Abstract
Molecular umbrella compounds may function as novel topical microbicides to prevent human immunodeficiency virus (HIV) and herpes simplex virus (HSV) infections. In a preliminary structure-activity investigation, one umbrella compound, designated Spm8CHAS, was identified which inhibited both HIV and HSV infections with no cellular toxicity. The objectives of the current studies were to define its spectrum of antiviral activity, characterize its mechanism of action, and explore the possibility of combining Spm8CHAS with HIV-specific reverse transcriptase inhibitors. Spm8CHAS inhibited infections by laboratory and clinical R5 and X4 clade B and clade C HIV strains in cell culture. Ectocervical tissue explants exposed to HIV-1BaL in the presence of Spm8CHAS were completely protected (50% inhibitory concentration [IC50], 13.6 μg/ml), and transfer of virus to target T cells via migratory cells was abolished (IC50, 3.8 μg/ml). Spm8CHAS inhibited HSV-2 infection of epithelial cells 10,000-fold if present throughout the infection. Notably, adding Spm8CHAS to cultures following HSV entry significantly reduced viral infection, indicating that the drug also acts postentry. Subsequent studies indicated that Spm8CHAS blocks cell-to-cell spread of HSV. Confocal microscopy using a fluorescently labeled analog of Spm8CHAS demonstrated that this conjugate crosses the plasma cell membrane and is transported to the nucleus. Combinations of Spm8CHAS with UC-781 or 9-[R-2-(phosphonylmethoxy)propyl] adenine monohydrate in vitro exhibited additive anti-HIV activity with preserved anti-HSV activity. The abilities of Spm8CHAS to inhibit primary isolates of HIV, block HSV infection postentry, and cross cell membranes support the development of a combination microbicide containing Spm8CHAS with an HIV-specific reverse transcriptase inhibitor to prevent both HIV and HSV infections by multiple mechanisms.
The disproportionate burden borne by women for human immunodeficiency virus (HIV), genital herpes, and other sexually transmitted infections (STIs) has promoted the development of prophylactic vaginal microbicides as a critical health priority. In sub-Saharan Africa, home to 64% of individuals living with HIV, more women than men are HIV infected, and the prevalence of HIV in pregnant women attending prenatal clinics in Swaziland increased from 4% in 1992 to 43% in 2004 (18). Heterosexual transmission remains the primary method of acquisition; 80% of HIV infections in India are attributed to heterosexual transmission (18). The dismal epidemiology of the HIV pandemic clearly demands a novel, female-controlled approach to HIV prevention.
The synergistic relationship between HIV and herpes simplex virus (HSV) infections also supports the need for a microbicide that inhibits both pathogens. A meta-analysis of the relationship between HSV-2 and HIV infection confirmed that in all subgroups (including heterosexual men, women, and individuals in both developing and developed countries), HSV-2 seropositivity increases the risk of HIV-1 acquisition (9). Epidemiological studies consistently demonstrate an increased risk of HIV-1 acquisition associated with HSV-2 infection. A study of 2,732 patients attending STI clinics in India found a recent incident of HSV-2 infection to be the most significant risk factor for acquiring HIV-1 infection (32). Thus, a microbicide that targets both viruses may have a greater impact on the HIV pandemic than that achieved by an agent that targets HIV alone.
While several compounds are currently in clinical trials, it is unlikely that a single agent will prove fully protective. Among the more promising first-generation microbicides are the polyanions PRO 2000 and Carraguard (13, 27). These compounds primarily act by competitively blocking HIV and HSV binding to cell surface receptors and thus are likely to provide only partial protection (1). Development of effective microbicides will likely require a combination of drugs that target different steps in the HIV life cycle and provide protection against HSV or other STIs known to facilitate HIV infection.
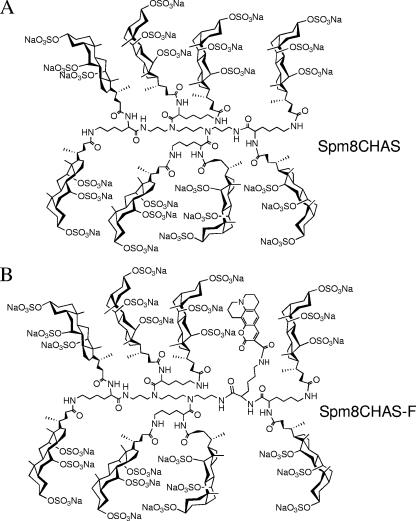
We previously screened a series of molecular umbrella compounds and identified one, Spm8CHAS, as a candidate based on its significant in vitro anti-HIV and anti-HSV activities (17). Although Spm8CHAS contains persulfations, it is chemically distinct from polyanionic polymers (Fig. 1A). It is not a polymer but a pure single molecule with a facially amphiphilic structure, which could potentially facilitate its transport across phospholipid bilayers. The studies described here were designed to (i) further define the antiviral properties of Spm8CHAS, (ii) identify the mechanism(s) of action of Spm8CHAS, (iii) explore the in vitro activity of Spm8CHAS in combination with other candidate microbicides, and (iv) investigate the toxicity of Spm8CHAS in both cell culture and cervical explant models.
FIG. 1.
Schematic diagram of the chemical structures of Spm8CHAS (A) and the fluorescently labeled analog, Spm8CHAS-F (B).
MATERIALS AND METHODS
Microbicides.
Nonoxynol-9 (N-9) was purchased from Sigma (St. Louis, MO). Spm8CHAS was provided by the Department of Chemistry at Lehigh University (Bethlehem, PA); its synthesis has been described previously (17). 9-[R-2-(phosphonylmethoxy)propyl] adenine monohydrate (PMPA, or tenofovir) was obtained from Gilead Sciences, Inc. (Foster City, CA), and UC-781 was obtained from Biosyn, Inc. (Philadelphia, PA).
HIV and cervical explant experiments. (i) Cell and virus culture.
All reagents used were from Sigma-Aldrich Ltd., Poole, United Kingdom, and cells and viruses were from the AIDS Reagent Project, National Institute for Biological Standards and Control, Potters Bar, United Kingdom, unless stated otherwise. TZM-bl cells, a HeLa cell line stably expressing CD4 and CCR5 and used for quantitative analysis of HIV-1 with luciferase as a reporter (29), were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (complete DMEM). PM-1 cells, a T-cell line susceptible to both X4- and R5-utilizing HIV-1 strains, were cultured in RPMI 1640 medium supplemented as the DMEM was (complete RPMI). Cells were passaged every 3 to 4 days. The primary HIV-1 isolates belonging to clades B and C, a gift from John P. Moore (Weill Medical College, Cornell University, NY), were grown in peripheral blood mononuclear cells obtained by density gradient centrifugation of healthy donor blood (buffy coat). Three days prior to use, the cells were activated with 5 μg/ml phytohemagglutinin and, after infection, cultured in the presence of 50 U/ml of human recombinant interleukin-2 (R&D Systems, Minneapolis, MN). The laboratory-adapted HIV-1 strains HIV-1RF (X4-utilizing strain) and HIV-1BaL (R5-utilizing strain) were grown in PM-1 cells and stored at −180°C after filtration through 0.2-μm filters (Millipore, MA).
(ii) Culture of human genital tract tissue and infection with HIV-1 and HSV-2.
Cervical mucosal tissue was collected from informed, consenting premenopausal women undergoing therapeutic hysterectomies at St. George's, St. Helier's, and Kingston Hospitals (London, United Kingdom). Tissue was cut into explants of approximately 3 by 3 by 2 mm prior to culture as previously described (11). Explants used for infection included the epithelial layer and underlying stromal tissue and were from the ectocervical area of the cervix. For HIV infection, explants were exposed to 105 50% tissue culture infective doses (TCID50) of cell-free HIV-1BaL in the presence or absence of different concentrations of Spm8CHAS in complete RPMI for 2 h, washed three times with 200 μl phosphate-buffered saline (PBS), transferred into new round-bottom 96-well plates, and cultured overnight in 200 μl complete RPMI at 37°C. Explants were then transferred into new flat-bottom 96-well plates and cultured for a further 10 days. Migratory cells present in the overnight culture plate were washed twice with 200 μl PBS and cocultured with 4 × 104 PM-1 cells in a new plate at 37°C for 10 days. Explants were fed with fresh medium every 2 to 3 days (50% replacement), and culture supernatants were collected 10 days postinfection from both the explant cultures and the migratory cell cocultures and assessed for viral replication using a p24 enzyme-linked immunosorbent assay (p24 antigen capture assay kit; NCI-Frederick Cancer Research and Development Center, AIDS Vaccine Program). For HSV infection, explants were exposed to HSV-2(G) (107 PFU/explant) in the presence of different amounts of Spm8CHAS in a total volume of 200 μl complete RPMI for 2 h. After removal of unbound virus and compound by extensive washing, explants were cultured for 7 days in 200 μl complete RPMI and fed with fresh medium every 2 to 3 days. Culture supernatants were harvested 7 days postinfection and assessed for infectious HSV-2 by plaque assay.
(iii) Luciferase assay for detection of HIV-1 infection.
TZM-bl cells were plated at 3 × 104/well and allowed to adhere overnight before exposure to 103 TCID50 HIV-1 in the presence of various concentrations of Spm8CHAS alone or in combination with the reverse transcriptase inhibitors UC-781 and PMPA. Virus and drugs were left in culture for 48 h at 37°C and then removed by washing once with 200 μl PBS. A 100-μl volume of luciferase cell culture lysis reagent (Promega, Southampton, United Kingdom) was added to each well, and the plates were stored at −20°C until assessed for luciferase activity. Fifty microliters of cell lysate was transferred to white high-bind 96-well plates (Corning Life Sciences, B.V., Schiphol-Rijk, The Netherlands) followed by addition of 50 μl luciferase assay buffer (Promega) immediately prior to reading in a Synergy-HT plate reader from Bio-Tek using KC4 software.
(iv) Anti-HIV activity of Spm8CHAS in cell models.
A monoclonal antibody to human HLA-DR was produced from the mouse hybridoma L243 (American Type Culture Collection) as described elsewhere (8). Antibody was bound to 96-well flat-bottom plates for 1 h at room temperature. After removal of excess antibody with 200 μl PBS, 104 TCID50 HIV-1 grown in HLA-DR+ cells (PM-1) were added to each well, and the plates were centrifuged for 90 minutes at 2,000 × g. Unbound virus was removed, and the plate was washed twice before addition of 100 μl of serial dilutions of Spm8CHAS for 1-h incubation at 37°C. To assess direct virucidal activity, the compound was removed and the plates washed four times with 200 μl PBS before addition of 4 × 104 Jurkat-Tat-CCR5 cells per well. Alternatively, cells were added without removal of compound or, to assess cell protection, Jurkat-Tat-CCR5 cells (4 × 104 cells/well) were exposed to the same concentrations of compound in U-bottom 96-well plates and washed in the same way before transfer to plates with immobilized virus. Viral replication was assessed by measuring reverse transcriptase (RT) levels in culture supernatants 7 days postinfection as described previously (29).
HSV experiments. (i) Cells and viruses.
CaSki cells (a human cervical cell line) and Vero cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained as described previously (14). The cell line 79VB4, provided by P. Spear (Northwestern University, Chicago, IL) is a gL-expressing Vero cell line, which was grown in DMEM supplemented with 10% fetal bovine serum in the presence of 200 μg/ml G418 sulfate (24). The viral strains were the two well-characterized laboratory strains HSV-2(G) and HSV-1(KOS) and HSV-1(KOS)gL86, a mutated virus in which the Escherichia coli β-galactosidase gene has replaced part of the gL open reading frame (24). Vesicular stomatitis virus Indiana was a gift from P. Palese (Mount Sinai, New York, NY) and was grown on Vero cells.
(ii) Plaque assays.
CaSki cells in 24-well dishes were exposed in duplicate to serial dilutions (1 μg/ml to 500 μg/ml) of Spm8CHAS for 15 min at 37°C and then challenged (without washing) with serial 10-fold dilutions of HSV (0.01 to 1,000 PFU/cell). After incubation for 1 h, the inoculum and drug were removed, and the cells were washed three times and overlaid with fresh medium again in the absence or presence of drug. The viral titers in the presence of each concentration of drug when present only during the initial 1-h adsorption period or throughout the experiment were determined by counting plaques 48 h postinfection (14). Only wells in which the number of plaques ranged from 20 to 100 were used to calculate the viral titer.
To determine if Spm8CHAS maintains its anti-HSV activity in the presence of PMPA or UC-781, plaque assays were performed with Spm8CHAS alone (dose range, 0.01 μg/ml to 100 μg/ml) or in combination with PMPA (dose range, 0.1 μg/ml to 100 μg/ml) and UC-781 (dose range, 0.1 ng/ml to 100 ng/ml). The plaques were counted at 48 h postinfection, and the concentrations of drug that inhibited 50 and 90% of the viral infection (IC50 and IC90, respectively) were determined from dose-response curves generated from three independent experiments.
(iii) Synchronized infection assays.
Time course assays were conducted to determine which steps in viral infection were inhibited by Spm8CHAS (13). CaSki cells were precooled to 4°C, and each well was inoculated with ≈1,000 PFU of virus for 3 h. Unbound virus was removed by washing the cells, and the cells were then shifted to 37°C to allow penetration for 15 min. The cell monolayers were exposed to citrate buffer (pH 3.0) for 1 minute to inactivate any nonpenetrated virus. The cells were washed and overlaid with medium. Spm8CHAS was added at doses of 10 μg/ml, 50 μg/ml, and 100 μg/ml either during the 4°C period, at the time that the cells were transferred to 37°C, or with the final medium overlay, to determine if the drug acts at the level of binding, penetration, or postentry, respectively.
(iv) Preincubation of virus or cells with Spm8CHAS.
To examine whether Spm8CHAS interacts primarily with HSV, with epithelial cells, or with both, Spm8CHAS was preincubated with ≈104 PFU of HSV-2(G) per ml for 1 hour at 37°C, and the mixture was then diluted 50-fold to yield ≈200 PFU/well on control plates and inoculated onto monolayers of CaSki cells in duplicate on 12-well dishes (4). For comparison, virus and drug combinations were immediately diluted 50-fold and then plated (without the 1-h incubation) onto the cells. Alternatively, Spm8CHAS (or PBS as a control) was preincubated with the cells for 1 hour at 37°C. The cells were then either washed extensively or not washed prior to inoculation with HSV-2(G).
(v) Confocal microscopy.
To determine whether Spm8CHAS traverses cellular membranes, confocal studies were conducted with a fluorescently labeled analog, designated Spm8CHAS-F (Fig. 1B); a manuscript describing detailed synthesis is in preparation. CaSki cells were stained for 30 min at room temperature in EZ-Link Sulfo-NHS-biotin reagent (1:1000; Pierce Chemicals), which reacts with primary amines on cell surface proteins. Cells were then cooled to 4°C and treated with Spm8CHAS-F and then shifted to 37°C for the indicated time periods. Subsequently, the cells were fixed with 4% paraformaldehyde, 1% Triton-X and reacted with an Alexa Fluor 647-conjugated streptavidin antibody (1:1,000; Molecular Probes, Eugene, OR) to detect the biotinylated membranes. Nuclei were detected by staining with 4′,6′-diamidino-2-phenylindole (DAPI) nucleic acid stain (Molecular Probes).
(vi) Cell-to-cell spread.
To assess the effect of Spm8CHAS on cell-to-cell spread of HSV, CaSki cells were infected with HSV-1(KOSVP26GFP) or HSV-2(G) (multiplicity of infection [MOI], 10 PFU/cell). Then, 4 to 5 h after infection, the infected cells were detached with trypsin-EDTA, counted, and mixed with uninfected cells at a ratio of 1:25, and ∼104 cells were plated in DMEM supplemented with 10% heat-inactivated fetal bovine serum and 1% anti-human immunoglobulin G (IgG; Calbiochem, San Diego, CA) in the absence or presence of Spm8CHAS or acyclovir. The pooled human immunoglobulin neutralizes infection by virus released into the medium. Cells were fixed 72 h after plating and analyzed for ability of virus to spread cell to cell by using confocal imaging or by counting plaques in a black plaque immunoassay. For confocal imaging, HSV-2(G) was detected by a mouse anti-VP5 antibody (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA) and an Alexa 488-conjugated anti-mouse antibody (1:500; Molecular Probes). Plasma cell membranes were detected by treating cells with EZ-Link Sulfo-NHS-biotin reagent prior to fixation and then reacting with an Alexa Fluor 647-conjugated streptavidin antibody to detect the biotinylated membranes. Nuclei were detected by staining with DAPI nucleic acid stain. Images were examined using a Zeiss LSM 510 Meta confocal microscope fitted with a 100× objective and analyzed using the LSM confocal software package as previously described (5).
(vii) Northern blotting.
CaSki cells were synchronously infected with HSV-1(KOS) at an MOI of 0.5 PFU/cell or an approximately equivalent number of viral particles of HSV-1(KOS)gL86 which had been grown on complementing cells (based on optical densitometry scanning of a Western blot of viral preparations) and treated with 100 μg/ml of Spm8CHAS, 100 μg/ml of acyclovir, or no drug immediately postentry. Total RNA was extracted using RNA-Stat 60 (Tel-Test Inc., TX) and propanol precipitation from 5 × 105 cells at either 3 or 18 h postinfection. Total RNA was measured in each sample using a NanoDrop ND-1000 UV-Vis spectrophotometer, resolved on a formaldehyde gel, photographed, and transferred to a GeneScreen Plus-NR membrane (Perkin-Elmer, MA) by capillary blotting. Hybridization was carried out using a DNA probe for glycoprotein C (1,452 bp; a late/γ gene) radiolabeled using [32P]dCTP (Perkin-Elmer, MA) and the Rediprime II random prime labeling system (Amersham-GE Healthcare, CA).
(viii) Cytotoxicity.
Cell viability following exposure of CaSki, TZM-bl, and Jurkat-Tat-CCR5 cells to microbicides alone or in combination was determined using a cell proliferation 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay (CellTiter96; Promega). Cells were exposed to drugs diluted in serum-free medium for 24 to 48 h (acute toxicity) or for 2 h daily for six consecutive days or 7 days (chronic toxicity). Controls included cells exposed to medium containing no compound and cells exposed to 0.001% N-9.
RESULTS
Spm8CHAS inhibits HIV-1 infection in cell culture.
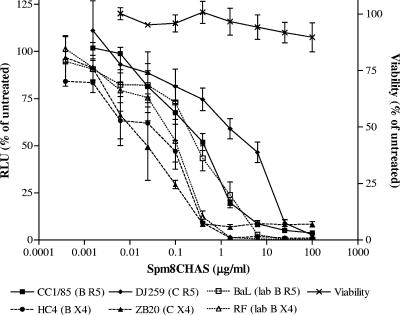
Spm8CHAS prevented infection by both HIV-1RF and HIV-1BaL, with IC50 values of 0.09 ± 0.03 μg/ml and 0.31 ± 0.14 μg/ml (mean ± standard error of the mean [SEM]), respectively (Fig. 2). Although higher concentrations of drug were required to inhibit the R5-using primary clade C isolate DJ259, the antiviral activity of Spm8CHAS against other primary isolates did not differ substantially from results obtained for the laboratory-adapted viruses. Importantly, at a concentration of 100 μg/ml, Spm8CHAS completely blocked infection by all viral isolates tested (Fig. 2). The IC50 for PMPA was comparable for all isolates tested, ranging from 0.6 to 1.1 μg/ml (not shown). No cytotoxicity was detected by MTS assay (Fig. 2).
FIG. 2.
Spm8CHAS inhibits HIV infection in cell culture. TZM-bl cells were infected with 103 TCID50 of each of the indicated HIV-1 strains in the presence or absence of various concentrations of Spm8CHAS. Virus and drug were left in culture for 48 h at 37°C, and infectivity was monitored by luciferase assay. Cell viability was determined by MTS assay following exposure to drug for 48 h. Results are means ± SEM obtained from three experiments conducted in triplicate.
Spm8CHAS inhibits HIV-1 infection of cervical mucosal tissue and dissemination of virus by migratory cells.
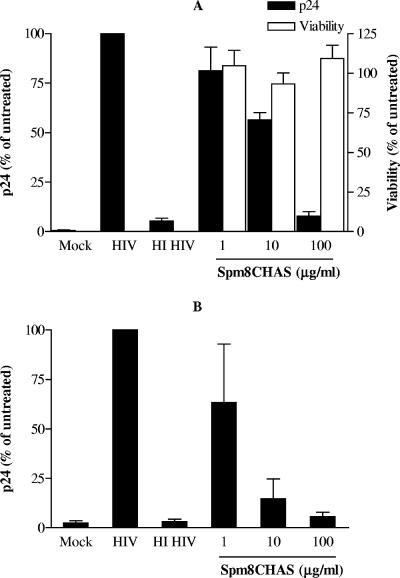
The possibility that Spm8CHAS may block infection in the female genital tract and prevent dissemination of virus by migratory cells was explored using an ex vivo model of mucosal HIV-1 transmission (11, 16, 25). An R5 isolate was selected for these studies, as R5 viruses predominate following sexual transmission (40). Ectocervical tissue explants were exposed to virus in the presence or absence of Spm8CHAS for 2 h. The unbound drug and virus were removed by washing, and infection was monitored by p24 release following 10 days in culture. Spm8CHAS inhibited infection in a dose-dependent manner with a calculated IC50 value of 13.55 ± 3.92 μg/ml (Fig. 3A); inhibition was almost complete when 100 μg/ml of drug was present during viral challenge. No cytotoxicity was detected at the highest concentration tested, 100 μg/ml. To determine if Spm8CHAS could also prevent transmission of HIV-1 via migratory cells emigrating from cervical explants to target cells, migratory cells were harvested from the same explant cultures at ≈16 h postinfection and then cocultured with permissive T cells (PM-1). Spm8CHAS reduced transmission of infectious virus from ectocervical explants to susceptible T cells (Fig. 3B). When tissue explants were exposed to virus in the presence of 100 μg/ml Spm8CHAS, amplification of infection by PM-1 cells in the coculture system was completely inhibited.
FIG. 3.
Spm8CHAS inhibits HIV infection of explants. Ectocervical tissue explants were mock infected or exposed to HIV-1BaL or heat-inactivated virus (HI) in the presence or absence of Spm8CHAS for 2 h. The unbound drug and virus were removed by washing, and infection was monitored by p24 release following 10 days in culture (A). To determine if Spm8CHAS could also prevent transmission of HIV-1 via migratory cells emigrating from cervical explants to target cells, migratory cells were harvested from the same explant cultures ≈16 h postinfection and then cocultured with permissive T cells (PM-1) (B). Results are means ± SEM obtained from three experiments conducted in triplicate.
Spm8CHAS targets HIV to prevent infection.
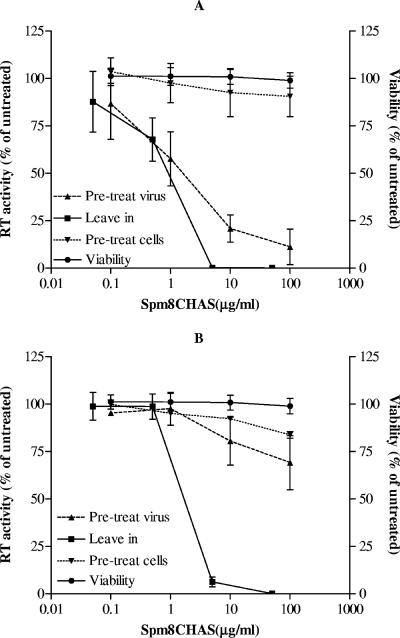
Spm8CHAS inhibited infection by both HIV-1BaL (R5) and HIV-1RF (X4) in target indicator T cells (Jurkat-Tat-CCR5) as assessed with solid-phase plate-based assays. For both viruses, less than 5 μg/ml of drug was needed for complete inhibition when the compound was present for the duration of the assay (Fig. 4). Pretreatment of the virus with Spm8CHAS, followed by removal of the drug by washing, resulted in almost complete inhibition of infection in target cells by HIV-1RF (IC50, 1.47 ± 1.43 μg/ml) (Fig. 4A), but not by HIV-1BaL (Fig. 4B), suggesting that the drug binds irreversibly to X4 viruses. Little antiviral activity was observed if the target T cells were treated with Spm8CHAS and then washed to remove drug prior to viral exposure, suggesting that the drug needs to be present at the time of viral exposure.
FIG. 4.
Spm8CHAS targets HIV to prevent infection. The effects of Spm8CHAS on HIV-1RF (A) or HIV-1BaL (B) infectivity were compared under three different conditions: (i) drug present throughout the experiment (leave in); (ii) pretreatment of virus followed by removal of the drug by washing (pretreat virus); or (iii) target T cells treated with Spm8CHAS and then washed to remove drug prior to viral exposure (pretreat cells). Cell viability was determined by MTS assay following exposure to drug throughout the experiment. Results are presented as mean RT activity or mean cell viability as a percentage of untreated cells and are means ± SEM obtained from two to five experiments conducted in six replicates.
Spm8CHAS blocks HSV infection.
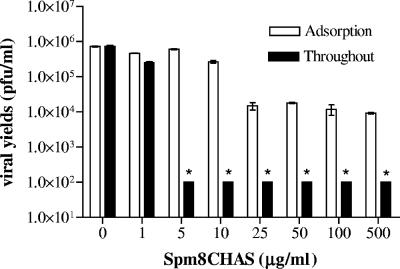
CaSki cells were exposed to serial dilutions of Spm8CHAS and then challenged with serial dilutions of HSV-2(G) for a 1-h adsorption period. Cells were then washed and overlaid with medium in the absence or presence of the same final concentration of Spm8CHAS. The viral titer (PFU/ml) if drug was present only during the 1-h adsorption period or throughout the experimental period was calculated after counting plaques 48 h postinfection and correcting for the viral dilution. At concentrations comparable to those easily achieved in formulations of other polyanionic drugs (100 to 300 μg/ml for PRO 2000) (19, 20), Spm8CHAS inhibited at least 4 logs of HSV-2 infection when present throughout the experimental period and ∼2 logs if present only during the adsorption period (Fig. 5). Note that the lowest limit of detection in the assay is 100 PFU/ml. The additive activity observed if drug was present throughout the experimental period suggests that Spm8CHAS must have a postentry antiviral activity. Spm8CHAS was also highly effective when tested against strains of HSV-1 and against two clinical HSV-2 isolates (data not shown). Spm8CHAS exhibited no activity against vesicular stomatitis virus (data not shown).
FIG. 5.
Anti-HSV activity of Spm8CHAS if present throughout the infection. CaSki cells were pretreated with the indicated dose of Spm8CHAS or PBS for 30 min and then challenged with serial 10-fold dilutions of HSV-2(G). After 1 h of incubation, the inoculum was removed and cells were overlaid with serum-free medium in the presence or absence of the drug at the indicated concentrations. Plaques were counted 48 h postinfection; only wells containing 25 to 100 plaques were used to calculate the viral titer. The results are the mean viral titer (PFU/ml) ± the standard deviation obtained from two independent experiments conducted in duplicate. The asterisks denote a significant reduction in the viral titer compared to the titer obtained in the presence of control buffer (P < 0.01).
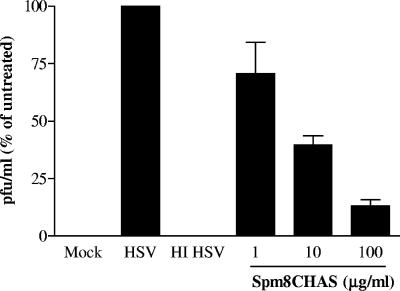
Human cervical explants are relatively resistant to HSV infection, primarily due to tight junctions and inaccessibility to viral coreceptors (10). In preliminary studies we found that exposure to 1 × 107 PFU/explant resulted in productive infection and that the infection was inhibited by acyclovir (data not shown). Explants were inoculated with 1 × 107 PFU/explant in the absence or presence of Spm8CHAS, and supernatants were collected at different time points and evaluated for infectious virus by plaque assays. Viral production peaked 4 days postinfection, with yields of ≈2 × 104 PFU/ml. Spm8CHAS inhibited HSV infection of human ectocervical explants in a dose-dependent manner, with an IC50 of 5.5 ± 1.93 μg/ml following exposure to this high viral inoculum (Fig. 6).
FIG. 6.
Spm8CHAS prevents HSV infection of cervical explants. Cervical explant cultures were exposed to 107 PFU/explant HSV-2(G) in the absence or presence of the indicated concentration of drug. Supernatants were collected 7 days postinfection and assayed for HSV by plaque assay on ME180 cells. Results are presented as PFU formed in the presence of drug as a percentage of PFU formed in the presence of medium alone and are means ± SEM obtained from three experiments conducted in triplicate.
Mechanisms of anti-HSV activity.
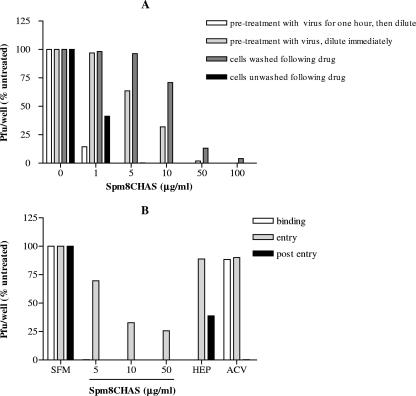
Anti-HSV activity persisted if viral particles were first preincubated with Spm8CHAS and then diluted to a subtherapeutic concentration of drug, suggesting that the drug acts irreversibly on the virus to prevent infection (Fig. 7A). These results are similar to those obtained with HIV-1RF. Pretreatment of target CaSki cells with drug, followed by washing and subsequent HSV exposure, led to a reduction in antiviral activity at low drug concentrations (Fig. 7A). However, substantial anti-HSV activity was still observed at Spm8CHAS concentrations of ≥50 μg/ml. These findings suggest that Spm8CHAS interacts not only with virus but also, at higher concentrations, with the target epithelial cell to render it less susceptible to infection.
FIG. 7.
Spm8CHAS primarily targets the HSV viral particle and blocks multiple steps in viral infection. Spm8CHAS was preincubated with ≈104 PFU of HSV-2(G) per ml an for 1 hour at 37°C, then diluted 50-fold to yield ≈200 PFU/well on control plates, and inoculated onto monolayers of CaSki cells in duplicate in 12-well dishes. As a control, Spm8CHAS was exposed to virus and then immediately diluted 50-fold and inoculated onto cells, with no preincubation period. Alternatively, cells were preincubated with the indicated concentrations of drug for 1 h at 37°C and then either washed extensively or not washed prior to inoculation with HSV-2(G). Results are presented as PFU/well as a percentage of PFU formed in the presence of medium alone and are means ± standard deviations (SD) obtained from three experiments conducted in duplicate (A). Synchronized infection assays were also conducted, and Spm8CHAS (5, 10, or 50 μg/ml), heparin (100 μg/ml), or acyclovir (50 mg/ml) was added at the time of binding (for 4 h at 4°C), entry (for 30 min at the time of temperature shift to 37°C), or immediately postentry (after citrate treatment) for the remaining duration of the experiment period. Plaques were counted at 48 h, and results are presented as PFU/well as a percentage of PFU formed in the presence of medium alone and are means ± SD obtained from three experiments conducted in duplicate (B).
HSV binding, which occurs at 4°C, can be experimentally differentiated from entry, which occurs after a temperature shift to 37°C, and from postentry events by treating cells with a low pH citrate buffer that inactivates noninternalized virus (12). Spm8CHAS was added at the time of binding (for 4 h at 4°C), entry (for 30 min at the time of temperature shift to 37°C), or immediately postentry (after citrate treatment) for the remaining duration of the experiment (Fig. 7B). Heparin, a competitive inhibitor of HSV binding, and acyclovir, an inhibitor of viral DNA replication, served as controls. As expected, heparin significantly inhibited HSV plaque formation if present during binding but had little effect if added at entry or postentry. In contrast, acyclovir was only effective if present postentry. Spm8CHAS completely inhibited HSV infection when added during binding or when added postentry. Notably, Spm8CHAS also retained some antiviral activity if present in the cultures only briefly during the 15-min entry period.
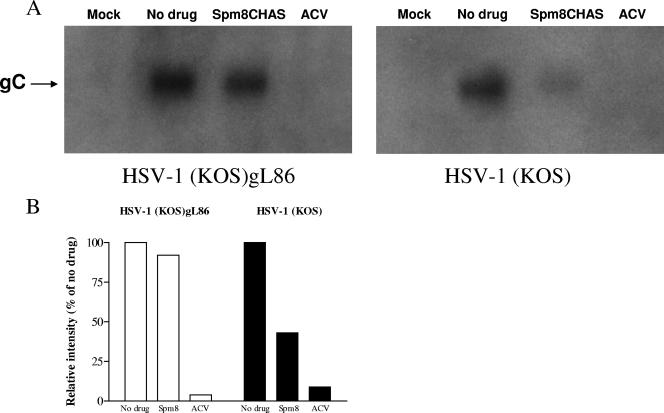
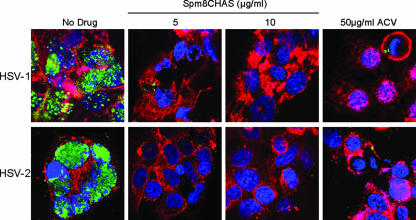
The substantial activity observed postentry could reflect blockade of later steps of the viral replicative cycle and/or inhibition of cell-to-cell spread of virus. The impact of Spm8CHAS on viral gene expression was evaluated following infection with HSV-1(KOS) or HSV-1(KOS)gL86, which is impaired in cell-to-cell spread. Spm8CHAS and acyclovir were added immediately postinfection. Both Spm8CHAS and acyclovir inhibited viral gC RNA expression 18 h postinfection with KOS, but only acyclovir blocked gC gene expression following infection with the gL-deletion virus (Fig. 8). These findings suggest that Spm8CHAS had little effect on viral gene expression in the initially infected cells but interfered with subsequent rounds of infection, possibly by preventing cell-to-cell spread. To directly assess this, infected cells were cocultured with uninfected cells at a ratio of 1:25 in the presence of pooled immunoglobulin and in the absence or presence of Spm8CHAS or acyclovir. In the presence of IgG, HSV presumably spreads via intercellular junctions created by local fusion events between the plasma membranes of infected and uninfected cells, but the virus released is neutralized by the IgG (33). Spm8CHAS completely prevented cell-to-cell spread (Fig. 9). Similar results were obtained by examining the effects of Spm8CHAS on plaque formation by black plaque immunoassay (not shown). These findings suggest that the primary postentry effects of Spm9CHAS are to prevent cell-to-cell spread of virus and suggest that the drug may be effective at inhibiting transmission of cell-associated HSV or viral spread following reactivation.
FIG. 8.
Spm8CHAS inhibits viral gene expression by wild-type HSV-1(KOS). CaSki cells were mock infected or infected with HSV-1(KOS) or HSV-1(KOS)gL86 grown on complementing cells at an MOI of 0.5 PFU/cell for KOS or an approximately equivalent number of viral particles of the gL deletion virus. Immediately postentry, 100 μg/ml Spm8CHAS, acyclovir (ACV), or no drug was added. Total RNA was extracted 18 h postinfection, and hybridization was performed using a radiolabeled DNA probe for viral glycoprotein C (A). The blots were scanned, and results were corrected for background (B). Results are representative of three independent experiments.
FIG. 9.
Spm8CHAS blocks cell-to-cell spread. CaSki cells infected with HSV-1(VP26GFP KOS) or HSV-2(G) were mixed with uninfected cells at a ratio of 1:25 in medium containing pooled human IgG in the absence or presence of 5 to 10 μg/ml Spm8CHAS or 50 μg/ml acyclovir (ACV). Cells were incubated with EZ-Link Sulfo-NHS-biotin reagent and fixed. Cellular biotinylated membranes were detected by an Alexa Fluor 647-conjugated streptavidin antibody (red). Nuclei were visualized by staining with DAPI (blue), HSV-1 capsids were observed by monitoring for GFP (green), and HSV-2 capsids were incubated with an anti-VP5 antibody and an Alexa 488-conjugated anti-mouse antibody (green). Results are representative of those obtained by examining 100 cells.
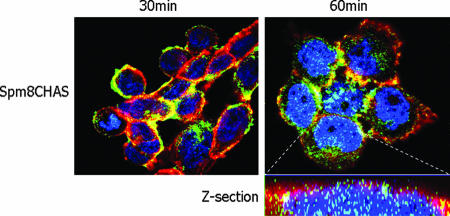
The observation that Spm8CHAS blocks events postentry and renders cells resistant to infection, even if they are washed following incubation with drug, supports the notion that Spm8CHAS may accumulate intracellularly in human epithelial cells. The ability to cross cell membranes would be consistent with the drug's amphiphilic structure. CaSki cells were treated with fluorescently labeled Spm8CHAS (Fig. 1B) and used in confocal microscopy studies (Fig. 10). Plaque assays demonstrated that Spm8CHAS-F retained anti-HSV activity comparable to that observed with the unlabeled drug (not shown). Spm8CHAS-F was easily detected intercalating with the cell membrane shortly after exposure and was readily detected within the nucleus 1 h postexposure. In contrast, a fluorescently labeled sulfonated polymer, having a similar size, negative charge, and antiviral activity as Spm8CHAS-F but lacking facial amphiphilicity, does not enter cells under similar conditions (data not shown). This suggests that facial amphiphilicity may also contribute to postentry activity.
FIG. 10.
Spm8CHAS accumulates intracellularly. CaSki cells were preincubated with EZ-Link Sulfo-NHS-biotin reagent to identify cell membranes, then cooled to 4°C and treated with Spm8CHAS-F(green), and then shifted to 37°C for the indicated times. Subsequently, the cells were fixed and reacted with an Alexa Fluor 647-conjugated streptavidin antibody to detect the biotinylated membranes (red). Nuclei were detected by staining with DAPI (blue).
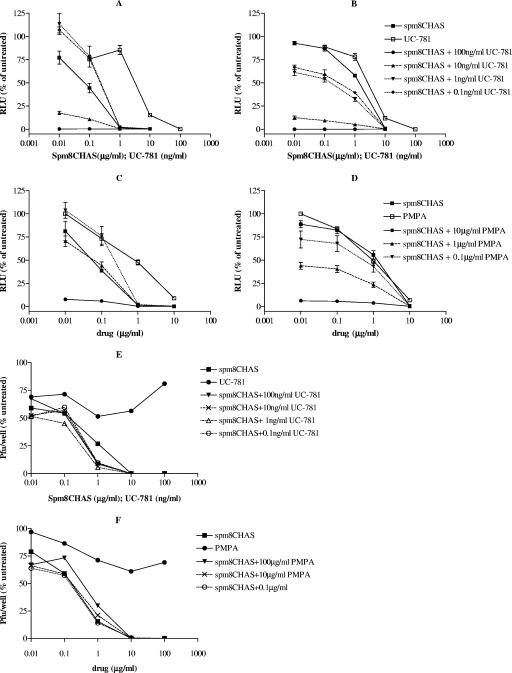
Spm8CHAS is active in combination with the RTIs UC-781 and PMPA.
The therapeutic success of highly active antiretroviral therapy suggests that a similar approach may be necessary for effective microbicide development. Combination vaginal microbicides could inhibit virus at multiple stages of the life cycle and have been tested in macaque studies (39). Synergy is not necessary, but it is essential that the combinations not be antagonistic. Spm8CHAS was tested in combination with the nucleotide reverse transcriptase inhibitor (RTI) PMPA and with the nonnucleoside RTI (NNRTI) UC-781. Spm8CHAS retained antiviral activity against HIV-1RF (Fig. 11A and C) and HIV-1BaL (Fig. 11B and D) in the presence of both RTIs. Low concentrations of UC-781 (0.1 to 1 ng/ml) and PMPA (0.1 to 1 μg/ml) led to a slight decrease in the activity of Spm8CHAS against the X4 virus, although the clinical relevance of results at these low concentrations is not known. More importantly, inhibition of HIV-1BaL was clearly enhanced by the addition of RTIs. The anti-HSV activity of Spm8CHAS was retained in the presence of both RTIs; Spm8CHAS completely inhibited HSV plaque formation at a concentration of 10 μg/ml alone or when combined with either UC-781 or PMPA (Fig. 11E and F). UC-781 showed a low level of anti-HSV activity, inhibiting plaque formation by 25% at all concentrations tested, which may have contributed to the observed increase in anti-HSV activity when 1 μg/ml Spm8CHAS was combined with UC-781. Similar additive anti-HIV activity was observed when Spm8CHAS was combined with the NNRTI TMC-120, a candidate microbicide also under development (not shown).
FIG. 11.
Combining Spm8CHAS with PMPA or UC-781 provides additive protection against HIV in vitro. TZM-bl cells were infected with 103 TCID50 of HIV-1RF (A and C) or HIV-1BaL (B and D) in the presence or absence of various concentrations of Spm8CHAS alone or in combination with the reverse transcriptase inhibitors UC-781 (A and B) and PMPA (C and D). Viral infection was monitored by detecting luciferase activity. Additionally, CaSki cells were exposed to HSV-2(G) in the presence or absence of Spm8CHAS alone or in combination with the reverse transcriptase inhibitors UC-781 (E) and PMPA (F) and infection monitored by counting plaques 48 h postinfection. Results are means ± standard deviations from at least three independent experiments conducted in triplicate.
Toxicity studies.
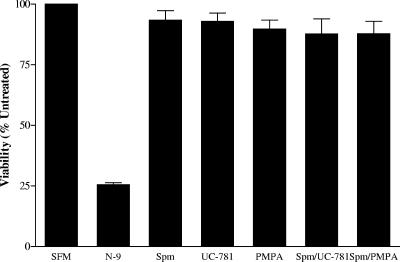
No reductions in cell or explant viability were observed following acute (24-h) or chronic (7-day) exposure to Spm8CHAS as assessed by an MTS assay. Moreover, no cellular toxicity was observed following 24-hour exposure of cells to Spm8CHAS in combination with UC-781 or PMPA. In contrast, exposure of explant tissue or cells to 0.01% N-9 significantly reduced cell and tissue viability. Representative results following 24-hour exposure of CaSki cells in culture are shown in Fig. 12.
FIG. 12.
Spm8CHAS alone and in combination is not cytotoxic. CaSki cells were cultured for 24 h with 100 μg/ml Spm8CHAS alone or in combination with 100 ng/ml UC781 or 100 μg/ml PMPA or with 0.001% N-9, and cell viability and proliferation were assayed by MTS assay. Results are means ± standard deviations obtained from two independent experiments conducted in duplicate.
DISCUSSION
The high HIV inoculum present in seminal plasma during acute infection combined with increases in viral loads associated with symptomatic or clinically silent primary or recurrent HSV-2 substantially increase the risk for HIV transmission (28). The epidemiological link between these two pathogens has prompted the initiation of clinical trials to determine whether acyclovir administered to HIV and HSV coinfected individuals might reduce HIV transmission and, conversely, whether acyclovir prophylaxis administered to HSV-2-seropositive individuals at high risk for HIV might reduce acquisition. Development of vaginal microbicides, such as Spm8CHAS, that target both pathogens should provide a realistic strategy to reduce infection in individuals at risk for HIV and HSV.
Spm8CHAS is a structurally unique molecular umbrella compound active against HIV and HSV. Spm8CHAS inhibited HIV-1 infection in cell culture and explant models and, importantly, prevented dissemination by migratory cells. Sexual transmission of HIV-1 requires that virus infecting mucosal sites gain access to immune cells permissive for infection. Migratory cells, which include immature dendritic cells located in the epithelium and subepithelium, play a critical role in the transmission of HIV-1 to target T cells in lymphoid tissue. Thus, the ability of microbicides to block this pathway is crucial in preventing HIV infection. The observations that Spm8CHAS is active at lower concentrations against X4 versus R5 virus and irreversibly inhibits HIV-1RF, but not HIVBaL, in cultures may be attributed to the greater avidity of the persulfations for the more cationic regions on the V3 loop of gp120 in X4 compared with R5 viruses (25, 34, 35). Some of the anti-HIV activity of Spm8CHAS may be mediated by electrostatic interaction between the compound and the gp120 V3 loop and the ability of Spm8CHAS to bind to the CD4-induced coreceptor binding site (38). However, the ability of Spm8CHAS to completely inhibit infection by the primary clade C and clade B isolates tested, coupled with the observed increased activity against both R5 and X4 viruses if the drug is present throughout the infection (Fig. 4), distinguish this compound from sulfated polymers in clinical trials. Additionally, the intriguing observation that molecules of Spm8CHAS efficiently penetrate cell membranes suggests the possibility that Spm8CHAS might sequester less-soluble antiretroviral drugs, effectively enhancing their transport across the cell plasma membrane if coformulated.
Although concentrations of ∼100 μg/ml were needed to completely inhibit infection by HIV-1Bal or R5-using primary isolates in vitro, these concentrations should be achievable in formulations. For example, in a recent study, we found that the concentration of PRO 2000 found in cervicovaginal lavage fluid 1 hour postapplication of a 0.5% gel ranged from ∼100 to 300 μg/ml (19). The substantial in vitro activity against clade C virus is noteworthy, as clade C remains the most prevalent HIV strain worldwide and in Central and South Africa. Few published studies have documented the antiviral activity of microbicides against non-clade B isolates prior to the initiation of clinical trials (21). A recent in vitro study indicated that Carraguard, which is currently in large-scale effectiveness trials, displays no activity against a clade C isolate (7). Whether these in vitro findings will translate to a loss in activity in vivo is not known.
The best strategy to prevent HIV infection may be to target multiple steps in the viral life cycle. This approach has clearly been successful for systemic HIV therapy. This strategy may also reduce the likelihood of selecting for resistant viruses. To evaluate this possibility, we examined the antiviral activity of Spm8CHAS combined with either UC-781 or PMPA. UC-781, a nonnucleoside reverse transcriptase inhibitor, exhibits excellent dose-dependent activity against R5 and X4 infections of T cells and also blocks transfer of HIV infection by migratory cells (8). Moreover, the drug shows substantial activity against clade C and clade A isolates in vitro (7). However, NNRTI resistance is increasingly prevalent in the HIV-infected population, and a concern for NNRTI-based microbicides is that they will be ineffective against drug-resistant virus and may in fact promote selection of NNRTI-resistant virus. In a recent study, UC-781 was 10- to 100-fold less effective against NNRTI-resistant HIV-1 compared to wild-type virus (15). Breakthrough experiments using UC-781-pretreated cells and mixtures of wild-type and NNRTI-resistant HIV-1 showed that UC-781 pretreatment selected for NNRTI-resistant HIV-1. This effect was overcome at concentrations greater than 25 μM; the amount of UC-781 in topical microbicide formulations under current development is approximately 100-fold greater than this concentration. Although it remains to be determined whether transmission of NNRTI-resistant viruses will become a clinical issue, the authors did conclude that additional antiviral agents should be included in NNRTI-based microbicide formulations. Our studies suggested additive in vitro activity against HIV-1BaL when Spm8CHAS was combined with UC-781, as well as additive activity against HIV-1RF at all but the lowest tested doses. Formulating Spm8CHAS with UC-781 could provide additive activity against multiple clades and prevent selection of NNRTI-resistant viruses, and the combination would provide significant activity against both HIV and HSV infection.
Similar additive activity was observed if Spm8CHAS was combined in vitro with PMPA, which is now in clinical development as a candidate vaginal microbicide. Although systemic experience suggests that PMPA induces less NRTI resistance than other drugs in its class, a recent study found that HIV-1 subtype C viruses rapidly develop K65R resistance to PMPA in cell culture (31). A formulated 1% PMPA vaginal gel, used twice daily, was found to be well tolerated in a study of both abstinent and sexually active HIV-negative and HIV-positive women (23). Notably, low serum PMPA levels were detected in 14 of the 25 women who participated in this study. Whether this low level of systemic adsorption could select for resistant viruses in women who use the gel and are HIV positive is not known. A combination of PMPA with Spm8CHAS could overcome this limitation by blocking HIV infection at distinct steps, thus decreasing the likelihood for selection of resistant viruses.
The anti-HSV activity of Spm8CHAS provides an additional benefit for a combination microbicides, as both UC-781 and PMPA are HIV specific. Although some of the anti-HSV activity of Spm8CHAS may be attributed to the persulfations and the ability to competitively block HSV binding, there was a significant postentry effect observed and Spm8CHAS completely prevented cell-to-cell spread of HSV. The postentry effect against HSV, if predictive of in vivo activity, suggests that the drug might be active if applied postexposure and might also reduce viral shedding during episodes of viral reactivation. Microbicides that must be applied shortly before sexual intercourse, or coitally dependent drugs, will have substantial limitations. However, the combination of Spm8CHAS, with its ability to cross epithelial cell membranes and block HSV infection postentry, combined with an RTI, which also acts against HIV postentry, could provide protection in a coitally independent manner. Moreover, if a combination of Spm8CHAS with an HIV-specific RTI could be delivered on a vaginal ring for sustained release, this would provide yet another advantage.
Together, these studies support further development of Spm8CHAS in combination with reverse transcriptase inhibitors or other HIV-specific drugs as topical microbicides. Preliminary safety studies demonstrated that Spm8CHAS alone and in combination with UC781 or PMPA was not cytotoxic and did not trigger any inflammatory responses in vitro (not shown). Expanding preclinical testing against additional clades and primary isolates, further defining the postentry mechanism of antiviral activity, determining how Spm8CHAS traverses cell membranes, and testing the effects of the drug on normal vaginal flora and mucosal immunity as well as its activity in the presence of seminal plasma, should provide important information to identify optimal combinations for formulation.
Acknowledgments
This work was supported by National Institutes of Health grants AI061679 (B.C.H.) and HD43733 (B.C.H.) and PHS grant GM 51814 (S.L.R.).
Footnotes
Published ahead of print on 9 May 2007.
REFERENCES
- 1.Baba, M., R. Snoeck, R. Pauwels, and E. de Clercq. 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 32:1742-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini, J., A. Holy, J. Jindrich, L. Naesens, R. Snoeck, D. Schols, and E. de Clercq. 1993. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 37:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzarini, J., H. Pelemans, S. Aquaro, C. F. Perno, M. Witvrouw, D. Schols, E. de Clercq, and A. Karlsson. 1996. Highly favorable antiviral activity and resistance profile of the novel thiocarboxanilide pentenyloxy ether derivatives UC-781 and UC-82 as inhibitors of human immunodeficiency virus type 1 replication. Mol. Pharmacol. 50:394-401. [PubMed] [Google Scholar]
- 4.Cheshenko, N., M. J. Keller, V. MasCasullo, G. A. Jarvis, H. Cheng, M. John, J. H. Li, K. Hogarty, R. A. Anderson, D. P. Waller, L. J. D. Zaneveld, A. T. Profy, M. E. Klotman, and B. C. Herold. 2004. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob. Agents Chemother. 48:2025-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheshenko, N., W. Liu, L. M. Satlin, and B. C. Herold. 2005. Focal adhesion kinase plays a pivotal role in HSV entry. J. Biol. Chem. 35:31116-31125. [DOI] [PubMed] [Google Scholar]
- 6.Corey, L., A. Wald, C. L. Celum, and T. C. Quinn. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35:435-445. [DOI] [PubMed] [Google Scholar]
- 7.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher, P., Y. Kiselyeva, G. Wallace, J. Romano, G. Griffin, L. Margolis, and R. Shattock. 2005. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J. Virol. 79:11179-11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman, E. E., H. A. Weiss, J. R. Glynn, P. L. Cross, J. A. Whitworth, and R. J. Hayes. 2006. Herpes simplex 2 virus infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73-83. [DOI] [PubMed] [Google Scholar]
- 10.Galen, B., N. Cheshenko, A. Tuyama, B. Ramratnam, and B. C. Herold. 2006. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J. Virol. 80:12209-12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazrati, E., B. Galen, W. Lu, Y. Ouyang, M. J. Keller, R. J. Lehrer, and B. C. Herold. 2006. Human α- and β-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 177:8658-8666. [DOI] [PubMed] [Google Scholar]
- 13.Herold, B. C., N. Bourne, D. Marcellino, R. Kirkpatric, D. M. Strauss, L. J. Zaneveld, D. P. Waller, R. A. Anderson, C. J. Chany, B. J. Barham, L. R. Stanberry, and M. D. Cooper. 2000. Poly(sodium 4-styrene sulfonate): an effective candidate topical antimicrobial for the prevention of sexually transmitted diseases. J. Infect. Dis. 181:770-773. [DOI] [PubMed] [Google Scholar]
- 14.Herold, B. C., A. Siston, J. Bremer, R. Kirkpatrick, G. Wilbanks, P. Fugedi, C. Peto, and M. Cooper. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 41:2776-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossain, M. M., and M. A. Parniak. 2006. In vitro microbicidal activity of the nonnucleoside reverse transcriptase inhibitor (NNRTI) UC781 against NNRTI-resistant human immunodeficiency virus type 1. J. Virol. 80:4440-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, Q., I. Frank, V. Williams, J. J. Santos, P. Watts, G. E. Griffin, and R. J. Shattock. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing, B., V. Janout, B. C. Herold, M. E. Klotman, T. Heald, and S. L. Regen. 2004. Persulfated molecular umbrellas as anti-HIV and anti-HSV agents. J. Am. Chem. Soc. 126:15930-15931. [DOI] [PubMed] [Google Scholar]
- 18.Joint United Nations Programme on HIV/AIDS. 2006. 2006 report on the global AIDS epidemic. http://www.unaids.org/en/hiv_data/2006globalreport/default.asp.
- 19.Keller, M. J., B. Zerhouni-Layachi, N. Cheshenko, M. John, K. Hogarty, A. Kasowitz, C. L. Goldberg, S. Wallenstein, A. T. Profy, M. E. Klotman, and B. C. Herold. 2006. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J. Infect. Dis. 193:27-35. [DOI] [PubMed] [Google Scholar]
- 20.Lacey, C. J., A. Wright, J. N. Weber, and A. T. Profy. 2006. Direct measurement of in-vivo vaginal microbicides levels of PRO 2000 achieved in a human safety study. AIDS 20:1027-1030. [DOI] [PubMed] [Google Scholar]
- 21.Lu, H., Q. Zhao, G. Wallace, S. Liu, Y. He, R. Shattock, A. R. Neurath, and S. Jiang. 2006. Cellulose acetate 1,2-benzenedicarboxylate inhibits infection by cell-free and cell-associated primary HIV-1 isolates. AIDS Res. Hum. Retrovir. 22:411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margolis, L., and R. Shattock. 2006. Selective transmission of CCR5-utilizing HIV-1: the “gatekeeper” problem resolved? Nat. Rev. Microbiol. 4:312-317. [DOI] [PubMed] [Google Scholar]
- 23.Mayer, K. H., L. A. Maslankowski, F. Gai, W. M. El-Sadr, J. Justman, A. Kwiecien, B. Masse, S. H. Eshleman, C. Hendrix, K. Morrow, J. F. Rooney, L. Soto-Torres, et al. 2006. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS 20:543-551. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 25.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp 120. J. Virol. 74:1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palacio, J., B. E. Souberbielle, R. J. Shattock, G. Robinson, I. Manyonda, and G. E. Griffin. 1994. In vitro HIV-1 infection of human cervical tissues. Res. Virol. 145:155-161. [DOI] [PubMed] [Google Scholar]
- 27.Pearce-Pratt, R., and D. M. Phillips. 1996. Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of human immunodeficiency virus-1. Biol. Reprod. 54:173-182. [DOI] [PubMed] [Google Scholar]
- 28.Pilcher, C. D., H. C. Tien, J. J. Eron, Jr., P. L. Vernazza, S. Y. Leu, P. W. Stewart, L. E. Goh, M. S. Cohen, et al. 2004. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J. Infect. Dis. 189:1785-1792. [DOI] [PubMed] [Google Scholar]
- 29.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophage-tropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potts, B. 1990. Mini reverse transcriptase (RT) assay, p. 103-106. In Aldovini and B. Walker (ed.), Techniques in HIV research. Stockton Press/MacMillan Publishers, New York, NY.
- 31.Quan, Y., B. G. Brenner, R. G. Marlink, M. Essex, T. Kurimura, and M. A. Wainberg. 2003. Drug resistance profiles of recombinant reverse transcriptases from human immunodeficiency virus type 1 subtypes A/E, B, and C. AIDS Res. Hum. Retrovir. 19:743-753. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds, S. J., A. R. Risbud, M. E. Shepherd, J. M. Zenilman, R. S. Brookmeyer, R. S. Paranjape, A. D. Divekar, R. R. Gangakhedkar, M. V. Ghate, R. C. Bollinger, and S. M. Mehendale. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J. Infect. Dis. 187:1513-1521. [DOI] [PubMed] [Google Scholar]
- 33.Roller, R. J., and B. C. Herold. 1997. Characterization of a BHK(TK−) cell clone resistant to postattachment entry by herpes simplex virus types 1 and 2. J. Virol. 71:5805-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 35.Shaunak, S., M. Thornton, I. Teo, B. Chandler, M. Jones, and S. Steel. 2003. Optimization of the degree of sulfation of a polymer based construct to block the entry of HIV-1 into cells. J. Drug Target. 11:443-448. [DOI] [PubMed] [Google Scholar]
- 36.Terhune, S. S., K. T. Coleman, R. Sekulovich, R. L. Burke, and P. G. Spear. 1998. Limited variability of glycoprotein gene sequences and neutralizing targets in herpes simplex virus type 2 isolates and stability on passage in cell culture. J. Infect. Dis. 178:8-15. [DOI] [PubMed] [Google Scholar]
- 37.Todd, J., H. Grosskurth, J. Changalucha, A. Obasi, F. Mosha, R. Balira, K. Orroth, S. Hugonnet, M. Pujades, D. Ross, A. Gavyole, D. Mabey, and R. Hayes. 2006. Risk factors influencing HIV infection incidence in a rural Africa population: a nested case-control study. J. Infect. Dis. 193:458-466. [DOI] [PubMed] [Google Scholar]
- 38.Vives, R. R., A. Imberty, O. J. Sattentau, and H. Lortat-Jacob. 2005. Heparan sulfate targets the HIV-1 envelope glycoprotein gp 120 coreceptor binding site. J. Biol. Chem. 280:21353-21357. [DOI] [PubMed] [Google Scholar]
- 39.Zeazey, R. S., O. J. Klasse, S. M. Schader, Q. Hu, T. J. Ketas, M. Lu, P. A. Marx, J. Dufour, R. J. Colonno, R. J. Shattock, M. S. Springer, and L. P. Moore. 2005. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 438:99-102. [DOI] [PubMed] [Google Scholar]
- 40.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]