Abstract
The entry of inhaled virions into airway cells is presumably the initiating step of varicella-zoster infection. In order to characterize viral entry, we studied the relative roles played by lipid rafts and clathrin-mediated transport. Virus and target cells were pretreated with agents designed to perturb selected aspects of endocytosis and membrane composition, and the effects of these perturbations on infectious focus formation were monitored. Infectivity was exquisitely sensitive to methyl-β-cyclodextrin (MβCD) and nystatin, which disrupt lipid rafts by removing cholesterol. These agents inhibited infection by enveloped, but not cell-associated, varicella-zoster virus (VZV) in a dose-dependent manner and exerted these effects on both target cell and viral membranes. Inhibition by MβCD, which could be reversed by cholesterol replenishment, rapidly declined as a function of time after exposure of target cells to VZV, suggesting that an early step in viral infection requires cholesterol. No effect of cholesterol depletion, however, was seen on viral binding; moreover, there was no reduction in the surface expression or internalization of mannose 6-phosphate receptors, which are required for VZV entry. Viral entry was energy dependent and showed concentration-dependent inhibition by chlorpromazine, which, among other actions, blocks clathrin-mediated endocytosis. These data suggest that both membrane lipid composition and clathrin-mediated transport are critical for VZV entry. Lipid rafts are likely to contribute directly to viral envelope integrity and, in the host membrane, may influence endocytosis, evoke downstream signaling, and/or facilitate membrane fusion.
The process by which varicella-zoster (VZ) virions enter host cells is critical to the establishment of primary and latent infections, but the intensely cell-associated nature of VZ virus (VZV) in vitro (11, 47) has hindered study of this important stage of the virus life cycle. In most cell types, both in vitro and within infected hosts, VZV spreads by cell-to-cell fusion; enveloped virions are released but have generally been rendered noninfectious by trafficking through the late endosomal compartment, a process that is mannose 6-phosphate receptor (MPRci) dependent (9). It is the natural downregulation of MPRci expression in maturing keratinocytes that instead allows newly enveloped VZ virions to be released constitutively within the skin lesions of infected persons (18, 47). These virions are believed to convey infection to the respiratory tracts of naive hosts (47) and to establish latent infection of sensory neurons innervating the epidermis (8).
In order to produce infection, any incoming virion must deliver its genetic material to the interior of the appropriate host cell. Whereas some viruses are able to fuse directly with the plasma membrane following receptor binding, fusion of others is triggered only after internalization in a specific intracellular organelle (38). A single virus may also use different routes, depending on the type of target cell and receptor (35). The process of viral entry has been examined in detail for a number of herpesviruses, but few studies have previously addressed the VZV entry mechanism (not least because of the difficulty of obtaining infectious virus that is uncontaminated with infected cells).
In the present work, we sought to determine the pathway of VZV entry to provide a context for previous observations of its dependence on cell surface heparan sulfate proteoglycan (HSPG) (50), the MPRci (9, 50), and insulin-degrading enzyme (IDE) (25). Some evidence was recently provided that VZ virion entry into a nonpermissive cell type depends on a low-pH compartment, since it was partially inhibited by lysosomotropic agents (14). We sought to clarify whether VZ virions gain entry by fusing directly with the plasma membrane or instead undergo endocytosis prior to fusion with the membrane delimiting an intracellular compartment. In addition, we wished to investigate the degree to which VZV entry is cholesterol dependent, as has been demonstrated for certain other viruses, including herpes simplex virus (HSV) (4), Epstein-Barr virus (23), and human immunodeficiency virus (49). Cholesterol is a major constituent of lipid rafts, dynamic microdomains within cellular membranes that participate in diverse cellular processes by virtue of their distinctive lipid and protein composition (5). While the ingress of some nonenveloped viruses (such as simian virus 40 and echovirus 1) entails lipid raft-dependent endocytosis (2, 30), the fact that certain enveloped viruses that enter by other routes share this cholesterol dependence implies alternative roles for cholesterol (7). A physical association between viral glycoproteins and host cell lipid rafts has been demonstrated in some cases (e.g., HSV) (4), while in others, receptor clustering within lipid rafts appears to be important (e.g., human immunodeficiency virus) (49). Raft association may be required for downstream effects of viral interaction with cellular receptors, such as cell signaling and internalization.
Our observations suggest that VZV infection of several cell types proceeds largely through clathrin-dependent endocytosis but that lipid raft integrity is also essential for viral entry.
MATERIALS AND METHODS
Cells and virus.
Human neonatal lung fibroblasts, human embryonic lung fibroblasts (HELF; both from Diagnostic Hybrids, Inc.), MeWo cells, and U373MG cells (American Type Culture Collection) were grown in Eagle's minimum essential medium (MEM) with Earle's salts supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, and Plasmocin at 5 μg/ml.
A low-passage clinical isolate of VZV (MLS) was propagated in lung fibroblasts and stored as infected cells at −80°C. To prepare cell-free virus, a heavily infected monolayer was trypsinized, passed 1:3 to 1:5 onto fresh monolayers in maintenance medium, and harvested when the cytopathic effect (CPE) was extensive (24 to 48 h). Infected cells were scraped into cold Hanks' buffered salt solution (HBSS, pH 7.4); spun briefly, if necessary, to reduce the total volume to 1.5 to 2 ml/150-cm2 flask; and sonicated on ice at a power setting of 2.2 to 2.6 for 30 s (Branson sonicator, model 875). The sonicate was immediately transferred to a polystyrene centrifuge tube and spun at 500 × g for 2 min with no brake. The cleared supernatant was aliquoted, stored at −80°C, and used within 3 months. Each batch was checked for heparin sensitivity to confirm that infectious virus was predominantly cell free.
Reagents.
All reagents were from Sigma unless otherwise stated. Methyl-β-cyclodextrin (MβCD) was dissolved in HBSS at 0.1 M and stored at 4°C. Chlorpromazine was dissolved in dimethyl sulfoxide (DMSO) at 50 mg/ml, stored frozen in aliquots at −20°C, and diluted to 2 mM in water as a working stock immediately prior to use. Other reagents were dissolved in DMSO or ethanol as advised by the manufacturer. In solvent-only control experiments, DMSO and ethanol were found not to affect VZV infectivity. For preincubation of target cells, inhibitors were diluted in maintenance medium (MEM, 2% FBS, 1% sodium bicarbonate plus antibiotics), except where indicated otherwise. For experiments on metabolic inhibition, HEPES-buffered saline (HBS; 50 mM HEPES, 150 mM NaCl, 5 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, pH 7.4) replaced tissue culture medium and sodium azide (10 mM) and 2-deoxyglucose (50 mM) were added as indicated.
Infectious-focus assay.
Confluent cell monolayers in 12-well plates were preincubated with inhibitors in maintenance medium. The supernatant was withdrawn prior to the addition of cell-free virus, freshly thawed, and diluted in HBSS at 4°C. The infection volume was typically 300 μl per well of a 12-well plate. Experiments were generally done in triplicate and at several multiplicities of infection (MOIs), in the range of 25 to 200 PFU per well. Where indicated, virus and cells were held at 4°C for up to 60 min before a shift to 37°C and 5% CO2. The viral inoculum was withdrawn after 1.5 to 2.5 h and replaced with maintenance medium in the presence or absence of inhibitors.
Culture was continued until plaques of CPE were clearly evident (3 to 5 days) and then stained as previously described (45). Plates were rinsed with phosphate-buffered saline (PBS) and fixed with fresh 4% formaldehyde (from paraformaldehyde) in 0.1 M phosphate buffer. After further washes with PBS, cells were incubated with blocking buffer (10% FBS in PBS) for 1 h. A murine monoclonal antibody against VZV gE, 13B1 (ViruSys), was applied at a dilution of 1:1,500 in blocking buffer for 1 h, and the cells were washed several times with PBS. An alkaline phosphatase-conjugated anti-mouse immunoglobulin G (IgG) antibody (Novagen) was then applied at 1:10,000, and after washing, plaques were revealed by using SigmaFast AP substrate. Plaques were visualized with a dissection microscope and counted.
Fluorescence microscopy.
For experiments on surface MPRci expression, subconfluent neonatal lung fibroblasts growing on glass chamber slides (Nunc) were preincubated with inhibitors in serum-free medium for 30 min, washed, and chilled to 4°C. Antibodies to MPRci (MEM-238; Abcam Inc.) were applied with rocking at 10 μg/ml in PBS for 30 min on ice. After two washes in PBS, medium or HBS was reapplied with or without inhibitors and cells were chased at 37°C as indicated. The slides were then washed with PBS and fixed in 4% formaldehyde (from paraformaldehyde) in 0.1 M phosphate buffer. After several washes, cells were blocked and permeabilized with 4% FBS-0.1% Triton X-100 in PBS-0.1% azide for 1 h and then incubated with goat anti-mouse IgG-Alexa Fluor488 (1:1,000; Molecular Probes) and bisbenzimide. After a further 30 min, slides were washed prior to mounting and viewing with a Leica DMRXA2 microscope.
Measurement of MPR internalization by fluorescence-activated cell sorter (FACS) analysis.
Confluent neonatal lung fibroblasts growing in 12-well plates were preincubated with inhibitors in serum-free medium for 30 min and then rapidly chilled and washed with PBS. Primary antibodies to MPRci were applied as described above, followed by three washes in ice-cold PBS and a further 30-min incubation with goat anti-mouse IgG-Alexa Fluor488. After washing away the secondary antibody, cells were processed immediately (t = 0) or covered with warm medium containing the relevant inhibitor and chased at 37°C in 5% CO2 for 15 min (t = 15).
Samples were prepared for FACS analysis on ice by detachment with cold 2 mM EDTA in PBS. This solution was withdrawn from the monolayer and replaced with PBS-1% FCS-0.1% sodium azide (FacsWash), and cells were dislodged by pipetting. Cells were pelleted by centrifugation and resuspended in 0.3 ml FacsWash. This suspension was split into two tubes and diluted 1:1 immediately prior to analysis with either FacsWash (unquenched) or 0.4% trypan blue (quenched). Samples were analyzed on a Becton Dickinson Excalibur FACScan instrument, and the data were handled with CellQuest software.
Virus binding assay.
Confluent lung fibroblasts growing in 12-well plates were preincubated with inhibitors as indicated, chilled on ice, and washed twice with cold PBS. Cell-free virus, freshly thawed from −80°C, was diluted 1:1 with HBSS and inoculated onto the cells at 100 μl per well. Where indicated, heparin at 100 μg/ml was included in the inoculum. Binding of the virus to cells was subsequently revealed by sequential application of primary mouse monoclonal antibodies to VZV gE (13B1, 1:1,000) and secondary antibodies (goat anti-mouse IgG-Alexa Fluor488, 1:1,000). All incubations were for 30 min, with rocking, on ice. Cells were washed three times with PBS between incubations. Finally, cells were detached as described above and suspended in FacsFix (PBS, 1% FCS, 0.1% sodium azide, 2% formaldehyde) prior to analysis.
TEM.
Subconfluent neonatal lung fibroblasts growing on ACLAR plastic discs in tissue culture plates were chilled and inoculated with freshly thawed virus at high titer (approximately 105 PFU/ml; MOI, ∼0.2). Samples were centrifuged at 4°C at 700 × g for 30 min prior to incubation for 0 to 5 min at 37°C in 5% CO2. Subsequently, cells were rapidly cooled on ice, washed twice with PBS, and fixed with 4% formaldehyde (from paraformaldehyde) plus 2.5% glutaraldehyde for 1 h at room temperature. Processing for transmission electron microscopy (TEM) was carried out as previously described (15). Cells were postfixed with 1% osmium tetroxide in 0.1 M phosphate buffer prior to dehydration in a graded series of ethanol concentrations, clearing by propylene oxide, and embedding in Epon 812. Thin sections were cut, stained with lead citrate and uranyl acetate, and examined with a JEOL 1200 EX electron microscope.
RESULTS
Inhibition of infection by heparin and mannose 6-phosphate in an infectious focus assay authenticates VZV preparations as cell free.
For simplicity, we chose to use an infectious-focus assay in our initial studies of VZ virion entry. In such an assay, healthy cell monolayers are exposed to virus at a variety of input concentrations; several days later, the cells are scored for the formation of infectious foci (plaques) that are each presumed to result from a single infecting event. Manipulations that prevent or delay virion entry lead to a reduced efficiency of infection, which is manifested as reduced numbers of infectious foci.
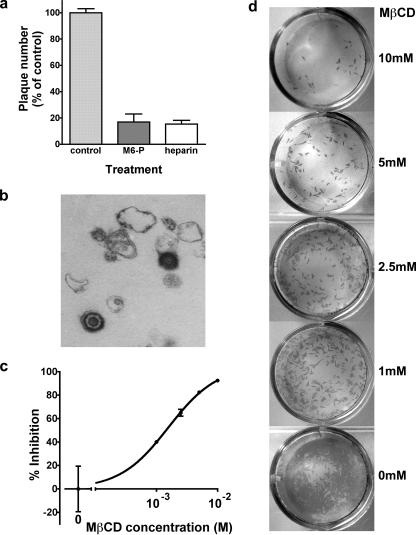
Any study of VZ virion entry requires a rigorously cell-free preparation of virus. In order to validate our assay and our cell-free virus, it was first confirmed that heparin and mannose 6-phosphate (known inhibitors of VZV attachment and entry, respectively) (50) could prevent the formation of ∼80% of infectious foci compared with controls (Fig. 1a). In contrast, cell-associated virus transmission was unaffected by the presence of either heparin or mannose 6-phosphate (data not shown). TEM of the cell-free viral inoculum revealed numerous apparently intact virions along with many misshapen and incomplete virus particles and small fragments of cellular debris (Fig. 1b).
FIG. 1.
The infectivity of a sonicated VZV preparation is attributable to free virions and can be inhibited by pretreatment of target cells with MβCD. (a) Infectious-focus formation by cell-free virus is inhibited by known antagonists of VZ virion entry. Cell-free virus (prepared as described in Materials and Methods) was inoculated onto HELF in the presence or absence of mannose 6-phosphate (10 mM) or heparin (100 μg/ml). Five days later, infectious foci were stained with an anti-VZV antibody and enumerated. Data shown are mean counts ± the standard error of triplicate wells, normalized to the control. (b) Transmission electron micrograph of a cell-free virus preparation, demonstrating apparently intact enveloped virions along with cellular debris and viral fragments. (c and d) Susceptibility to cell-free VZV infection is reduced in a concentration-dependent manner by pretreatment of target cells with MβCD. (c) Quantitation of infectious foci (mean ± standard error of triplicate wells, normalized to the control). Data are representative of three experiments. (d) Appearance of stained foci of VZV infection. Representative wells at a single input MOI are shown, with progressively greater concentrations of MβCD from the bottom (no drug) to the top (10 mM MβCD).
Cholesterol is required for an early step in infection by VZ virions.
In order to disrupt lipid rafts, we depleted target cell membranes of cholesterol by preincubating cells in serum-free medium containing MβCD. The cells were then washed prior to the addition of cell-free VZV. Concentration-dependent inhibition of infectious-focus formation occurred over a concentration range in keeping with MβCD's expected effects on membrane cholesterol (Fig. 1c and d).
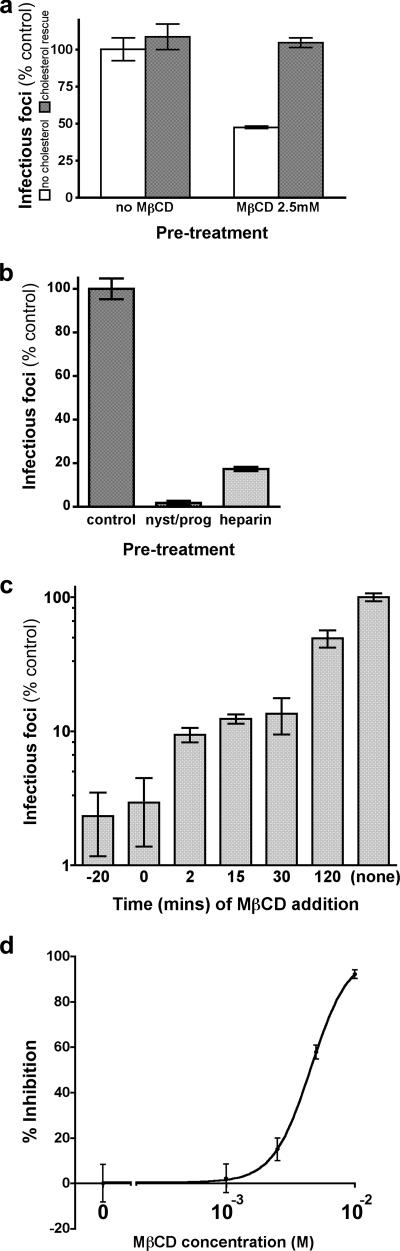
The inhibitory effect of MβCD was reversed by replenishment of cellular cholesterol during a 30-min incubation with water-soluble cholesterol at 0.1 M prior to virus addition (Fig. 2a), confirming that MβCD had acted specifically. As expected, cholesterol depletion by other means (overnight treatment with nystatin at 25 μg/ml and progesterone at 10 μg/ml) (39) produced a similarly marked reduction of viral infectivity (Fig. 2b). Of note, concentrations of MβCD that were completely nontoxic to cells regularly produced even greater inhibition of viral infectivity than did heparin at 100 μg/ml.
FIG. 2.
Mechanism of action of MβCD. Cholesterol depletion from target cells or virions inhibits viral entry. (a) Inhibition of viral infectivity by pretreatment with MβCD (2.5 mM) can be completely reversed by cholesterol replenishment (30 min at 0.1 mM compared with 0 mM cholesterol). (b) Inhibition of viral infectivity by overnight pretreatment of target cells with nystatin (25 μg/ml) and progesterone (10 μg/ml), compared with heparin (100 μg/ml, present in the viral inoculum). (c) Delayed addition of MβCD (2.5 mM) after inoculation of virus onto cells shows progressive loss of susceptibility to inhibition of infectivity, suggesting that cholesterol is required for an early step. (d) Viral infectivity is impaired by direct exposure of the viral inoculum to cholesterol-depleting concentrations of MβCD, suggesting a distinct role for envelope cholesterol in virion infectivity.
In order to determine whether the cholesterol-dependent step occurs early in infection (compatible with entry), we reasoned that the inhibitory effect of MβCD should be lost as the drug is added at progressively later times after the initiation of infection; such a decline in susceptibility was indeed observed (Fig. 2c). Cell-associated VZV infection was not affected by MβCD at these concentrations (data not shown). Collectively, these data indicate that a cholesterol-dependent step occurs during the entry of VZ virions.
Both target cell cholesterol and viral membrane cholesterol play a role in virion entry.
VZV acquires its envelope from the trans-Golgi network, membranes of which are typically rich in cholesterol and other components of lipid rafts (13, 22). We therefore examined the effect of cholesterol depletion upon virion integrity by preincubating cell-free virus with MβCD for 20 min and then diluting this mixture immediately before inoculation onto cells for infectious focus assay. While some inhibition of infectivity was indeed produced, virions appeared to be relatively insensitive to MβCD (Fig. 2d). Their exposure to trace amounts of drug above washed, MβCD-treated target cells could not, therefore, account for the profound inhibition of viral infectivity we observed under that condition.
Cholesterol depletion does not impair virus binding.
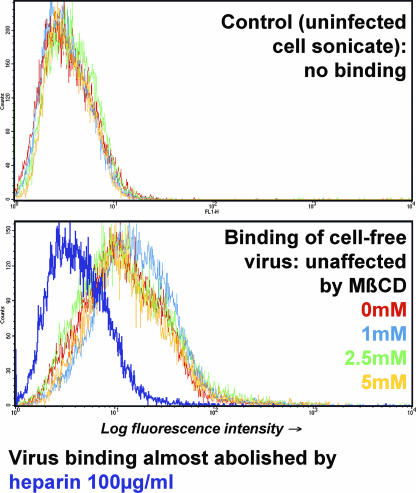
The possible influence of cholesterol depletion on the availability of cell surface receptors for VZV was investigated. Virus binding was monitored in a FACS-based assay in which inhibition by heparin was readily demonstrated (Fig. 3), in keeping with the previous finding that HSPG is the predominant determinant of virion binding (50). In contrast, target cells that had been incubated with MβCD prior to virion addition retained the ability to bind virus at levels similar to those of controls (Fig. 3). Cholesterol depletion, therefore, does not impair infectivity at the level of virus binding.
FIG. 3.
Virus binding is not affected by cholesterol depletion from target cells. The binding of cell-free VZV to target cells was analyzed by a FACS-based immunoassay (see Materials and Methods). Heparin completely inhibited virus binding (purple trace, lower panel), but MβCD pretreatment had no effect, even at concentrations that strongly inhibit viral infectivity.
Cholesterol depletion does not prevent surface expression or internalization of the MPRci.
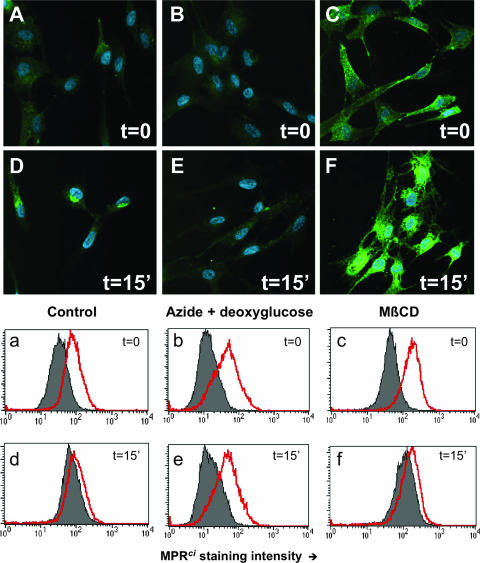
In a second set of experiments, the influence of cholesterol depletion upon the expression and trafficking of the MPRci was explored. The MPRci does not contribute significantly to overall binding of virions to cells that express HSPG (50) but is required for the establishment of infection (9) and is likely to function as a cell surface receptor for the virus. Far from being downregulated, surface MPRci immunoreactivity was increased in a dose-dependent manner by MβCD, as assessed both by FACS analysis and by fluorescence microscopy (Fig. 4C and c; compare with weaker surface staining in panels A and a). This finding suggests that cholesterol is likely to be required for normal MPRci distribution within cells.
FIG. 4.
Cholesterol depletion neither reduces the surface expression of the MPRci nor prevents its internalization. (A to F) Fluorescence microscopy following the incubation of live cells with antibodies to MPRci, with fixation before (A to C) or after (D to F) a 15-min (15′) chase. Green, anti-MPRci; blue, nuclei counterstained with bisbenzimide. (a to f) Corresponding FACS experiment with anti-MPRci and labeled secondary antibodies and analysis in the presence (gray fill) or absence (red line) of the membrane-impermeant quenching agent trypan blue as described in Materials and Methods. Similar results were obtained with Zenon-Alexa Fluor-labeled anti-MPRci antibody. A, D, a, and d, untreated target cells; B, E, b, and e, pretreatment with sodium azide (10 mM) and deoxyglucose (50 mM); C, F, c, and f, target cell pretreatment with MβCD (2.5 mM).
To investigate the effect of MβCD upon MPRci internalization from cell surfaces, live cells were exposed to labeled antibodies to MPRci in the cold and their distribution was monitored following a chase. By wide-field fluorescence microscopy (Fig. 4D), we confirmed that the MPRci of control cells was rapidly internalized, as previously reported, to a perinuclear compartment (27). In separate experiments, control murine IgG yielded negligible surface staining only (not shown). Internalization of the MPRci did not appear to be diminished significantly by cholesterol depletion (Fig. 4F); however, this interpretation was somewhat complicated by the accompanying overall increase in MPRci immunoreactivity. To corroborate this result, we therefore used FACS analysis in the presence and absence of the membrane-impermeant quenching agent trypan blue to distinguish between internalized and surface-exposed green fluorescence. Following incubation with labeled antibodies to MPRci and a 15-min chase period, control cells lost most of the susceptibility to quenching by trypan blue that had been evident prior to the chase (Fig. 4d, compare with panel a). In contrast, cells subjected to metabolic poisoning by sodium azide and 2-deoxyglucose remained fully sensitive to surface quenching (Fig. 4e), indicating, as expected, that they failed to internalize the MPRci. Cholesterol-depleted cells retained the ability to internalize the MPRci, as demonstrated by insensitivity to trypan blue by 15 min (Fig. 4f). Internalization was specific since control antibody produced negligible surface staining that remained fully quenchable by trypan blue (data not shown).
We conclude that the ability of MβCD to inhibit viral entry results neither from reduced viral binding to HSPG nor from diminution of MPRci expression and internalization. Although it is conceivable that cholesterol depletion impairs the ability of VZV to interact with another viral receptor (such as IDE), it seems more likely that other cholesterol-requiring steps exist, such as lipid raft-dependent endocytosis and/or membrane fusion itself.
VZV infection is sensitive to inhibitors of endocytosis.
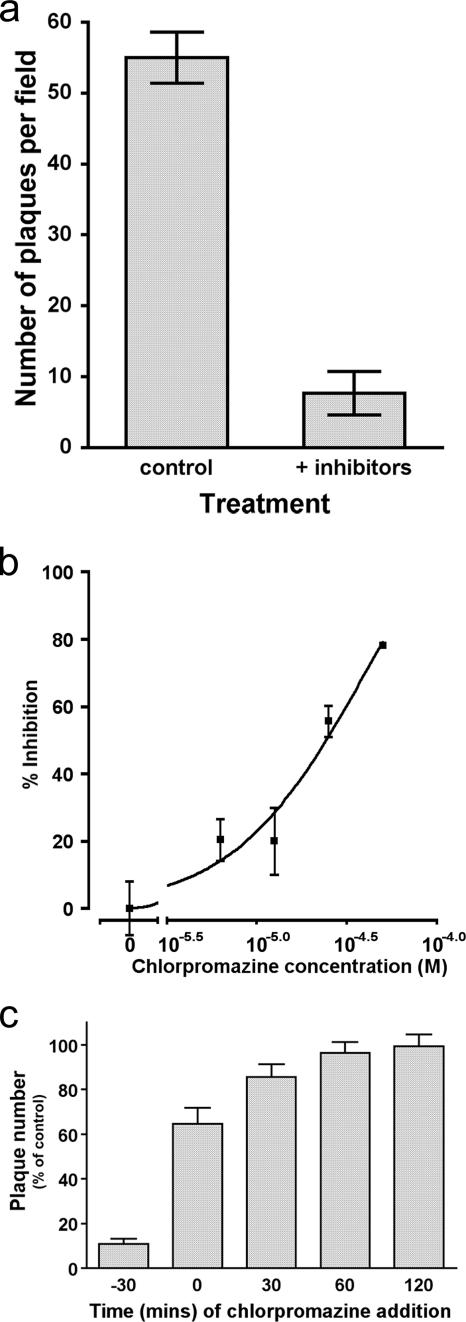
We reasoned that if VZV entry requires endocytosis, infection should be sensitive to metabolic poisoning. We therefore treated target cells with sodium azide and 2-deoxyglucose immediately prior to inoculation with cell-free virus (Fig. 5a). As expected, marked inhibition of infectious-focus formation was produced; however, this inhibitory effect was lost if metabolic poisoning was delayed after virus addition. Infection by cell-associated virus was not significantly affected by metabolic poisoning. These findings are consistent with a model of VZ virion entry that includes an energy-requiring step such as endocytosis.
FIG. 5.
A possible role for endocytosis in VZV entry. (a) Metabolic poisoning with sodium azide (10 mM) and deoxyglucose (50 mM) prevents establishment of infection with VZV. (b) Target cell treatment with chlorpromazine produces a concentration-dependent reduction in VZV infectivity. (c) The chlorpromazine-dependent step occurs early in the infection process, compatible with a role in viral entry. Addition of chlorpromazine (final concentration, 25 μM) was delayed to the indicated times with respect to viral inoculation of target cells.
Clathrin-mediated endocytosis might be expected to play a role in VZV entry because the MPRci traffics from the cell surface by this means (27). In order to disrupt clathrin-mediated endocytosis, cells were incubated with chlorpromazine prior to and during virus exposure. Subsequent infectious-focus formation by cell-free VZV was inhibited in a concentration-dependent fashion (Fig. 5b), with approximately 50% inhibition at 25 μM chlorpromazine. Although the specificity of the drug's effects have been questioned, this is a concentration typically used to inhibit clathrin-dependent endocytosis (1). These results are thus consistent with the idea that clathrin-dependent endocytosis is required for VZV infection of neonatal lung fibroblasts or HELF.
The observed effect of chlorpromazine on VZV infection was linked to an entry step, since >50% of the sensitivity to chlorpromazine inhibition was lost when its addition was delayed up to 2 h (Fig. 5c). Although consistent with a requirement for clathrin-dependent endocytosis of VZV during entry, it was also conceivable that chlorpromazine might alter the availability of VZV receptors at the cell surface. By FACS analysis, chlorpromazine-treated cells expressed the MPRci at levels similar to controls and retained the ability to bind virus in a heparin-sensitive manner (data not shown). Changes in HSPG and/or MPRci availability thus do not explain the inhibitory effect of chlorpromazine toward viral entry.
Electron microscopic analysis of virion entry.
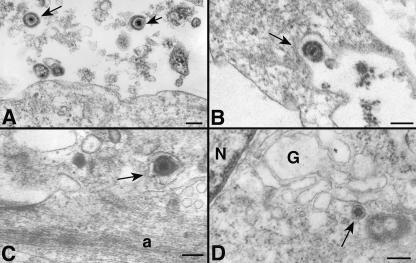
To obtain further information about the route of VZ virion entry, we sought to visualize viral trafficking ultrastructurally. Cells were exposed to cell-free virus in the cold, warmed briefly to 37°C, and then washed and fixed prior to processing for TEM. The maximum MOI that could be achieved was approximately 0.2. Plentiful viral particles were seen adjacent to cell surfaces, and occasional individual virions were closely associated with the plasmalemma of a potential target cell (Fig. 6A). Rarely, enveloped viral particles were seen within a plasmalemmal invagination (Fig. 6B), within a vesicle in the target cell (Fig. 6 C), or uncoated as nucleocapsids near the Golgi apparatus (Fig. 6D). Unfortunately, viral profiles were encountered at a frequency that was too low to permit quantitation. No instances of virion fusion at the plasmalemma were encountered. These results are compatible with, although not confirmation of, the hypothesis that VZ virions enter by endocytosis. To our knowledge, these are the first electron micrographs to have been obtained that appear to show VZ virion ingress.
FIG. 6.
TEM images are compatible with endocytosis of VZ virions. (A) Intact enveloped VZ virions (arrows) are found extracellularly in proximity to the plasma membrane of a target cell. (B) An enveloped virion is found in a coated pit (arrow) formed by the plasma membrane of a target cell. (C) An enveloped virion is found in a vesicle in the cortical cytoplasm of a target cell. The appearance of the vesicle is compatible with that of an early endosome. (D) An unenveloped nucleocapsid is found in the region of the Golgi apparatus of a target cell. Bars = 200 nm. N, nucleus; G, Golgi apparatus; a, cortical actin filaments.
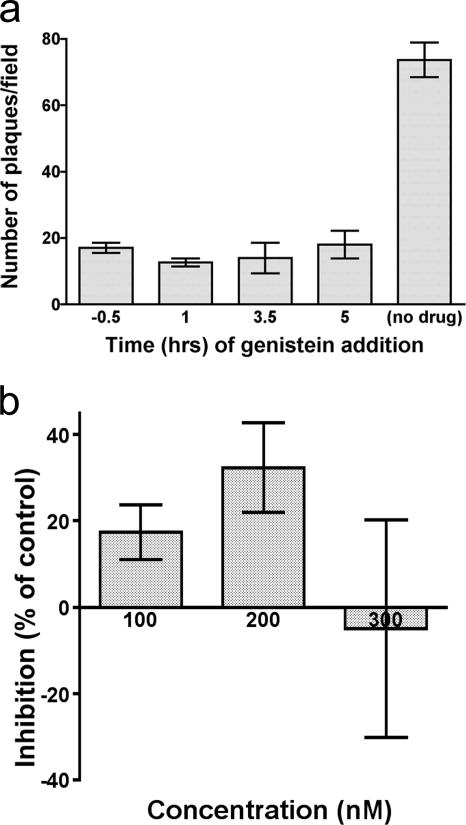
Tyrosine kinase inhibition affects a stage of infection that follows VZV entry.
Several viruses have been shown to require cellular tyrosine kinase activity for efficient entry, on the basis of their susceptibility to inhibition by the specific kinase inhibitor genistein; for example, this inhibitor blocks the endocytic entry of HSV into keratinocytes (35). VZV infection proved to be sensitive to genistein, but with a time course that was quite different from that of sensitivity to chlorpromazine and MβCD (Fig. 7a). Unlike the latter inhibitors, genistein was as effective when added several hours after exposure to cell-free VZV as when it was present throughout. Genistein's effects, furthermore, were reversible until at least 4 h postinfection (data not shown); the inhibition displayed in Fig. 7a resulted from genistein treatment until 20 h postinfection. Consistent with these findings, genistein also produced significant inhibition of infectious focus formation by cell-associated VZV. Tyrosine kinase activity, therefore, does not appear to be required during entry of VZV but is necessary later during viral replication. The relevant substrates for tyrosine phosphorylation remain to be explored; among the candidates would be VZV gE, since dimers of this essential viral glycoprotein have been shown to be tyrosine phosphorylated (37).
FIG. 7.
Cellular kinase activity is required for viral infectivity beyond an entry step. (a) A delay in the addition of genistein (100 μM) of up to 5 h after inoculation of virus onto target cells produced no diminution of its inhibitory effect on infectious focus formation. Treatment was continued until 20 h postinfection. (b) Target cells preincubated with increasing concentrations of the PI3K inhibitor wortmannin showed no consistent inhibition of VZV infectivity.
PI3K activity plays a minor role in VZ virion entry.
Phosphatidylinositol metabolism is important for a variety of intracellular trafficking processes, including the appropriate endosomal sorting of such molecules as the MPRci (16). Inhibitors of phosphatidylinositol 3-kinase (PI3K) have been used to show that these pathways are required for the ingress of certain viruses; for example, blockade of productive HSV infection by wortmannin has been reported (35). With the infectious focus assay, we were unable to demonstrate significant inhibition of VZV entry by wortmannin (Fig. 7b) or by the alternative PI3K inhibitor LY294002 (data not shown), present during the first 2.5 h of infection. Of note, these agents exhibited significant toxicity over the 4 to 5 days following treatment, limiting the concentrations that could be analyzed. The lack of inhibition produced by concentrations of wortmannin that prevent HSV infection (35) implies that PI3K-dependent trafficking is not required during entry of VZV.
Many viruses undergo pH-dependent fusion following internalization, which can be inhibited by lysosomotropic agents (31). We found that preincubation of target cells with such agents (e.g., bafilomycin A at 10 nM) prior to the introduction of virus completely blocked infectivity. Similar findings have recently been reported by Finnen et al. (14). Although this is compatible with a role for endosomal acidification in viral fusion, alternative explanations include a change in the trafficking of viral receptors following endosomal alkalinization.
Applicability to other cell types.
To determine whether our observations are specific for lung fibroblasts, we also investigated MeWo cells and U373MG cells (melanoma and astrocytoma derived, respectively). VZV infection of both of these cell lines showed similar marked sensitivity to inhibition by MβCD, chlorpromazine, and genistein. Cholesterol and endocytosis thus appear to play significant roles in the infection of more that a single cell type.
DISCUSSION
The present work demonstrates the importance of membrane cholesterol for the establishment of infection by VZ virions and suggests that clathrin-mediated endocytosis is a major route of viral entry into several cell types. These findings invite comparison with VZV's alphaherpesvirus relative HSV, which expresses orthologues of VZV's glycoproteins (gB, gC, gE, gH, gI, gK, gL, gM) but relies on a different glycoprotein (gD) for binding to its cellular receptors (the Ig superfamily members nectin-1 and -2, the herpesvirus entry mediator protein [a tumor necrosis factor receptor family member], and 3-O-sulfated heparan sulfate) (43). VZV lacks a gD orthologue. Analysis of the requirements for HSV entry has been complicated by possible differences of receptor usage in different cell types, albeit the common result appears to be a major conformational change in gD; this permits engagement of the membrane fusion machinery, which also includes gH, gL, and gB.
Like the entry of VZV, that of HSV requires both cellular and virion cholesterol, as shown in elegant work by Bender and colleagues (4). The concentration dependence of inhibition of HSV infection by MβCD and its time course and reversibility by cholesterol replenishment appear very similar to those of VZV. Bender et al. have also explored the molecular basis of HSV's cholesterol dependence and demonstrated that the HSV glycoprotein gB becomes associated with lipid rafts during viral entry (4). Since HSV gB's recently solved crystal structure (20) and its behavior in transfected cells (44, 46) strongly imply a fusogenic function, it is plausible that cholesterol plays a role in the fusion process. It is noteworthy that cholesterol is increasingly recognized to participate in endogenous membrane fusion events such as regulated exocytosis (41). A variety of models has been put forward to account for this relationship, including an organizing or scaffolding role (via raft proteins) and a direct participation of raft lipid moieties in the fusion process (40, 41). Certainly, gB has been linked to VZV-induced membrane fusion events (29) and is one of the most conserved glycoproteins among the human herpesviruses. It is thus possible that lipid rafts become involved in VZV entry through the participation of gB in the fusion of the viral envelope with cellular membranes. The lipid raft association of VZV glycoproteins within infected cells, however, remains to be determined.
Endocytosis appears to play a rather variable role in HSV entry, as judged by differences in the extent to which HSV infection can be inhibited by lysosomotropic agents and metabolic poisoning in different cell types. For example, HSV entry into Vero cells does not require endocytosis (48), while the virus must traverse a low-pH endocytic compartment in CHO nectin-1 and HeLa cells (36); C10 cells become infected via a third, pH-independent and yet endocytic, route (10, 32). These differences do not arise solely because of alternative receptor usage because a single receptor can support entry via different mechanisms (17). One suggestion is that the subplasmalemmal cortical microfilament network may present a variable barrier to the penetration of virions which can be overcome by endocytosis. Another possibility is that the rate of gD-dependent fusion is modulated by other cellular factors, such as pH, membrane lipid composition, and possibly coreceptor availability. Among the latter, the existence of a coreceptor for gB has been inferred from the residual, heparin-resistant binding of gB to cells that do not express its major ligand, HSPG, and the fact that HSV infection of such cells is inhibited by soluble gB (3). This does not detract from the central role played by gD in enabling target cell recognition and subsequent membrane fusion.
Since VZV has no gD orthologue, another glycoprotein must serve the important functions gD performs for HSV. Attention has recently focused on gE, a glycoprotein that is essential for VZV, which is reflected in the fact that a gE knockout virus cannot be propagated (34). A role for gE in facilitating membrane fusion is suggested by the finding that its coexpression with gB potentiates the ability of gB to confer a syncytium-forming phenotype upon transfected cells in culture (29). Recently, Li et al. (25) demonstrated specific binding of VZV gE to the ubiquitous Zn2+ metalloproteinase IDE and provided evidence that such an interaction is required for VZV infectivity. Proteolytic enzymes play two types of role in the entry of other viruses, sometimes functioning as pure binding receptors (26) and sometimes activating fusion by means of proteolytic cleavage of viral fusogens (6, 21, 42). The data of Li et al. (25) suggest that IDE does not degrade gE; therefore, a receptor recognition role appears more likely. The mechanism by which a soluble protein such as IDE could effect virion-cell recognition appears to require a membrane-anchoring adaptor molecule (or coreceptor); such a molecule has yet to be identified but presumably may also be responsible for the observed association of IDE with the extracellular face of the plasmalemma in certain cell types, despite the absence of an IDE signal sequence (28). IDE is also found within endosomes (19).
The relationship of IDE to the MPRci is unclear. The MPRci is a type I transmembrane protein with multiple roles, important among which is the delivery of phosphomannosylated cargo proteins from the trans-Golgi network and extracellular space to late endosomes. Downregulation of the MPRci prevents infection of cells by enveloped, but not cell-associated, VZV (9), and virion entry is inhibited by free mannose 6-phosphate (50). VZV glycoproteins, moreover, bear phosphomannosyl sugars (15), and alkaline phosphatase treatment destroys virion infectivity (50). It has thus been postulated that direct binding to the MPRci occurs.
The MPRci undergoes efficient internalization from the plasma membrane (27), and its known function in the delivery of a variety of ligands to the endosomal compartment (12, 24) immediately suggests a possible mechanism for virion endocytosis. MPRci trafficking is generally clathrin mediated, and although cholesterol depletion has been shown to affect the steady-state distribution of the MPRci (33), we have found that internalization of the MPRci from the cell surface does not require cholesterol.
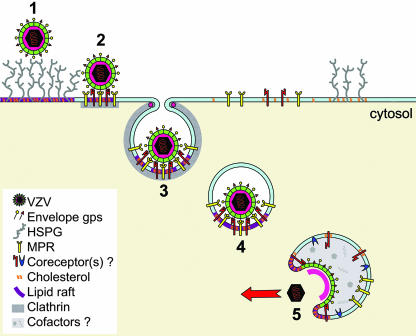
We propose that VZ virions undergo receptor-mediated endocytosis after binding to the MPRci and are thereby delivered to an intracellular compartment in which fusion is triggered by unknown factors (possibly including a coreceptor such as IDE). This scheme is shown diagrammatically in Fig. 8. The identity of the fusion compartment is of interest, and one intriguing possibility is that the same endosomal conditions that inactivate outgoing virions—including an acidic pH and/or other factors (11, 14)—are responsible for triggering fusion of incoming infectious virus with the endosomal membrane.
FIG. 8.
A suggested mechanism of VZ virion entry. The incoming virion initially attaches to cellular HSPG (step 1), facilitating specific interaction with a cellular receptor, e.g., MPRci (step 2). This leads to receptor-mediated (clathrin-dependent) endocytosis (step 3) and delivery to an endosomal compartment (step 4). Within this structure, the virion is exposed to cofactors for membrane fusion, possibly including IDE and/or an altered pH (step 5). Following triggered membrane fusion, the VZ nucleocapsid is delivered to the cytoplasm. Cholesterol may play a role at each stage.
It is noteworthy that in the case of VZV, the molecular requirements for fusion of the viral envelope with target cells appear to differ from those for cell-associated viral spread, which involves the fusion of adjacent cells. In contrast to the infection of cells by enveloped virions, cell-associated viral spread is insensitive to inhibition by free mannose 6-phosphate or downregulation of the MPRci (9, 50), and we now find that it is also relatively independent of membrane cholesterol. Naturally, the immediate molecular context of viral glycoproteins within the virion envelope must be different from that of those within the plasma membrane, in terms of lipid and protein composition, as well as curvature. Cell-to-cell and virion-to-cell fusion events are further distinguished by the receiving cellular membrane, since the requirement for endocytosis implies that VZ virions fuse with the membrane delimiting an intracellular compartment and not with the plasmalemma.
Further work is needed to characterize fully the cellular requirements for VZ virion entry and to develop an understanding of virion-to-cell fusion at the molecular level. In contrast to the striking divergence of cellular receptor usage among the human alphaherpesviruses, our findings suggest similarities in the downstream entry mechanism that likely reflect conservation of major elements of the viral fusogenic machinery. An important corollary of this conservation is that potential therapeutic targets for fusion inhibitors may be shared between HSV and VZV.
Acknowledgments
We are grateful to Wanda Setlik for expert technical assistance in electron microscopy.
This work was supported by NIH grants AI27187 and AI24021.
Footnotes
Published ahead of print on 9 May 2007.
REFERENCES
- 1.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 77:7978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, F. C., J. C. Whitbeck, M. Ponce de Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 77:9542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, D. A. 2006. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology 21:430-439. [DOI] [PubMed] [Google Scholar]
- 6.Chandran, K., N. J. Sullivan, U. Felbor, S. P. Whelan, and J. M. Cunningham. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chazal, N., and D. Gerlier. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67:226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, J. J., A. A. Gershon, Z. S. Li, O. Lungu, and M. D. Gershon. 2003. Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J. Med. Virol. 70(Suppl. 1):S71-S78. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J. J., Z. Zhu, A. A. Gershon, and M. D. Gershon. 2004. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell 119:915-926. [DOI] [PubMed] [Google Scholar]
- 10.Clement, C., V. Tiwari, P. M. Scanlan, T. Valyi-Nagy, B. Y. Yue, and D. Shukla. 2006. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J. Cell Biol. 174:1009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook, M. L., and J. G. Stevens. 1968. Labile coat: reason for noninfectious cell-free varicella-zoster virus in culture. J. Virol. 2:1458-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahms, N. M., P. Lobel, and S. Kornfeld. 1989. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J. Biol. Chem. 264:12115-12118. [PubMed] [Google Scholar]
- 13.Fielding, C. J., and P. E. Fielding. 1997. Intracellular cholesterol transport. J. Lipid Res. 38:1503-1521. [PubMed] [Google Scholar]
- 14.Finnen, R. L., K. R. Mizokami, B. W. Banfield, G. Y. Cai, S. A. Simpson, L. I. Pizer, and M. J. Levin. 2006. Postentry events are responsible for restriction of productive varicella-zoster virus infection in Chinese hamster ovary cells. J. Virol. 80:10325-10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabel, C. A., L. Dubey, S. P. Steinberg, D. Sherman, M. D. Gershon, and A. A. Gershon. 1989. Varicella-zoster virus glycoprotein oligosaccharides are phosphorylated during posttranslational maturation. J. Virol. 63:4264-4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaffet, P., A. T. Jones, and M. J. Clague. 1997. Inhibition of calcium-independent mannose 6-phosphate receptor incorporation into trans-Golgi network-derived clathrin-coated vesicles by wortmannin. J. Biol. Chem. 272:24170-24175. [DOI] [PubMed] [Google Scholar]
- 17.Gianni, T., G. Campadelli-Fiume, and L. Menotti. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 78:12268-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grose, C., M. Ye, and J. Padilla. 2000. Pathogenesis of primary infection, p. 105-122. In A. M. Arvin and A. A. Gershon (ed.), Varicella-zoster virus: virology and clinical management, 1st ed. Cambridge University Press, Cambridge, United Kingdom.
- 19.Hamel, F. G., M. J. Mahoney, and W. C. Duckworth. 1991. Degradation of intraendosomal insulin by insulin-degrading enzyme without acidification. Diabetes 40:436-443. [DOI] [PubMed] [Google Scholar]
- 20.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 21.Huang, I. C., B. J. Bosch, W. Li, M. Farzan, P. M. Rottier, and H. Choe. 2006. SARS-CoV, but not HCoV-NL63, utilizes cathepsins to infect cells: viral entry. Adv. Exp. Med. Biol. 581:335-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida-Yamamoto, A., M. Simon, M. Kishibe, Y. Miyauchi, H. Takahashi, S. Yoshida, T. J. O'Brien, G. Serre, and H. Iizuka. 2004. Epidermal lamellar granules transport different cargoes as distinct aggregates. J. Investig. Dermatol. 122:1137-1144. [DOI] [PubMed] [Google Scholar]
- 23.Katzman, R. B., and R. Longnecker. 2003. Cholesterol-dependent infection of Burkitt's lymphoma cell lines by Epstein-Barr virus. J. Gen. Virol. 84:2987-2992. [DOI] [PubMed] [Google Scholar]
- 24.Kornfeld, S. 1987. Trafficking of lysosomal enzymes. FASEB J. 1:462-468. [DOI] [PubMed] [Google Scholar]
- 25.Li, Q., M. A. Ali, and J. I. Cohen. 2006. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell 127:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, W., M. J. Moore, N. Vasilieva, J. Sui, S. K. Wong, M. A. Berne, M. Somasundaran, J. L. Sullivan, K. Luzuriaga, T. C. Greenough, H. Choe, and M. Farzan. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, S. X., W. G. Mallet, A. Y. Huang, and F. R. Maxfield. 2004. Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes. Mol. Biol. Cell 15:721-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch, J. A., A. M. George, P. B. Eisenhauer, K. Conn, W. Gao, I. Carreras, J. M. Wells, A. McKee, M. D. Ullman, and R. E. Fine. 2006. Insulin degrading enzyme is localized predominantly at the cell surface of polarized and unpolarized human cerebrovascular endothelial cell cultures. J. Neurosci. Res. 83:1262-1270. [DOI] [PubMed] [Google Scholar]
- 29.Maresova, L., T. J. Pasieka, and C. Grose. 2001. Varicella-zoster virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression. J. Virol. 75:9483-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marjomäki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matlin, K. S., H. Reggio, A. Helenius, and K. Simons. 1981. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 91:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milne, R. S., A. V. Nicola, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 79:6655-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miwako, I., A. Yamamoto, T. Kitamura, K. Nagayama, and M. Ohashi. 2001. Cholesterol requirement for cation-independent mannose 6-phosphate receptor exit from multivesicular late endosomes to the Golgi. J. Cell Sci. 114:1765-1776. [DOI] [PubMed] [Google Scholar]
- 34.Mo, C., J. Lee, M. Sommer, C. Grose, and A. M. Arvin. 2002. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology 304:176-186. [DOI] [PubMed] [Google Scholar]
- 35.Nicola, A. V., J. Hou, E. O. Major, and S. E. Straus. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 79:7609-7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson, J. K., G. A. Bishop, and C. Grose. 1997. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J. Virol. 71:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 39.Pietiäinen, V., V. Marjomaki, P. Upla, L. Pelkmans, A. Helenius, and T. Hyypia. 2004. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol. Biol. Cell 15:4911-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohrbough, J., and K. Broadie. 2005. Lipid regulation of the synaptic vesicle cycle. Nat. Rev. Neurosci. 6:139-150. [DOI] [PubMed] [Google Scholar]
- 41.Salaün, C., D. J. James, and L. H. Chamberlain. 2004. Lipid rafts and the regulation of exocytosis. Traffic 5:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schornberg, K., S. Matsuyama, K. Kabsch, S. Delos, A. Bouton, and J. White. 2006. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J. Virol. 80:4174-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 44.Subramanian, R. P., and R. J. Geraghty. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. USA 104:2903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor, S. L., P. R. Kinchington, A. Brooks, and J. F. Moffat. 2004. Roscovitine, a cyclin-dependent kinase inhibitor, prevents replication of varicella-zoster virus. J. Virol. 78:2853-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weller, T. H., H. M. Witton, and E. J. Bell. 1958. The etiologic agents of varicella and herpes zoster; isolation, propagation, and cultural characteristics in vitro. J. Exp. Med. 108:843-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wittels, M., and P. G. Spear. 1991. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 18:271-290. [DOI] [PubMed] [Google Scholar]
- 49.Yi, L., J. Fang, N. Isik, J. Chim, and T. Jin. 2006. HIV gp120-induced interaction between CD4 and CCR5 requires cholesterol-rich microenvironments revealed by live cell fluorescence resonance energy transfer imaging. J. Biol. Chem. 281:35446-35453. [DOI] [PubMed] [Google Scholar]
- 50.Zhu, Z., M. D. Gershon, R. Ambron, C. Gabel, and A. A. Gershon. 1995. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc. Natl. Acad. Sci. USA 92:3546-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]