRE motifs contribute to XBP-1s-PKD activation of Zp. (A) HeLa cells were cotransfected with reporter gene constructs containing the wild-type Zp (−221 to +12) inserted upstream of the CAT gene or containing site-directed mutations in the Zp altering the MEF2D or CRE motifs and either a vector control or the XBP-1s expression vector. CAT activity was quantitated 2 days later. Results are presented as the increase (n-fold) in the amount of CAT activity after transfection with XBP-1s compared with the amount of activity after transfection with the control vector as indicated for each promoter construct. +, present; −, absent. (B) EMSA was performed using in vitro-translated XBP-1s (+) or untranslated rabbit reticulocyte lysate (−) in combination with either the positive control oligonucleotide probe containing a consensus XBP-1s-binding motif or a probe containing the Zp CRE motif in the absence (−) or presence (+) of anti-XBP-1 antibody (Ab). (C) HeLa cells were transfected with Zp-LUC constructs with either wild-type Zp or Zp containing a site-directed mutation in the ZEB binding motif (ΔZEB) along with either empty vector or vector expressing XBP-1s. Luciferase activity was measured 2 days later.
PKD reverses the inhibitory effect of MEF2D motifs in Zp.
To further examine why PKD is required for efficient XBP-1s activation of the Zp promoter, we examined the effect of mutating the MEF2D (ZI)-binding motifs in Zp (5, 52), which have previously been shown to inhibit Zp activity when MEF2D interacts with class II HDACs (5, 15). As shown in Fig. 5A, a vector containing the wild-type Zp (from −221 to +12) linked to the CAT reporter gene responded minimally to XBP-1s or PKD alone but was efficiently activated by the XBP-1s-PKD combination. In contrast, when two MEF2D-binding motifs in Zp-CAT were mutated, the ability of XBP-1s alone to stimulate the promoter was enhanced, and the combination of PKD and XBP-1s together was no more effective than XBP-1s alone. These results indicate that the MEF2D sites act to inhibit XBP-1s activation of Zp and that PKD reverses this effect.
In addition to MEF2D, Zp activity is positively regulated by a CRE motif (ZII) and negatively regulated by a ZEB-binding site (ZV). The mutation of the CRE (ZII) site in Zp, which has been previously shown to be bound by ATF1, ATF2, CREB, and c-jun (23), decreased Zp activation by the XBP-1s-PKD combination, suggesting that the CRE motif is required for an efficient XBP-1s-PKD effect (Fig. 5A). However, we found that XBP-1s binds only weakly, if at all, to the Zp CRE motif in EMSAs (Fig. 5B). The mutation of the Zp ZEB-binding site (in the context of the Zp-LUC construct) enhanced the ability of the XBP-1s-PKD combination to activate Zp (Fig. 5C). These results confirm that ZEB binding to Zp, as previously described (28), acts as a negative regulator of Zp activity.
CaMKIV and valproic acid also augment the ability of XBP-1s to induce EBV reactivation.
The two isoforms of PKD expressed in B cells are thought to account for the majority of class II HDAC inhibitory activity in uninfected B cells (11, 21, 37). However, as PKD affects a number of cellular pathways in addition to HDAC proteins (3, 8, 19, 35, 39, 48), we determined if other HDAC inhibitors could likewise augment the ability of XBP-1s to induce lytic EBV gene expression. CaMKIV, like activated PKD, directly phosphorylates class II HDAC proteins and inhibits their function (34, 38). Although CaMKIV is not normally expressed in B cells, its transcription is induced by a latent EBV protein, LMP-1 (40), and B-cell receptor engagement converts the CaMKIV protein into its active form (40). Therefore, in B cells with type II or type III latent EBV infection (in which LMP-1 is expressed), CaMKIV (in addition to PKD) may function as a class II HDAC inhibitor.
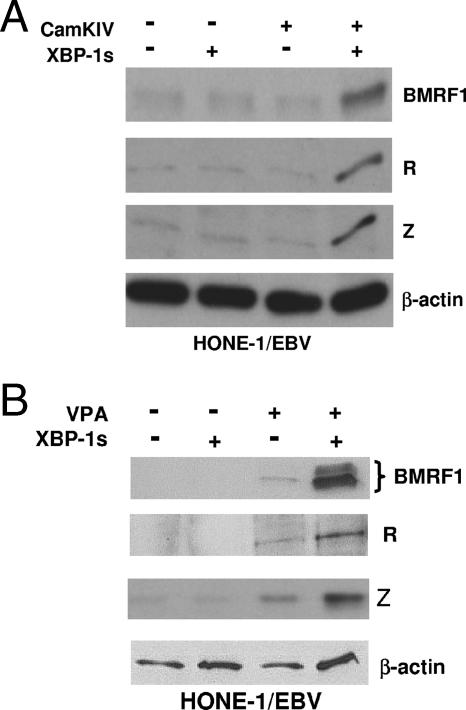
The transfection of HONE-1/EBV cells with a vector expressing a constitutively active form of CaMKIV by itself produced minimal expression of lytic EBV BMRF1, R, and Z genes but greatly augmented the ability of transfected XBP-1s to induce lytic viral gene expression (Fig. 6A). In addition, transfected XBP-1s induced the expression of lytic EBV BMRF1, R, and Z genes in the presence, but not the absence, of a pharmacologic HDAC inhibitor, valproic acid (Fig. 6B). These results suggest that it is the HDAC-inhibitory activity of PKD and CaMKIV that allows them to complement XBP-1s in reactivating EBV lytic infection.
FIG. 6.
XBP-1s and HDAC inhibitors CaMKIV and valproic acid activate lytic EBV gene expression. (A) HONE-1/EBV cells were transfected with empty vector (−), XBP-1s, or constitutively active CaMKIV either alone or in combination, as indicated. Cell extracts were examined by immunoblotting for the expression of the lytic EBV proteins (BMRF1, R, and Z) and cellular β-actin 2 days later. +, present. (B) HONE-1/EBV cells were transfected with empty vector or XBP-1s in the presence (+) or absence (−) or valproic acid (VPA; 1 mM). Extracts were analyzed by immunoblotting 2 days later for the lytic EBV proteins (BMRF1, R, and Z) and cellular β-actin.
XBP-1s expression contributes to lytic EBV gene expression in early-passage LCLs.
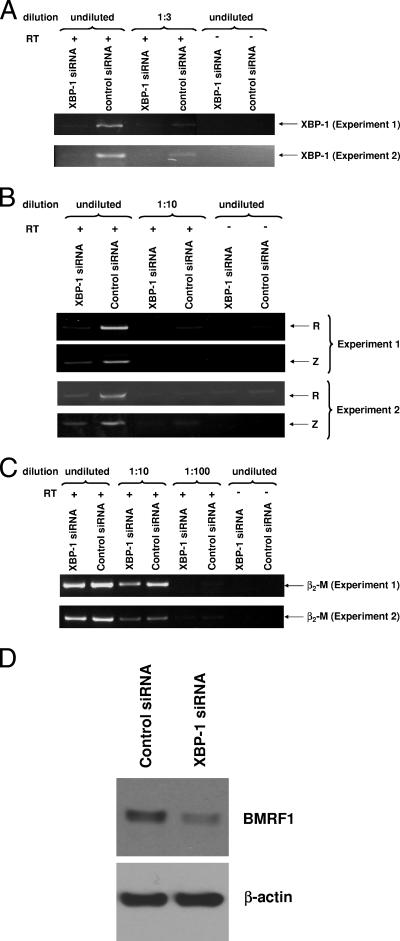
EBV-transformed B-cell lines (LCL lines) usually secrete immunoglobulin (53), and early-passage lines commonly express a small amount of lytic EBV genes (20). XBP-1s may therefore contribute to the low-level constitutive lytic EBV gene expression that occurs in some LCLs. To determine if this is the case, we transfected early-passage LCLs with an XBP-1 siRNA or equivalent amounts of a control siRNA (Fig. 7). Cells transfected with the XBP-1 siRNA expressed less XBP-1 RNA than cells transfected with the control siRNA (Fig. 7A). Furthermore, the expression of the lytic EBV Z and R genes was decreased in cells transfected with XBP-1 siRNA, while the β2-microglobulin message was not affected (Fig. 7B and C). In addition, the transfection of LCLs with XBP-1 siRNA led to a decrease in the expression of the EBV lytic BMRF1 gene as shown by the immunoblot in Fig. 7D. These results indicate that constitutive XBP-1s expression in early-passage EBV-transformed B cells contributes to the constitutive lytic viral gene expression in these cells.
FIG. 7.
XBP-1 is required for lytic EBV gene expression in LCLs. EBV-transformed B cells were transfected with an siRNA directed against XBP-1 or equal amounts of a control siRNA. Results from two separate experiments are shown. (A) The level of XBP-1 expression was examined by RT-PCR analysis 2 days after siRNA delivery. PCR amplifications were performed using undiluted cDNA, as well as various dilutions of the cDNA, as indicated. +, present; −, absent. (B) The expression level of the lytic viral Z and R genes was examined by RT-PCR. (C) The expression of the β2-microglobulin (β2-M) message was examined by RT-PCR. (D) The expression of the early lytic EBV BMRF1 protein, as well as cellular actin, was examined by immunoblot analysis 2 days after siRNA delivery.
DISCUSSION
Tightly latent EBV infection, which cannot easily be recognized and eliminated by the immune response, enables long-term persistence of the virus in the host. Latent EBV infection is established in the long-lived memory B-cell compartment, reducing the likelihood that the reservoir of latently infected cells will ever be lost. However, to ensure that the virus is transmitted from host to host, EBV must be periodically reactivated to the lytic form of infection. Increasing evidence suggests that the lytic form of EBV infection in humans occurs in antibody-secreting plasma cells (30) and tonsillar epithelial cells (42). Nevertheless, the molecular mechanism(s) underlying the reactivation of lytic EBV infection during plasma cell differentiation has not previously been explained. In this work, we show that XBP-1, in combination with activated PKD, is sufficient to induce lytic EBV gene expression in latently infected epithelial cells as well as LCLs. Furthermore, we demonstrate that XBP-1 is required for the small amount of constitutive lytic viral gene expression that occurs in early-passage LCLs. Since XBP-1 is converted into its active (spliced) form during plasma cell differentiation, while PKD is activated by B-cell receptor engagement, these results help explain why the lytic form of EBV infection is so strongly associated with plasma cell differentiation in humans.
XBP-1 activation and plasma cell differentiation are intricately linked. On the one hand, the activity of the XBP-1 transcription factor is dramatically enhanced by both transcriptional and splicing mechanisms as B cells differentiate into plasma cells. The production of antibodies by plasma cells results in the accumulation of unfolded proteins in the endoplasmic reticulum (ER), leading to ER stress and activation of the unfolded-protein response (UPR) (24). The UPR then enhances XBP-1 function through at least two different mechanisms. First, the UPR induces proteolytic cleaving of the ER protein ATF6α, allowing the amino-terminal portion of the protein to transit from the ER to the nucleus, where it binds to the promoter of the XBP-1 gene and activates its transcription (55, 56). Second, the UPR induces the homodimerization and transphosphorylation of interferon gene regulatory element 1α, converting it into an atypical splicing enzyme which mediates the cytoplasmic splicing of XBP-1. Only this spliced form of XBP-1, XBP-1s, contains a transcriptional activator domain and can activate the transcription of downstream target genes (2, 31).
On the other hand, XBP-1 is also clearly required for full plasma cell differentiation, although its effect is downstream of that of another essential plasma cell differentiation factor, Blimp-1 (27, 46). B cells derived from XBP-1 knockout mice mature normally but are unable to differentiate into antibody-secreting plasma cells (43). The spliced form of XBP-1 not only activates the transcription of numerous ER chaperone genes (55) but also increases the size of the cellular mitochondrial, ER, and secretory apparatus compartments (46). These downstream effects of XBP-1s, which presumably help the plasma cell to efficiently produce and secrete large amounts of antibody (54, 55), may also promote the large-scale protein synthesis required for the synthesis of lytic herpesvirus proteins. Interestingly, cytomegalovirus was recently shown to modulate and enhance certain aspects of XBP-1s function (22). By selectively converting into the lytic form of viral infection in plasma cells, EBV has likewise found a mechanism for ensuring that sufficient XBP-1s activity is available in cells with the lytic form of viral replication.
XBP-1s is known to activate most downstream target genes, including ER chaperone genes and some major histocompatibility complex class II genes, via direct binding to the promoters of these genes (9, 25, 41, 54, 55). XBP-1s may also activate transcription through indirect mechanisms, including enhancing the transcriptional effect of estrogen receptor alpha (12) and inducing the expression of CCAAT/enhancer-binding protein beta (7). Our EMSA experiments suggest that XBP-1s does not bind directly to Rp and binds minimally to the CRE motif in Zp. We speculate that XBP-1s indirectly activates Zp and/or Rp by inducing the transcription of a cellular factor that activates one or both promoters or by interacting with one or more cellular proteins that directly bind to Zp and/or Rp. Dissecting the exact mechanism(s) by which XBP-1s increases Z and R transcription will require further study.
Although XBP-1s alone activates the EBV Rp IE promoter in reporter gene assays and high-level expression of R is sufficient to induce lytic EBV infection in many cell types (including HONE-1/EBV cells) (59), the combination of both XBP-1s and activated PKD is required for the induction of lytic EBV infection in latently infected HONE-1/EBV cells. PKD, which belongs to a group of serine/threonine protein kinases (44), is activated by B-cell receptor engagement via a pathway involving phospholipase C. Phospholipase C hydrolyzes phosphatidylinositol (4,5)-bisphosphate to produce diacylglycerol, activating novel PKC isoforms, which in turn phosphorylate and activate PKD (50, 52, 60). Activated PKD can then travel to the nucleus and phosphorylate and inactivate class II HDACs.
The ability of PKD to disrupt viral latency in conjunction with XBP-1s is likely mediated through the inhibition of class II HDAC proteins, since a similar effect was observed using two other HDAC inhibitors, CaMKIV and valproic acid. The inhibition of class II HDAC proteins would be expected to enhance the ability of XBP-1s to activate lytic viral gene transcription in the context of the intact latent genome through at least two different mechanisms. First, Rp is highly methylated with an inactive (unacetylated) chromatin structure in the context of the intact latent EBV genome in many cell lines (4, 6, 58). Chromatin accessibility to transcription factors is regulated by the acetylation or deacetylation of nucleosomal histones, and HDAC proteins inhibit the ability of many transcription factors to activate target promoters by converting the chromatin into a less accessible (deacetylated) form. The acetylation of the chromatin around Rp (and possibly Zp) in the context of the intact latent viral genome presumably enhances the ability of XBP-1s to activate the promoter.
In addition, certain transcription factors, including members of the MEF2 family, directly interact with class II (but not class I) HDAC proteins and tether these HDACs to promoters with MEF2-binding sites. Zp contains a series of MEF2D-binding sites (known as ZI motifs), which function as negative regulators when complexed with class II HDAC proteins (5, 13, 15, 18). The finding that the removal of the MEF2D sites in Zp allowed XBP-1s alone to activate Zp without PKD strongly suggests that PKD enhances XBP-1s activation of Zp at least partially by inhibiting the interaction between MEF2D and class II HDAC proteins.
As XBP-1s can also be activated by the UPR in non-B cell types, it remains possible that XBP-1s also contributes to the induction of lytic EBV infection in other cell types in response to stimuli such as chemotherapy and radiation (13, 14). In any event, the activation of the two EBV IE promoters by the combination of two factors (spliced XBP-1 and activated PKD) that are induced by B-cell receptor engagement and plasma cell differentiation helps to ensure that EBV pathogenesis is strongly linked to the state of B-cell maturation and activation.
Acknowledgments
We thank Lawrence Young and Chris Dawson for providing the HONE-1/EBV cell line.
This work was supported by National Institutes of Health grants RO1 CA58853 and R01-CA66519.
Footnotes
Published ahead of print on 9 May 2007.
REFERENCES
- 1.Amon, W., U. K. Binne, H. Bryant, P. J. Jenkins, C. E. Karstegl, and P. J. Farrell. 2004. Lytic cycle gene regulation of Epstein-Barr virus. J. Virol. 78:13460-13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Back, S. H., K. Lee, E. Vink, and R. J. Kaufman. 2006. Cytoplasmic IRE1alpha-mediated XBP1 mRNA splicing in the absence of nuclear processing and endoplasmic reticulum stress. J. Biol. Chem. 281:18691-18706. [DOI] [PubMed] [Google Scholar]
- 3.Baron, C. L., and V. Malhotra. 2002. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295:325-328. [DOI] [PubMed] [Google Scholar]
- 4.Bhende, P. M., W. T. Seaman, H. J. Delecluse, and S. C. Kenney. 2004. The EBV lytic switch protein, Z, preferentially binds to and activates the methylated viral genome. Nat. Genet. 36:1099-1104. [DOI] [PubMed] [Google Scholar]
- 5.Bryant, H., and P. J. Farrell. 2002. Signal transduction and transcription factor modification during reactivation of Epstein-Barr virus from latency. J. Virol. 76:10290-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, L. K., and S. T. Liu. 2000. Activation of the BRLF1 promoter and lytic cycle of Epstein-Barr virus by histone acetylation. Nucleic Acids Res. 28:3918-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C., E. E. Dudenhausen, Y. X. Pan, C. Zhong, and M. S. Kilberg. 2004. Human CCAAT/enhancer-binding protein beta gene expression is activated by endoplasmic reticulum stress through an unfolded protein response element downstream of the protein coding sequence. J. Biol. Chem. 279:27948-27956. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J., G. Lu, and Q. J. Wang. 2005. Protein kinase C-independent effects of protein kinase D3 in glucose transport in L6 myotubes. Mol. Pharmacol. 67:152-162. [DOI] [PubMed] [Google Scholar]
- 9.Clauss, I. M., M. Chu, J. L. Zhao, and L. H. Glimcher. 1996. The basic domain/leucine zipper protein hXBP-1 preferentially binds to and transactivates CRE-like sequences containing an ACGT core. Nucleic Acids Res. 24:1855-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford, D. H., and I. Ando. 1986. EB virus induction is associated with B-cell maturation. Immunology 59:405-409. [PMC free article] [PubMed] [Google Scholar]
- 11.Dequiedt, F., J. Van Lint, E. Lecomte, V. Van Duppen, T. Seufferlein, J. R. Vandenheede, R. Wattiez, and R. Kettmann. 2005. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J. Exp. Med. 201:793-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding, L., J. Yan, J. Zhu, H. Zhong, Q. Lu, Z. Wang, C. Huang, and Q. Ye. 2003. Ligand-independent activation of estrogen receptor alpha by XBP-1. Nucleic Acids Res. 31:5266-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, W. H., J. I. Cohen, S. Fischer, L. Li, M. Sneller, R. Goldbach-Mansky, N. Raab-Traub, H. J. Delecluse, and S. C. Kenney. 2004. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J. Natl. Cancer Inst. 96:1691-1702. [DOI] [PubMed] [Google Scholar]
- 14.Feng, W. H., G. Hong, H. J. Delecluse, and S. C. Kenney. 2004. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J. Virol. 78:1893-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flemington, E., and S. H. Speck. 1990. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flemington, E. K., A. E. Goldfeld, and S. H. Speck. 1991. Efficient transcription of the Epstein-Barr virus immediate-early BZLF1 and BRLF1 genes requires protein synthesis. J. Virol. 65:7073-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grogan, E., H. Jenson, J. Countryman, L. Heston, L. Gradoville, and G. Miller. 1987. Transfection of a rearranged viral DNA fragment, WZhet, stably converts latent Epstein-Barr viral infection to productive infection in lymphoid cells. Proc. Natl. Acad. Sci. USA 84:1332-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruffat, H., E. Manet, and A. Sergeant. 2002. MEF2-mediated recruitment of class II HDAC at the EBV immediate early gene BZLF1 links latency and chromatin remodeling. EMBO Rep. 3:141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hausser, A., P. Storz, S. Martens, G. Link, A. Toker, and K. Pfizenmaier. 2005. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat. Cell Biol. 7:880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong, G. K., P. Kumar, L. Wang, B. Damania, M. L. Gulley, H. J. Delecluse, P. J. Polverini, and S. C. Kenney. 2005. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 79:13984-13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huynh, Q. K., and T. A. McKinsey. 2006. Protein kinase D directly phosphorylates histone deacetylase 5 via a random sequential kinetic mechanism. Arch. Biochem. Biophys. 450:141-148. [DOI] [PubMed] [Google Scholar]
- 22.Isler, J. A., A. H. Skalet, and J. C. Alwine. 2005. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 79:6890-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israel, B. F., and S. C. Kenney. 2005. EBV lytic infection. In E. S. Robertson (ed.), Epstein-Barr virus. Caister Academic Press, Norwich, United Kingdom.
- 24.Iwakoshi, N. N., A. H. Lee, P. Vallabhajosyula, K. L. Otipoby, K. Rajewsky, and L. H. Glimcher. 2003. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 4:321-329. [DOI] [PubMed] [Google Scholar]
- 25.Kanemoto, S., S. Kondo, M. Ogata, T. Murakami, F. Urano, and K. Imaizumi. 2005. XBP1 activates the transcription of its target genes via an ACGT core sequence under ER stress. Biochem. Biophys. Res. Commun. 331:1146-1153. [DOI] [PubMed] [Google Scholar]
- 26.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2574. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Strauss (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 27.Klein, U., S. Casola, G. Cattoretti, Q. Shen, M. Lia, T. Mo, T. Ludwig, K. Rajewsky, and R. Dalla-Favera. 2006. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat. Immunol. 7:773-782. [DOI] [PubMed] [Google Scholar]
- 28.Kraus, R. J., J. G. Perrigoue, and J. E. Mertz. 2003. ZEB negatively regulates the lytic-switch BZLF1 gene promoter of Epstein-Barr virus. J. Virol. 77:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laichalk, L. L., D. Hochberg, G. J. Babcock, R. B. Freeman, and D. A. Thorley-Lawson. 2002. The dispersal of mucosal memory B cells: evidence from persistent EBV infection. Immunity 16:745-754. [DOI] [PubMed] [Google Scholar]
- 30.Laichalk, L. L., and D. A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, K., W. Tirasophon, X. Shen, M. Michalak, R. Prywes, T. Okada, H. Yoshida, K. Mori, and R. J. Kaufman. 2002. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16:452-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, Q. X., L. S. Young, G. Niedobitek, C. W. Dawson, M. Birkenbach, F. Wang, and A. B. Rickinson. 1992. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature 356:347-350. [DOI] [PubMed] [Google Scholar]
- 33.Liou, H. C., M. R. Boothby, P. W. Finn, R. Davidon, N. Nabavi, N. J. Zeleznik-Le, J. P. Ting, and L. H. Glimcher. 1990. A new member of the leucine zipper class of proteins that binds to the HLA DR alpha promoter. Science 247:1581-1584. [DOI] [PubMed] [Google Scholar]
- 34.Lu, J., T. A. McKinsey, R. L. Nicol, and E. N. Olson. 2000. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc. Natl. Acad. Sci. USA 97:4070-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marklund, U., K. Lightfoot, and D. Cantrell. 2003. Intracellular location and cell context-dependent function of protein kinase D. Immunity 19:491-501. [DOI] [PubMed] [Google Scholar]
- 36.Matthews, S. A., R. Dayalu, L. J. Thompson, and A. M. Scharenberg. 2003. Regulation of protein kinase C by the B-cell antigen receptor. J. Biol. Chem. 278:9086-9091. [DOI] [PubMed] [Google Scholar]
- 37.Matthews, S. A., P. Liu, M. Spitaler, E. N. Olson, T. A. McKinsey, D. A. Cantrell, and A. M. Scharenberg. 2006. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol. Cell. Biol. 26:1569-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinsey, T. A., C. L. Zhang, J. Lu, and E. N. Olson. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408:106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mihailovic, T., M. Marx, A. Auer, J. Van Lint, M. Schmid, C. Weber, and T. Seufferlein. 2004. Protein kinase D2 mediates activation of nuclear factor kappaB by Bcr-Abl in Bcr-Abl+ human myeloid leukemia cells. Cancer Res. 64:8939-8944. [DOI] [PubMed] [Google Scholar]
- 40.Mosialos, G., S. H. Hanissian, S. Jawahar, L. Vara, E. Kieff, and T. A. Chatila. 1994. A Ca2+/calmodulin-dependent protein kinase, CaM kinase-Gr, expressed after transformation of primary human B lymphocytes by Epstein-Barr virus (EBV) is induced by the EBV oncogene LMP1. J. Virol. 68:1697-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono, S. J., H. C. Liou, R. Davidon, J. L. Strominger, and L. H. Glimcher. 1991. Human X-box-binding protein 1 is required for the transcription of a subset of human class II major histocompatibility genes and forms a heterodimer with c-fos. Proc. Natl. Acad. Sci. USA 88:4309-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pegtel, D. M., J. Middeldorp, and D. A. Thorley-Lawson. 2004. Epstein-Barr virus infection in ex vivo tonsil epithelial cell cultures of asymptomatic carriers. J. Virol. 78:12613-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reimold, A. M., N. N. Iwakoshi, J. Manis, P. Vallabhajosyula, E. Szomolanyi-Tsuda, E. M. Gravallese, D. Friend, M. J. Grusby, F. Alt, and L. H. Glimcher. 2001. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412:300-307. [DOI] [PubMed] [Google Scholar]
- 44.Rey, O., R. Papazayan, R. T. Waldron, S. H. Young, J. Lippincott-Schwarz, R. Jacamo, and E. Rozengurt. 2006. The nuclear import of protein kinase D3 requires its catalytic activity. J. Biol. Chem. 281:5149-5157. [DOI] [PubMed] [Google Scholar]
- 45.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Strauss (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 46.Shaffer, A. L., M. Shapiro-Shelef, N. N. Iwakoshi, A. H. Lee, S. B. Qian, H. Zhao, X. Yu, L. Yang, B. K. Tan, A. Rosenwald, E. M. Hurt, E. Petroulakis, N. Sonenberg, J. W. Yewdell, K. Calame, L. H. Glimcher, and L. M. Staudt. 2004. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21:81-93. [DOI] [PubMed] [Google Scholar]
- 47.Stewart, S., C. W. Dawson, K. Takada, J. Curnow, C. A. Moody, J. W. Sixbey, and L. S. Young. 2004. Epstein-Barr virus-encoded LMP2A regulates viral and cellular gene expression by modulation of the NF-kappaB transcription factor pathway. Proc. Natl. Acad. Sci. USA 101:15730-15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storz, P., and A. Toker. 2003. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 22:109-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldron, R. T., and E. Rozengurt. 2003. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J. Biol. Chem. 278:154-163. [DOI] [PubMed] [Google Scholar]
- 51.Walling, D. M., C. M. Flaitz, C. M. Nichols, S. D. Hudnall, and K. Adler-Storthz. 2001. Persistent productive Epstein-Barr virus replication in normal epithelial cells in vivo. J. Infect. Dis. 184:1499-1507. [DOI] [PubMed] [Google Scholar]
- 52.Wang, Q. J. 2006. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol. Sci. 27:317-323. [DOI] [PubMed] [Google Scholar]
- 53.Wroblewski, J. M., A. Copple, L. P. Batson, C. D. Landers, and J. R. Yannelli. 2002. Cell surface phenotyping and cytokine production of Epstein-Barr Virus (EBV)-transformed lymphoblastoid cell lines (LCLs). J. Immunol. Methods 264:19-28. [DOI] [PubMed] [Google Scholar]
- 54.Ye, J., R. B. Rawson, R. Komuro, X. Chen, U. P. Dave, R. Prywes, M. S. Brown, and J. L. Goldstein. 2000. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6:1355-1364. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida, H., T. Matsui, A. Yamamoto, T. Okada, and K. Mori. 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881-891. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida, H., T. Okada, K. Haze, H. Yanagi, T. Yura, M. Negishi, and K. Mori. 2000. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20:6755-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan, J., E. Cahir-McFarland, B. Zhao, and E. Kieff. 2006. Virus and cell RNAs expressed during Epstein-Barr virus replication. J. Virol. 80:2548-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zalani, S., A. Coppage, E. Holley-Guthrie, and S. Kenney. 1997. The cellular YY1 transcription factor binds a cis-acting, negatively regulating element in the Epstein-Barr virus BRLF1 promoter. J. Virol. 71:3268-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zugaza, J. L., J. Sinnett-Smith, J. Van Lint, and E. Rozengurt. 1996. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J. 15:6220-6230. [PMC free article] [PubMed] [Google Scholar]