Abstract
Dendritic cells (DCs) act as a portal for invasion by human immunodeficiency virus type-1 (HIV-1). Here, we investigated whether virion-incorporated host cell membrane proteins can affect virus replication in DC-T-cell cocultures. Using isogenic viruses either devoid of or bearing host-derived leukocyte function-associated antigen 1 (LFA-1), we showed that HIV-1 production is augmented when LFA-1-bearing virions are used compared to that for viral entities lacking this adhesion molecule. This phenomenon was observed in immature monocyte-derived DCs (IM-MDDCs) only and not in DCs displaying a mature phenotype. The increase is not due to higher virus production in responder CD4+ T cells but rather is linked with a more important productive infection of IM-MDDCs. We provided evidence that virus-associated host LFA-1 molecules do not affect a late event in the HIV-1 life cycle but rather exert an effect on an early step in virus replication. We demonstrated that the enhancement of productive infection of IM-MDDCs that is conferred by virus-anchored host LFA-1 involves the protein kinase A (PKA) and PKC signal transduction pathways. The biological significance of this phenomenon was established by performing experiments with virus stocks produced in primary human cells and anti-LFA-1 antibodies. Together, our results indicate that the association between some virus-bound host proteins and their natural cognate ligands can modulate de novo HIV-1 production by IM-MDDCs. Therefore, the additional interactions between virus-bound host cell membrane constituents and counter receptors on the surfaces of DCs can influence HIV-1 replication in IM-MDDC-T-cell cocultures.
Dendritic cells (DCs) play a pivotal role in the establishment and dissemination of human immunodeficiency virus type 1 (HIV-1) infection as well as in the development of a virus-specific immune response (58). The involvement of this cell type in the overall pathogenesis of the disease was described soon after the discovery of this retrovirus, but its exact contribution remains elusive (58). The mechanism by which HIV-1 is transmitted from the mucosa to CD4+ T cells is not entirely understood. Three possibilities have been proposed to explain how mucosal DCs come in contact with HIV-1. The first proposes a selective transcytosis of R5-tropic virions through the mucosal cells (8). The second suggests that the initial transmission of R5 virions can occur by infection of mucosal epithelial cells via the galactosylceramide and/or CCR5 receptors (50). The third alternative promotes the idea that DCs present in the submucosal tissue capture HIV-1 particles with their dendrites (61, 62). In all three pathways, the crucial events in both virus entry and transmission are the binding and capture of viruses by specific cell surface receptors. It is now well established that internalization of HIV-1 into target cells requires the formation of a fusion pore resulting from a high-affinity interaction between envelope spike glycoproteins (i.e., gp120) and a complex consisting of the CD4 receptor and a seven-transmembrane coreceptor (e.g., CXCR4 or CCR5) (15). However, it is becoming clear that the initial attachment step is more complex than first thought, since it is modulated by a number of interactions between the viral entity and the target cell surface (15, 75). The most convincing example is the association between the gp120 oligosaccharides and different C-type lectin receptors, such as mannose receptor (CD206), langerin (CD207), and DC-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN; also called CD209), which are all expressed on DCs. This association results in the capture of HIV-1 and its subsequent transmission to CD4+ T cells, preferentially in a trans-infectious mode (3, 11, 19, 28, 34). Following its capture by C-type lectin receptors, a virus particle is rapidly taken up into endolysosomal vacuoles, where it remains infectious for 1 to 3 days (74), which is approximately the time required for the migration of DCs to the draining lymph nodes (42). When these DCs encounter CD4+ T cells, the internalized viruses rapidly relocate to the DC-T-cell contact zone, the local region between the two cell types where viruses concentrate, referred to as the virological synapse (4, 49). Such a close encounter between cells and viruses leads to efficient transfer, subversion of the immune system, and virus production in both cell types, particularly in responder CD4+ T lymphocytes. The reported low levels of CD4, CXCR4, and CCR5 on DCs are probably responsible for their weaker susceptibility to productive HIV-1 infection in vitro compared to that of CD4+ T cells (59, 73). Interestingly, a recent work has shown that HIV-1 transfer from DCs to CD4+ T cells occurs in two distinct phases (74). In the initial transfer phase (i.e., early transfer) viruses located within endosomal compartments in DCs are transported to the DC-T-cell synapse as described above. This is followed by a second phase (i.e., late transfer) that is dependent on productive infection of DCs and eventual transfer of progeny virus to CD4+ T cells.
The process of HIV-1 attachment to DCs could also be influenced by costimulatory molecules such as ICAM-1 and LFA-1 (leukocyte function-associated antigen 1). These adhesion molecules, which are present on both virus-infected cells and the viral entity itself (6, 7, 71), actively participate in the regulation of immune responses as well as in various aspects of HIV-1 pathogenesis. For example, their expression levels are increased in advanced HIV-1-associated diseases (57) compared to those of the asymptomatic phase (1). The interaction between ICAM-1 and LFA-1 can affect the progression of the disease in several ways. It has been shown to enhance cell-to-cell virus transmission, augment virus production (22, 40, 43), facilitate syncytium formation (5, 38, 39), mediate a higher depletion of CD4+ T cells, and favor a more-dramatic destruction of the architecture of lymphoid organs (38, 56). Moreover, the interaction between ICAM-1 and LFA-1 largely contributes to the increased transmission of HIV-1 by DCs (39, 40, 64), and it plays a role in HIV-1 attachment to follicular DCs (25).
Previous studies have clearly established that HIV-1 incorporates several host cell membrane constituents, including the adhesion glycoproteins ICAM-1 and LFA-1. It has been demonstrated that such host components remain functional when located on viral entities (6, 10, 12, 24) and have profound effects on specific steps of the virus life cycle (13, 54, 55). In fact, there is evidence that virus biology is affected upon incorporation of host-derived LFA-1 (41, 44). Although a previous study has revealed that HIV-1 capture and transfer by DCs are not affected by virus-anchored host ICAM-1 (9), the contribution of other virus-bound host molecules to the complex interplay between DCs and HIV-1 remains to be scrutinized. In contrast to what was observed with virus-associated host ICAM-1, we demonstrated here for the first time that HIV-1 production is increased in immature monocyte-derived DC (IM-MDDC)-T-cell cocultures when LFA-1-bearing virions are used for infection. The mechanism responsible for this phenomenon is linked with more-efficient HIV-1 infection of IM-MDDCs in a cis mode. Experiments performed with specific pharmacologic inhibitors indicate that the more-important productive infection of IM-MDDCs by LFA-1-bearing viruses seems to be dependent on cellular signal transduction pathways modulated by protein kinase A (PKA) and PKC. The contribution of the LFA-1-mediated effect as well as the physiological significance of our findings were proven in studies carried out in the presence of anti-LFA-1 antibodies in combination with progeny virus produced in natural cellular reservoirs.
MATERIALS AND METHODS
Reagents.
3′-Azido-3′-deoxythymidine (AZT), phytohemagglutinin-L, lipopolysaccharide (LPS), and LPS-free dimethyl sulfoxide were purchased from Sigma (St. Louis, MO). H89 and Ro-318220 were obtained from Calbiochem (San Diego, CA). Recombinant human interleukin-2 (IL-2) was obtained through the AIDS Repository Reagent Program (Germantown, MD). IL-4 and gamma interferon were purchased from R&D Systems (Minneapolis, MN), and granulocyte-macrophage colony-stimulating factor (GM-CSF) was a generous gift from Cangene (Winnipeg, Manitoba, Canada). The complete culture medium consisted of RPMI 1640 supplemented with 10% fetal bovine serum, penicillin G (100 U/ml), streptomycin (100 U/ml), and glutamine (2 mM), which were all purchased from Wisent (St-Bruno, Quebec, Canada).
Antibodies.
The monoclonal anti-ICAM-1 antibody RR1/1.1.1 was kindly supplied by R. Rothlein (Boehringer Ingelheim, Ridgefield, CO) (63). Anti-p24 (clone 31-90-25), anti-CD3 (clone OKT3; specific for the zeta chain), anti-CD18 (clone TS1/18.1), anti-CD11a (clone TS1/22.1), and anti-HLA-DR (clone L243) hybridomas were obtained from the American Type Culture Collection (Manassas, VA). These antibodies were purified by using MAbTrap protein affinity columns according to the manufacturer's instructions (Pharmacia Technology AB, Uppsala, Sweden). Anti-CD86 (clone BU-63) was supplied by D. L. Hardie (University of Birmingham, Birmingham, United Kingdom). Commercial anti-DC-SIGN antibodies were obtained from eBioscience (San Diego, CA) and R&D Systems. Anti-CD19 (clone LT19) and anti-CD14 (clone MEM-18) were obtained from EXBIO Praha (Prague, Czech Republic), and anti-CD83 (clone HP15E) was purchased from Research Diagnostics (Concord, MA). Phycoerythrin-conjugated goat anti-mouse immunoglobulin G (IgG) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Cells.
Human DCs were generated from monocytes. Briefly, peripheral blood was obtained from normal healthy donors, and peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation on a Ficoll-Hypaque density gradient. Next, CD14+ cells were isolated by using a monocyte-positive selection kit according to the manufacturer's instructions (MACS CD14 microbeads; StemCell Technologies Inc., Vancouver, British Columbia, Canada). CD14+ cells were cultured in six-well plates at a density of 106 cells/ml. To generate IM-MDDCs, purified monocytes were cultured in complete culture medium that was supplemented every other day with GM-CSF (1,000 U/ml) and IL-4 (200 U/ml) for 7 days. The maturation of IM-MDDCs was induced on the fifth day by culturing them for 48 h with the above-described cytokines supplemented with gamma interferon (1,000 U/ml) and LPS (100 ng/ml). The final phenotype of IM-MDDCs and mature monocyte-derived DCs (M-MDDCs) was monitored by flow cytometry (data not shown). IM-MDDCs express HLA-DR, CD86, DC-SIGN, CD1a, and low levels of CD14, whereas M-MDDCs express CD83 and high levels of ICAM-1, HLA-DR, and CD86 but lower levels of DC-SIGN and CD14 than IM-MDDCs. The expression of CD3 and CD19 was measured to assess contamination with T and B cells, respectively. Adequate differentiation from monocytes to IM-MDDCs was based on the loss of CD14 and acquisition of CD1a and DC-SIGN. Autologous CD4+ T cells were isolated using a negative selection kit according to the manufacturer's instructions (StemCell Technologies, Inc.). These cells were activated with the mitogenic agent phytohemagglutinin-L (1 μg/ml) and maintained in complete culture medium supplemented with IL-2 (30 U/ml) at a density of 2 × 106 cells/ml.
Production of virus stocks.
Virions were produced by transient transfection in human embryonic kidney 293T cells as previously described (12). Briefly, the cells were transfected using a calcium phosphate coprecipitation kit according to the manufacturer's instructions (CalPhos mammalian transfection kit; Clontech Laboratories Inc., Palo Alto, CA). Plasmids used in this study include pNL4-3 (X4-tropic), pJR-CSF (R5-tropic), and pNL4-3balenv (R5-tropic). The pNL4-3balenv vector was generated by replacing the env gene of the T-tropic HIV-1 strain NL4-3 with that of the macrophage-tropic HIV-1 Bal strain, resulting in an infectious molecular clone with macrophage-tropic properties (kindly provided by R. Pomerantz, Thomas Jefferson University, Philadelphia, PA) (20). The NL4-3 and JR-CSF molecular constructs were obtained through the AIDS Repository Reagent Program. The mammalian expression vectors coding for human ICAM-1 (i.e., pCD1.8) or LFA-1 (i.e., pCDL1 for the αL/CD11a chain and pCDB1 for the β2/CD18 integrin subunit) were kindly provided by T. A. Springer (Harvard Medical School, Boston, MA). In some instances, virus stocks were made after acute infections of mitogen-treated PBMCs with NL4-3balenv. The virus-containing supernatants were filtered through a 0.22-μm cellulose acetate syringe filter and normalized for virion content by using a sensitive in-house, double-antibody sandwich enzyme-linked immunosorbent assay specific for the major viral core p24Gag protein (10). The physical presence of host-encoded LFA-1 and ICAM-1 on progeny virus was evaluated by an immunocapture assay as previously described (data not shown) (48). All virus preparations underwent only one freeze-thaw cycle before being used in subsequent experiments.
DC-T-cell cocultures.
DCs (IM-MDDCs and M-MDDCs) were first pulsed for 60 min at 37°C with isogenic virus particles either lacking or bearing host-derived LFA-1 molecules. In some experiments, DCs were pulsed with progeny virus originating from acutely infected PBMCs. Next, the virus-cell mixture was washed three times with phosphate-buffered saline (PBS) to remove untrapped virions. DCs were cocultured with autologous activated CD4+ T cells at a ratio of 1 to 3 in complete culture medium supplemented with IL-2 (30 U/ml) in 96-well plates in a final volume of 200 μl (except for that for Fig. 1, from which IL-2 was omitted). Every 2 days, half of the medium was removed and fresh medium was added to the culture. Virus replication was estimated by measuring p24Gag levels in cell-free culture supernatants.
FIG. 1.
HIV-1 production in DC-T-cell cocultures. IM-MDDCs (A) and M-MDDCs (B) (1 × 105 cells) were pulsed for 60 min with similar concentrations of isogenic JR-CSF either lacking or bearing host-derived LFA-1 (2 ng of p24Gag). After three washes with PBS, the cell-virus mixture was divided into three aliquots and cocultured with autologous CD4+ T cells at a DC-to-T-cell ratio of 1:3. Virus production was determined by measuring p24Gag levels in the culture supernatants at the indicated time points (D.P.I., day postinfection). The data shown are the means ± the standard deviations (SDs) of the results for triplicate samples and are representative of three independent experiments. *, P < 0.05; **, P < 0.01. A 2- to 10-fold increase in virus production at 4 days following initiation of the coculture was observed with LFA-1-bearing virions for the three donors tested.
Virus internalization assay.
DCs were pulsed for 60 min at 37°C with the studied isogenic virus stocks. The cells were then washed three times with PBS to remove untrapped virions. DCs were lysed with a hypotonic buffer as described previously (30), and the cell lysates were frozen at −20°C before the p24 contents were monitored. In some experiments, the extent of virus uptake was also quantified by flow cytometry. In brief, DCs pulsed with the tested virus stocks were incubated for 15 min at 4°C with a pool of human sera from 20 healthy donors to block Fc receptors. The cells were washed with PB buffer (PBS and 0.01% bovine serum albumin) before being incubated for 15 min at 4°C in the dark with a fluorescein isothiocyanate-tagged anti-DC-SIGN antibody (clone eB-h209; eBioscience, San Diego, CA). The cells were washed once, and the pellets were resuspended in 100 μl of a permeabilization/fixation solution (Cytofix/Cytoperm; BD PharMingen, San Diego, CA) and incubated for 20 min at 4°C in the dark. The cells were washed with a PBCP buffer (PBS, 0.01% bovine serum albumin, and 0.01% Cytoperm). The anti-p24 (clone 31-90-25) and IgG1 isotype-matched control antibodies were labeled with Alexa Fluor 546 according to the manufacturer's instructions (Molecular Probes, Eugene, OR) and diluted in the PBCP buffer before being added to the cells. The mixture was incubated for 30 min at 4°C. The cells were washed once more in the PBCP buffer before cytofluorometry analysis was performed (Coulter Epics XL).
Infection of IM-MDDCs and CD4+ T cells.
De novo virus production in IM-MDDCs was monitored by incubating the cells for 60 min at 37°C with concentrations of the studied virus stocks similar to those that were produced in either transiently transfected 293T cells or mitogen-stimulated PBMCs. After three washes with PBS, the cells were maintained in complete culture medium supplemented with GM-CSF (1,000 U/ml) and IL-4 (200 U/ml). Virus production was estimated by quantifying the p24Gag contents in the culture supernatants. In some studies, the percentage of IM-MDDCs productively infected with HIV-1 was monitored by estimating the number of cells positive for both cell surface DC-SIGN and intracellular p24 at 9 days postinfection. Briefly, IM-MDDCs were first labeled with a fluorescein isothiocyanate-tagged anti-DC-SIGN antibody (clone 120507 from R&D Systems) or an appropriate control antibody. Next, intracellular labeling was achieved by using a commercial monoclonal anti-p24 antibody (R-phycoerythrin-labeled KC57; Coulter Clone, Mississauga, Ontario, Canada) or an appropriate isotype-matched irrelevant control antibody. Finally, the cells were analyzed using a flow cytometer (Coulter Epics XL). Purified CD4+ T cells were incubated for 2 h with isogenic virus particles either lacking or bearing the studied host-derived molecule (i.e., LFA-1 or ICAM-1). After three washes with PBS, the cells were cultured in complete culture medium supplemented with IL-2 (30 U/ml) for 3 days, and virus production was estimated by assessing p24Gag levels in the culture supernatants.
Antibody-blocking assay.
For coculture studies and de novo virus production, NL4-3balenv particles produced in PBMCs or isogenic virions produced in 293T cells either lacking or bearing host-derived LFA-1 were first incubated for 15 min at 4°C with F(ab′)2 fragments of anti-CD18 (TS1/18.1) and/or anti-CD11a (TS1/22.1) antibodies. The F(ab′)2 fragments of an isotype-matched IgG1 antibody (i.e., anti-CD32; kindly provided by P. H. Naccache, CHUL Research Center) (29) were also used as controls. Next, the virus-antibody mixture was diluted in four volumes of RPMI medium before being incubated with IM-MDDCs for 60 min at 37°C. Finally, the pulsed IM-MDDCs were either cocultured with autologous CD4+ T cells or maintained in cultures as described above. Virus production was analyzed by measuring the p24Gag contents in the culture supernatants. In some experiments, the virus-antibody mixture was incubated with mitogen-stimulated CD4+ T cells, and virus production was assessed by measuring the p24 content in cell-free supernatants.
The F(ab′)2 fragments of anti-CD18, anti-CD11a, and anti-CD32 were prepared according to the manufacturer's instructions (Pierce, Rockford, IL). In brief, the antibodies were digested with ficin, and intact antibodies were eliminated by adding protein A and protein G beads. The integrity of the F(ab′)2 fragments was verified by assessing their ability to label intact human DCs, as determined by flow cytometry.
Real-time PCR.
The amount of integrated viral DNA was assessed with the real-time PCR approach described by Suzuki and colleagues (68). Briefly, the DNA in IM-MDDCs was extracted at day 12 postinfection with the QIAGEN DNeasy tissue kit according to the manufacturer's instructions. A first round of PCR was performed with an Alu sequence-specific sense primer (5′-TCC CAG CTA CTC GGG AGG CTG AGG-3′) (14) used in combination with an antisense HIV-1-specific primer (M661; 5′-CCT GCG TCG AGA GAT CTC CTC TG-3′, nucleotides 673 to 695). The cycling conditions of the first PCR consisted of a denaturation step (94°C for 3 min), followed by 22 cycles of denaturation (94°C for 30 s), annealing (66°C for 30 s), an extension (70°C for 10 min), and a final extension (72°C for 10 min). The first PCR products were diluted fivefold and subjected to a real-time PCR assay targeting the HIV-1 R/U5 region. The specific sense primer (M667; 5′-GGC TAA CTA GGG AAC CCA CTG C-3′, nucleotides 496 to 517) coupled to an antisense primer (AA55; 5′-CTG CTA GAG ATT TTC CAC ACT GAC-3′, nucleotides 612 to 635) was used with the fluorogenic probe TaqMan (FAM; 5′-TAG TGT GTG CCC GTC TGT TGT GTG AC-3′; BHQ-1) (Biosearch Technologies, Novato, CA). Next, PCR was performed using the Rotor-Gene 3000 four-channel multiplexing system (Corbett Research, Sydney, Australia). Cycling conditions included 40 denaturation cycles (95°C for 20 s) and an extension (60°C for 1 min). NL4-3 DNA was used for the standard curve (i.e., from 469 to 30,000 copies). HIV-1 standards contained 1 ng of DNA from uninfected cells as a carrier.
Statistical analysis.
The statistical significance of the differences between groups was determined by Student's nonpaired t test (two-tailed). Calculations were made with GraphPad Prism software. P values of less than 0.05 were considered statistically significant.
RESULTS
HIV-1 production in IM-MDDC-T-cell cocultures is augmented when LFA-1-bearing virions are used.
In an attempt to gain new information on the possible modulatory effect of virus-anchored host cell surface molecules on HIV-1 production in DC-T-cell cocultures, IM-MDDCs and M-MDDCs were pulsed with isogenic R5-tropic virions either lacking or bearing ICAM-1 or LFA-1. Virus production was not affected by the presence of host ICAM-1 on the surfaces of viruses (data not shown), which is in agreement with previous observations (9). However, HIV-1 production was enhanced when LFA-1-bearing virions were used compared to that for isogenic viruses devoid of host LFA-1 (Fig. 1A). Interestingly enough, a similar observation could not be made when studies were conducted with M-MDDCs (Fig. 1B) or X4-tropic virions (i.e., NL4-3) (data not shown). Therefore, the following series of investigations were performed exclusively with IM-MDDCs in combination with R5 viruses. The use of IM-MDDCs was also prompted by the idea that it is most likely the first cell type to come in contact with the virus, because these cells reside in mucosal tissues. Moreover, the following coculture experiments were performed in the presence of exogenous IL-2 to maintain the viability of autologous CD4+ T cells after HIV-1 infection. Although the observed differences are smaller at earlier time points in the presence of IL-2, this experimental strategy confirmed that virus production is statistically more important when infection is performed with LFA-1-bearing HIV-1 particles (data not shown).
Activated CD4+ T cells display comparable sensitivity to virions either lacking or bearing LFA-1.
The next studies were aimed at providing the mechanistic basis by which virus-associated LFA-1 can enhance HIV-1 production in IM-MDDC-T-cell cocultures. Previously published reports indicate that viruses bearing host-derived ICAM-1 are more infectious for CD4+ T cells than viruses lacking this adhesion molecule (10, 23, 24, 69). We examined whether part of the observed enhancement of HIV-1 replication with LFA-1-bearing virions could be due to a higher susceptibility of CD4+ T lymphocytes to such viral entities. In this set of experiments, viruses either lacking or bearing ICAM-1 were used as controls. As expected, viruses carrying host-derived ICAM-1 on their surfaces were found to be more infectious than isogenic viruses lacking ICAM-1 (Fig. 2). In contrast to what was observed in IM-MDDC-T-cell cocultures, virus production in CD4+ T cells was comparable for virions lacking or bearing LFA-1.
FIG. 2.
Virus replication in activated CD4+ T cells. CD4+ T cells (3 × 105 cells) were exposed for 2 h to similar concentrations of isogenic NL4-3balenv, JR-CSF, or NL4-3 either lacking or bearing host-derived ICAM-1 or LFA-1 (15 ng of p24Gag). Virus production was determined by measuring p24Gag levels in the culture supernatants at day 3 postinfection. The data shown are the means ± the SDs of the results for triplicate samples and are representative of four independent experiments. ***, P < 0.001.
Infection of IM-MDDCs in cis was promoted when LFA-1-bearing viruses were used.
To shed light on the putative mechanism(s) by which virus-associated host LFA-1 can enhance replication of R5 virions in IM-MDDCs-T-cell cocultures, we initially measured the overall uptake of the tested virus preparations by IM-MDDCs. To this end, IM-MDDCs were first pulsed with viruses either lacking or bearing host-derived LFA-1 before being subjected to a hypotonic lysis buffer. Note that such studies were carried out with NL4-3balenv, which is a highly infectious R5-tropic viral strain (unpublished observations). Measurements of the intracellular p24Gag contents revealed that LFA-1-bearing virions and viruses lacking host-encoded LFA-1 were internalized in IM-MDDCs with comparable efficiencies (Fig. 3A). These findings were confirmed by double-staining studies of IM-MDDCs at an early time point after they were pulsed with HIV-1 (i.e., 1 h). Indeed, data from flow cytometry analyses indicated that a comparable percentage of DC-SIGN-expressing IM-MDDCs was positive for intracellular p24 staining following incubation with isogenic viruses either lacking (i.e., 3.8%) or bearing (i.e., 2.8%) host LFA-1 (Fig. 3B).
FIG. 3.
Virus uptake by IM-MDDCs. IM-MDDCs (2 × 105 cells) were pulsed for 60 min with identical concentrations of isogenic JR-CSF virions either lacking or bearing host-derived LFA-1 (20 ng of p24Gag). After three washes with PBS, the cells were subjected to a hypotonic lysis buffer or analyzed by flow cytometry. The uptake of virions by IM-MDDCs was estimated by measuring the p24Gag content in cell lysates (A) or quantifying the percentage of IM-MDDCs positive for both cell surface DC-SIGN and intracellular p24 (B). The data shown are the means ± the SDs of the results for triplicate samples, and these results are representative of at least five independent experiments for panel A and two for panel B.
Previously published data revealed that HIV-1 is transferred from DCs to CD4+ T cells via a process involving two distinct phases (26, 74). An initial transfer phase (i.e., early transfer) occurs when the virus located within endosomal compartments in IM-MDDCs is transported to the DC-T-cell synapse and then transmitted directly to CD4+ T cells (Fig. 4A). This event is followed by a second kinetic phase (i.e., late transfer) that is dependent on productive virus infection of IM-MDDCs and eventual transfer of progeny virus to CD4+ T cells. To define whether the presence of host LFA-1 on HIV-1 particles affects the early and/or late transfer phase, IM-MDDCs were treated with the antiretroviral drug AZT during pulsing with R5 virions. This treatment blocks the late transfer phase (i.e., productive virus infection) without affecting the early transfer mode. As expected, LFA-1-bearing viruses were transferred in greater amounts than isogenic virions lacking host LFA-1 in coculture studies performed in the absence of AZT (Fig. 4B). However, the addition of AZT resulted in a comparable transmission level in the studied virus preparations, suggesting that IM-MDDCs are more efficiently infected with LFA-1-bearing virions than with viruses lacking host-derived LFA-1. Similar observations were made in experiments carried out with efavirenz, which is a nonnucleoside reverse transcriptase inhibitor (data not shown). To corroborate that the late-phase transfer is indeed the process that is modulated by the insertion of host LFA-1 within the virus envelope, IM-MDDCs were cocultured with autologous CD4+ T cells 5 days following pulsing of IM-MDDCs with viruses. This is based on the previous demonstration that endosome-associated virions are not present within IM-MDDCs for more than 3 days after virus pulsing (74). Data from this set of experiments confirmed that virus production is more important in IM-MDDC-T-cell cocultures following infection with virions carrying host-derived LFA-1 on their surfaces than in isogenic viruses lacking LFA-1 (Fig. 4C). This phenomenon was also observed when another R5-tropic isolate of HIV-1 (i.e., JR-CSF) was used (data not shown).
FIG. 4.
Early and late transfer of HIV-1 particles by IM-MDDCs. (A) Schematic representation showing early- and late-transfer processes of HIV-1 by DCs. iDC, immature DCs. (B) IM-MDDCs (1 × 105 cells) were either left untreated (empty bars) or treated with the antiviral agent AZT (10 μM; filled bars) for 10 min. The cells were then pulsed for 60 min with similar concentrations of isogenic NL4-3balenv either lacking or bearing host-encoded LFA-1 (10 ng p24Gag). After three washes with PBS, the cell-virus mixture was divided into three aliquots and cocultured with autologous CD4+ T cells at a DC-to-T-cell ratio of 1:3. Virus production was determined by measuring p24Gag levels in the culture supernatants at day 3 following initiation of the coculture. (C) The late transfer phase was assayed as follows. First, IM-MDDCs (5 × 105 cells) were pulsed with similar concentrations of isogenic NL4-3balenv either lacking or bearing host-encoded LFA-1 (50 ng p24Gag). Next, 5 days later, IM-MDDCs were cocultured with autologous CD4+ T cells at a DC-to-T-cell ratio of 1:3. Virus production was determined by measuring p24Gag levels in the culture supernatants at day 6 following initiation of the coculture. The data shown are the means ± the SDs of the results for triplicate samples and are representative of three independent experiments. *, P < 0.05; **, P < 0.01.
To substantiate what seems to be a more-important productive infection of IM-MDDCs with LFA-1-bearing HIV-1 particles, IM-MDDCs were infected with NL4-3balenv particles either lacking or bearing host-encoded LFA-1 and de novo virus production by this cell type was estimated by monitoring the extracellular p24 contents. The results depicted in Fig. 5A indicate that more-important de novo virus production was observed when IM-MDDCs were inoculated with LFA-1-bearing virions than with viruses devoid of host-derived LFA-1. Measurements of integrated virus DNA copies provided further evidence that infection in cis of IM-MDDCs was increased when virus particles carrying LFA-1 on their surfaces were used (Fig. 5B). Finally, to eliminate the possibility that the observed increase in virus production might be due to contaminating cells in our culture system (e.g., undifferentiated monocytes or macrophages), we estimated the number of IM-MDDCs productively infected with HIV-1 by quantifying the percentage of cells positive for both cell surface DC-SIGN and intracellular p24 at 9 days postinfection. As depicted in Fig. 5C, infection of IM-MDDCs with LFA-1-bearing virions resulted in close to 4% double-positive cells compared to less than 1% when infection was carried out with isogenic virions lacking host-derived LFA-1.
FIG. 5.
Virus replication in IM-MDDCs. IM-MDDCs (1 × 106 cells) were pulsed for 60 min with similar concentrations of isogenic NL4-3balenv either lacking or bearing host-derived LFA-1 (100 ng of p24Gag). After three washes with PBS, the cells were maintained in complete culture medium supplemented with GM-CSF and IL-4. (A) Virus production was assessed by measuring p24Gag levels in the culture supernatants at the indicated time points. D.P.I., day postinfection. (B) The amounts of integrated viral DNA were quantified at day 12 after virus exposure with real-time PCR assays, as described in Materials and Methods. The data shown represent the means ± the SDs of the results for duplicate samples and are representative of at least four independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) The percentage of IM-MDDCs positive for both cell surface DC-SIGN and intracellular p24 was quantified by flow cytometry at 9 days postinfection.
A higher level of productive infection of IM-MDDCs through the PKA and PKC signaling pathways was observed with LFA-1-bearing virions.
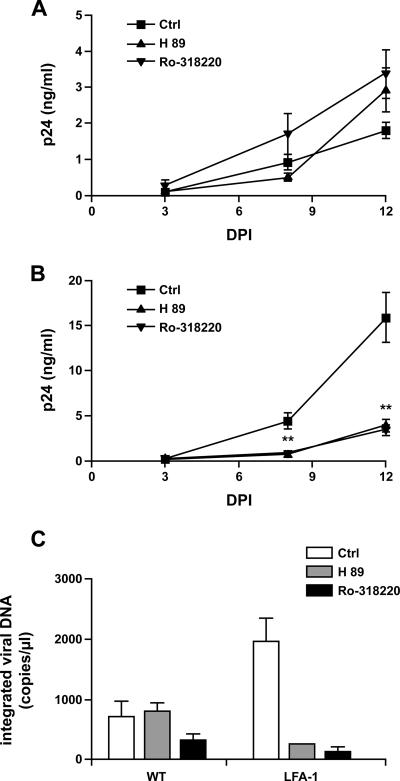
Tyrosine kinases (i.e., Src and Syk) and serine/threonine kinases (i.e., PKA and PKC) play essential roles in modulating signaling cascades in several cellular processes, such as antigen uptake, formation of the immunological synapse, and ICAM-1 internalization (17, 45, 52, 65, 67, 70). We explored the contribution of such signal transducers to the observed increase in productive infection of IM-MDDCs by pretreating cells with several pharmacological kinase inhibitors before exposing them to the virus. Virus production in IM-MDDCs following infection with virions bearing host-derived LFA-1 was not affected by the Src tyrosine kinase inhibitor PP2 or following inhibition of Syk by piceatannol (data not shown). However, although pretreatment of IM-MDDCs with the PKA inhibitor H89 or the PKC inhibitor Ro-318220 had no significant effect on the replication of virions lacking LFA-1 (Fig. 6A), replication of LFA-1-bearing viruses was markedly reduced upon pretreatment of IM-MDDCs with these two pharmacological agents (Fig. 6B). Comparable findings were made when the amounts of integrated viral DNA copies were measured (Fig. 6C). These findings suggest that the PKA- and PKC-mediated signal transduction pathways are key elements in the more-important productive HIV-1 infection in IM-MDDCs that is conferred by host-derived LFA-1.
FIG. 6.
Effects of PKA and PKC inhibitors on virus replication in IM-MDDCs. IM-MDDCs (1 × 106 cells) were either left untreated or treated with the PKA inhibitor H89 (30 μM) or the PKC inhibitor Ro-318220 (1 μM) for 10 min. Next, IM-MDDCs were pulsed for 60 min with similar concentrations of isogenic NL4-3balenv either lacking (A) or bearing (B) host-derived LFA-1 (100 ng of p24Gag). After three washes with PBS, the cells were cultured in complete culture medium supplemented with GM-CSF and IL-4. Virus production was determined by measuring p24Gag levels in the culture supernatants at the indicated time points. The amount of integrated viral DNA was quantified at day 12 after virus exposure with real-time PCR assays, as described in Materials and Methods (C). The data shown are the means ± the SDs of the results for triplicate samples and are representative of at least four independent experiments. **, P < 0.01; DPI, day postinfection.
The LFA-1-mediated effect was specific and still present when virus produced in a natural cellular reservoir was used.
The specificity of the LFA-1-ICAM-1 interaction in the observed phenomenon was addressed by performing coculture experiments with isogenic NL4-3balenv particles either lacking or bearing host LFA-1 in the presence of an antibody that can block interactions between LFA-1 and ICAM-1 (i.e., anti-CD11a). The data illustrated in Fig. 7 demonstrate that virus production in IM-MDDC-T-cell cocultures was reduced when LFA-1-bearing virions and the anti-CD11a antibody were used, whereas replication of viruses lacking host-derived LFA-1 was minimally affected.
FIG. 7.
Specificity of the LFA-1-mediated effect on virus production in DC-T-cell cocultures. NL4-3balenv (10 ng of p24Gag) either lacking or bearing host-derived LFA-1 was produced in 293T cells. Virus stocks were first incubated for 15 min at 4°C with the F(ab′)2 fragments of an anti-CD11a antibody (0.5 μg). The virus-antibody mixture was diluted in four volumes of RPMI medium before being incubated with IM-MDDCs (1 × 105 cells) for 60 min. After three washes with PBS, IM-MDDCs were cocultured with autologous CD4+ T cells at a DC-to-T-cell ratio of 1:3. Virus production was evaluated at day 2 by measuring p24Gag levels. The data shown are the means ± the SDs of the results for triplicate samples and are representative of at least two independent experiments. **, P < 0.01.
We addressed the biological significance of the observed phenomenon by performing additional coculture and infection studies with viruses produced in primary human cells in combination with blocking antibodies specific for the LFA-1 subunits (i.e., CD18 and CD11a). This task was achieved by testing NL4-3balenv particles amplified in acutely-infected PBMCs. The results shown in Fig. 8A indicate that HIV-1 replication in IM-MDDC-T-cell cocultures was reduced in the presence of the tested anti-LFA-1 antibodies but was not affected by a control antibody. Moreover, de novo virus production in IM-MDDCs was also reduced upon treatment with anti-CD18- and anti-CD11a antibodies (Fig. 8B), whereas an isotype-matched control antibody had no comparable effect. It is important to note that all experiments with anti-LFA-1-blocking antibodies were performed with the F(ab′)2 fragments of anti-CD18 or anti-CD11a to avoid any nonspecific effects mediated by the binding of antibodies to Fc receptors, which are highly expressed on IM-MDDCs. In some experiments, mitogen-stimulated CD4+ T cells were exposed to HIV-1 particles produced in either PBMCs or transiently transfected 293T cells in the absence or presence of anti-LFA-1 antibodies. The anti-LFA-1 antibodies had no effect whatsoever on the replication of the tested virus stocks in CD4+ T cells (data not shown). This suggests that the anti-LFA-1 antibodies did not induce the formation of clumps made of virus particles and were most likely acting by blocking the normal interaction between virus-anchored host LFA-1 and its normal counter ligand (i.e., ICAM-1) on the surfaces of IM-MDDCs.
FIG. 8.
Biological significance of the LFA-1-mediated effect. NL4-3balenv (10 ng of p24Gag for panel A and 100 ng of p24Gag for panel B) produced in PBMCs was first incubated for 15 min at 4°C with the F(ab′)2 fragments from the listed antibodies (0.5 μg each in panel A and 5 μg each in panel B). The virus-antibody mixture was diluted in four volumes of RPMI medium before being incubated with IM-MDDCs (1 × 105 cells in panel A and 1 × 106 cells in panel B) for 60 min. After three washes with PBS, IM-MDDCs were either divided into three aliquots and cocultured with autologous CD4+ T cells at a DC-to-T-cell ratio of 1:3 (A) or cultured in complete culture medium supplemented with GM-CSF and IL-4 (B). Virus production was evaluated at either day 2 (A) or day 8 (B) by measuring p24Gag levels. The data shown are the means ± the SDs of the results for triplicate samples and are representative of at least two independent experiments. *, P < 0.05; **, P < 0.01.
DISCUSSION
It is now well accepted that interactions between DCs and HIV-1 are complex and multifactorial. For example, they involve molecular interactions between gp120 and various cell surface constituents, including CD4, DC-SIGN, mannose receptor, heparan sulfate proteoglycans, and possibly other still-unknown receptors. Previous studies have clearly established that HIV-1 incorporates several functional host cell membrane proteins, including cell adhesion molecules, that can potentially have profound effects on some specific steps of the virus life cycle, such as attachment and entry (5, 12, 24, 44, 69). Even though a previous work revealed that adhesion molecules play an important role in achieving a very successful HIV-1 transmission from DCs to autologous CD4+ T cells (72), there is still minimal information on the contribution of virus-associated host molecules to this process. We provided evidence in this study that greater amounts of virus were produced by IM-MDDC-T-cell cocultures when infection was allowed to proceed with LFA-1-bearing virions than with isogenic virions devoid of host-derived LFA-1. The mechanism underlying this increased HIV-1 replication is not linked with higher susceptibility of CD4+ T cells to infection with LFA-1-bearing viruses but rather is due to a more-important productive infection of IM-MDDCs. Experiments performed with specific inhibitors indicated that the more-important productive infection of IM-MDDCs that is conferred by virus-anchored host LFA-1 seems to be dependent on the cellular signaling pathways modulated by PKA and PKC. Our observations are in agreement with those of previous studies showing that IM-MDDCs are not productively infected with X4-tropic virions (53) and that HIV-1 infection occurs almost exclusively in IM-MDDCs, not in M-MDDCs, in which virus replication is blocked at a postentry level (32, 33). It has been demonstrated that virus preparations contain various contaminants, such as microvesicles and/or exosomes (31). Exosomes might represent a confounding factor, since they can incorporate a wide variety of host cell surface molecules that remain functional (16). Therefore, studies were also performed with virus stocks that were subjected to a velocity gradient-based method to separate HIV-1 particles from cellular contaminants (i.e., a 6% to 18% Optiprep density gradient) (18, 37, 51, 76). A comparable increase in productive infection of IM-MDDCs was observed when highly purified LFA-1-bearing virions were used (data not shown), thereby indicating that the observed phenomenon is really due to virus-anchored host LFA-1 and not to LFA-1-bearing cellular contaminants. It is important to emphasize that the contribution of virus-incorporated host LFA-1 to the process of HIV-1 replication in DC-T-cell cocultures was carried out using in vitro-differentiated DCs (i.e., IM-MDDCs). Similar experiments using immature DCs obtained directly from the blood will need to be done to reconfirm this finding, because these cells display characteristics that might be different from those of IM-MDDCs. For example, blood-derived immature DCs do not express cell surface DC-SIGN.
Attachment of the virus to a target cell is a critical event in the infection process, and as the understanding of virus-host interactions expands, it is becoming clear that the initial binding step requires several specific and nonspecific cell surface structures for its completion. It is now recognized that the entry of HIV-1 into myeloid cells (e.g., DCs and macrophages) can occur through at least three distinct processes: cytosolic delivery, which results in productive infection (46, 47, 60); endocytosis, which is thought to lead to degradation by lysosomal enzymes (74); or targeting of internalized viruses into acidic nonlysosomal compartments, where virus infectivity is preserved to facilitate subsequent transmission during the formation of the virological synapse (4, 27, 28). It has been observed that virus-associated host proteins can increase HIV-1 adsorption onto host cells and modify the entry route (36, 69). The interactions between virus-anchored LFA-1 and its natural counterreceptors on the surfaces of IM-MDDCs do not seem to affect the first steps in the virus replicative cycle, as indicated by measuring cell-associated p24 contents and assessing the percentage of p24-positive IM-MDDCs. However, data from real-time PCR, double staining (i.e., cell surface DC-SIGN and intracellular p24), and infection studies demonstrate that virus-associated LFA-1 molecules modulate productive virus infection of IM-MDDCs. For example, flow cytometric analyses indicate that less than 4% of IM-MDDCs are positive for both DC-SIGN and p24 following infection with LFA-1-bearing virions compared to less than 1% following infection with viruses lacking LFA-1 (Fig. 5C). Note that a moderate productive infection of IM-MDDCs is in agreement with published observations, since it was shown that only 1 to 3% of DCs from healthy donors can be productively infected with HIV-1 in vitro, as determined by intracellular p24 staining (66).
The process through which virus-associated LFA-1 can lead to a more-productive infection of IM-MDDCs is still unclear, but different possibilities can be proposed. We postulate that the intracellular itinerary of HIV-1 within IM-MDDCs might be different for viruses bearing host-derived LFA-1 on their surfaces. We hypothesize that a higher proportion of LFA-1-bearing viruses gains access to the cytosol and/or a lower percentage of internalized LFA-1-bearing virions is destroyed in the endosomal apparatus. For example, the additional interactions between virus-associated LFA-1 and their natural counterligands on the surfaces of IM-MDDCs might favor the binding of gp120 to the complex made of CD4 and an appropriate coreceptor (e.g., CCR5), which would promote the cytosolic delivery of virus material through a pH-independent fusion of viral and cellular membranes. This postulate is supported by the ability of CCR5 antagonists to inhibit de novo virus production by IM-MDDCs (data not shown). Interestingly, ICAM-1 incorporation increases the efficiency of productive HIV-1 entry into primary CD4+ T cells by enhancing the level of virus material released into the cytosol (36, 69). A fractionation assay to separate the cytoplasmic and vesicular fractions upon entry of HIV-1 into IM-MDDCs could be attempted to shed light on the process through which virus-associated host LFA-1 can affect the route of entry of HIV-1. Unfortunately, the percentage of p24-positive cells detected following pulsing of IM-MDDCs with the tested virus preparations is too low to perform an informative cell fractionation assay (i.e., less than 4%) (Fig. 3B). Given that HIV-1 entry into macrophages is thought to be mediated primarily by macropinocytosis (47), it can also be hypothesized that virus-anchored host LFA-1 molecules promote this mode of virus internalization in IM-MDDCs. Note that, although most virions found in macropinosomes are degraded, a pH-independent productive virus infection has been shown to occur in macrophages (47). The observation that the uptake of anti-ICAM-1 conjugates by endothelial cells takes place via macropinocytosis (52) is supportive of this scenario. This possibility can be assessed through the use of dimethyl amiloride, an agent known to inhibit macropinocytosis. Unfortunately, productive infection of IM-MDDCs with HIV-1 particles lacking host LFA-1 is significantly reduced upon treatment with dimethyl amiloride (unpublished observations). Signaling through the cognate ligands of LFA-1 on IM-MDDCs could also favor the assembly and/or transport of the preintegration complex by yet-to-be-defined processes. Additional studies are needed to gain useful information on how de novo virus production can be enhanced in IM-MDDCs upon infection with LFA-1-bearing HIV-1 particles.
A fascinating observation of the current study relates to abolishment of the more important productive infection of IM-MDDCs with LFA-1-bearing viruses following treatment with PKA or PKC inhibitors. It has been reported that the specificity of protein phosphorylation/dephosphorylation events is achieved through the targeting of multienzyme signaling complexes containing, for example, PKA and PKC toward particular substrates at specific cellular microdomains, such as lipid rafts (reviewed in reference 65). The process of internalization of HIV-1 in DCs seems to be dependent on lipid raft integrity (35). PKA and its substrates are known for their role in modulating signaling cascades in several cellular processes, such as antigen-uptake and formation of immunological synapses, and also in the regulation of activities of several proteins, such as small RhoA GTPases, phospholipase C gamma 1, the adaptor protein Csk, and the transcription factors NF-κB and NFAT (70). To date, there is a very limited amount of data concerning the roles played by PKA and PKC in LFA-1-mediated signal transduction pathways and uptake of HIV-1 particles by DCs. Interestingly, activation of PKC following ligation of ICAM-1 by specific antibodies leads to cytoskeleton rearrangement in brain endothelial cell lines (21), and macropinocytosis is a process relying on cytoskeleton rearrangement (2).
In conclusion, our studies provide novel insights into the complex interactions between HIV-1 and DCs. Given the central role played by DCs in the pathogenesis of HIV-1 infection, a better understanding of the process by which HIV-1 can productively infect this cell type can certainly help in the discovery and development of new and better therapeutic agents. Indeed, the demonstrated involvement of virus-bound host LFA-1 in productive infection of IM-MDDCs should be taken into account when designing strategies aimed at blocking HIV-1 uptake by DCs.
Acknowledgments
We express our gratitude to Lahlou Hadji, Chantal Burelout, and Philippe Desaulnier for critical and constructive comments related to the present work. We thank Sylvie Méthot and Lahlou Hadji for their excellent technical assistance in writing this manuscript. We are grateful to Michael Imbeault and Mélanie Tardif for their assistance with the real-time PCR assay. We appreciate the excellent technical contributions of Maurice Dufour, Odette Simard, and Caroline Côté.
This work was performed by C.G. in partial fulfillment of her postdoctoral training and was supported by operating grants to M.J.T. from the Canadian Foundation for AIDS Research (grant number 015 026) and the Canadian Institutes of Health Research (CIHR) under the HIV/AIDS Research Program (grants MOP-14438 and MOP-79542). C.G. is the recipient of a Fellowship Award from the CIHR HIV/AIDS Research Program, and M.J.T. holds a Tier 1 Canada Research Chair in Human Immuno-Retrovirology.
Footnotes
Published ahead of print on 9 May 2007.
REFERENCES
- 1.Amiel, C., M. C. Bene, T. May, P. Canton, and G. C. Faure. 1988. LFA1 expression in HIV infection. AIDS 2:211-214. [PubMed] [Google Scholar]
- 2.Amyere, M., M. Mettlen, P. Van Der Smissen, A. Platek, B. Payrastre, A. Veithen, and P. J. Courtoy. 2002. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int. J. Med. Microbiol. 291:487-494. [DOI] [PubMed] [Google Scholar]
- 3.Ancuta, P., Y. Bakri, N. Chomont, H. Hocini, D. Gabuzda, and N. Haeffner-Cavaillon. 2001. Opposite effects of IL-10 on the ability of dendritic cells and macrophages to replicate primary CXCR4-dependent HIV-1 strains. J. Immunol. 166:4244-4253. [DOI] [PubMed] [Google Scholar]
- 4.Arrighi, J. F., M. Pion, E. Garcia, J. M. Escola, Y. van Kooyk, T. B. Geijtenbeek, and V. Piguet. 2004. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 200:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbeau, B., J. F. Fortin, N. Genois, and M. J. Tremblay. 1998. Modulation of human immunodeficiency virus type 1-induced syncytium formation by the conformational state of LFA-1 determined by a new luciferase-based syncytium quantitative assay. J. Virol. 72:7125-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastiani, L., S. Laal, M. Kim, and S. Zolla-Pazner. 1997. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J. Virol. 71:3444-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastiani Lallos, L., D. Cecilia, E. M. Fenyo, S. Laal, and S. Zolla-Pazner. 2000. HIV phenotype correlates with the relative amounts of lymphocyte function-related molecule 1 (LFA-1) and major histocompatibility complex (MHC) class II in the virion envelope. AIDS 14:1523-1531. [DOI] [PubMed] [Google Scholar]
- 8.Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 3:42-47. [DOI] [PubMed] [Google Scholar]
- 9.Bounou, S., J. F. Giguere, R. Cantin, C. Gilbert, M. Imbeault, G. Martin, and M. J. Tremblay. 2004. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. FASEB J. 18:1294-1296. [DOI] [PubMed] [Google Scholar]
- 10.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canque, B., Y. Bakri, S. Camus, M. Yagello, A. Benjouad, and J. C. Gluckman. 1999. The susceptibility to X4 and R5 human immunodeficiency virus-1 strains of dendritic cells derived in vitro from CD34+ hematopoietic progenitor cells is primarily determined by their maturation stage. Blood 93:3866-3875. [PubMed] [Google Scholar]
- 12.Cantin, R., J. F. Fortin, G. Lamontagne, and M. Tremblay. 1997. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J. Virol. 71:1922-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantin, R., S. Methot, and M. J. Tremblay. 2005. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J. Virol. 79:6577-6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clapham, P. R., and A. McKnight. 2001. HIV-1 receptors and cell tropism. Br. Med. Bull. 58:43-59. [DOI] [PubMed] [Google Scholar]
- 16.Clayton, A., J. Court, H. Navabi, M. Adams, M. D. Mason, J. A. Hobot, G. R. Newman, and B. Jasani. 2001. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods 247:163-174. [DOI] [PubMed] [Google Scholar]
- 17.Crowley, M. T., P. S. Costello, C. J. Fitzer-Attas, M. Turner, F. Meng, C. Lowell, V. L. Tybulewicz, and A. L. DeFranco. 1997. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J. Exp. Med. 186:1027-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dettenhofer, M., and X. F. Yu. 1999. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J. Virol. 73:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101:4505-4511. [DOI] [PubMed] [Google Scholar]
- 20.Dornadula, G., H. Zhang, S. Shetty, and R. J. Pomerantz. 1999. HIV-1 virions produced from replicating peripheral blood lymphocytes are more infectious than those from nonproliferating macrophages due to higher levels of intravirion reverse transcripts: implications for pathogenesis and transmission. Virology 253:10-16. [DOI] [PubMed] [Google Scholar]
- 21.Etienne-Manneville, S., J. B. Manneville, P. Adamson, B. Wilbourn, J. Greenwood, and P. O. Couraud. 2000. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J. Immunol. 165:3375-3383. [DOI] [PubMed] [Google Scholar]
- 22.Fecondo, J. V., N. C. Pavuk, K. A. Silburn, D. M. Read, A. S. Mansell, A. W. Boyd, and D. A. McPhee. 1993. Synthetic peptide analogs of intercellular adhesion molecule 1 (ICAM-1) inhibit HIV-1 replication in MT-2 cells. AIDS Res. Hum. Retrovir. 9:733-740. [DOI] [PubMed] [Google Scholar]
- 23.Fortin, J. F., R. Cantin, M. G. Bergeron, and M. J. Tremblay. 2000. Interaction between virion-bound host intercellular adhesion molecule-1 and the high-affinity state of lymphocyte function-associated antigen-1 on target cells renders R5 and X4 isolates of human immunodeficiency virus type 1 more refractory to neutralization. Virology 268:493-503. [DOI] [PubMed] [Google Scholar]
- 24.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujiwara, M., R. Tsunoda, S. Shigeta, T. Yokota, and M. Baba. 1999. Human follicular dendritic cells remain uninfected and capture human immunodeficiency virus type 1 through CD54-CD11a interaction. J. Virol. 73:3603-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganesh, L., K. Leung, K. Lore, R. Levin, A. Panet, O. Schwartz, R. A. Koup, and G. J. Nabel. 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 78:11980-11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geijtenbeek, T. B., A. Engering, and Y. Van Kooyk. 2002. DC-SIGN, a C-type lectin on dendritic cells that unveils many aspects of dendritic cell biology. J. Leukoc. Biol. 71:921-931. [PubMed] [Google Scholar]
- 28.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert, C., F. Barabe, E. Rollet-Labelle, S. G. Bourgoin, S. R. McColl, B. B. Damaj, and P. H. Naccache. 2001. Evidence for a role for SAM68 in the responses of human neutrophils to ligation of CD32 and to monosodium urate crystals. J. Immunol. 166:4664-4671. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert, C., E. Rollet-Labelle, and P. H. Naccache. 2002. Preservation of the pattern of tyrosine phosphorylation in human neutrophil lysates. II. A sequential lysis protocol for the analysis of tyrosine phosphorylation-dependent signalling. J. Immunol. Methods 261:85-101. [DOI] [PubMed] [Google Scholar]
- 31.Gluschankof, P., I. Mondor, H. S. Gelderblom, and Q. J. Sattentau. 1997. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virology 230:125-133. [DOI] [PubMed] [Google Scholar]
- 32.Granelli-Piperno, A., D. Chen, B. Moser, and R. M. Steinman. 1997. The HIV-1 life cycle is blocked at two different points in mature dendritic cells. Adv. Exp. Med. Biol. 417:415-419. [DOI] [PubMed] [Google Scholar]
- 33.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granelli-Piperno, A., V. Finkel, E. Delgado, and R. M. Steinman. 1999. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr. Biol. 9:21-29. [DOI] [PubMed] [Google Scholar]
- 35.Gummuluru, S., M. Rogel, L. Stamatatos, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 77:12865-12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo, M. M., and J. E. Hildreth. 1995. HIV acquires functional adhesion receptors from host cells. AIDS Res. Hum. Retrovir. 11:1007-1013. [DOI] [PubMed] [Google Scholar]
- 37.Hermens, W. T., O. ter Brake, P. A. Dijkhuizen, M. A. Sonnemans, D. Grimm, J. A. Kleinschmidt, and J. Verhaagen. 1999. Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system. Hum. Gene Ther. 10:1885-1891. [DOI] [PubMed] [Google Scholar]
- 38.Hildreth, J. E., and R. J. Orentas. 1989. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science 244:1075-1078. [DOI] [PubMed] [Google Scholar]
- 39.Hioe, C. E., L. Bastiani, J. E. Hildreth, and S. Zolla-Pazner. 1998. Role of cellular adhesion molecules in HIV type 1 infection and their impact on virus neutralization. AIDS Res. Hum. Retrovir. 3(Suppl 14):S247-S254. [PubMed] [Google Scholar]
- 40.Hioe, C. E., P. C. Chien, Jr., C. Lu, T. A. Springer, X. H. Wang, J. Bandres, and M. Tuen. 2001. LFA-1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J. Virol. 75:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hioe, C. E., J. E. Hildreth, and S. Zolla-Pazner. 1999. Enhanced HIV type 1 neutralization by human anti-glycoprotein 120 monoclonal antibodies in the presence of monoclonal antibodies to lymphocyte function-associated molecule 1. AIDS Res. Hum. Retrovir. 15:523-531. [DOI] [PubMed] [Google Scholar]
- 42.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalter, D. C., H. E. Gendelman, and M. S. Meltzer. 1991. Inhibition of human immunodeficiency virus infection in monocytes by monoclonal antibodies against leukocyte adhesion molecules. Immunol. Lett. 30:219-227. [DOI] [PubMed] [Google Scholar]
- 44.Liao, Z., J. W. Roos, and J. E. Hildreth. 2000. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res. Hum. Retrovir. 16:355-366. [DOI] [PubMed] [Google Scholar]
- 45.Majeed, M., E. Caveggion, C. A. Lowell, and G. Berton. 2001. Role of Src kinases and Syk in Fcγ receptor-mediated phagocytosis and phagosome-lysosome fusion. J. Leukoc. Biol. 70:801-811. [PubMed] [Google Scholar]
- 46.Maréchal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maréchal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin, G., and M. J. Tremblay. 2004. HLA-DR, ICAM-1, CD40, CD40L, and CD86 are incorporated to a similar degree into clinical human immunodeficiency virus type 1 variants expanded in natural reservoirs such as peripheral blood mononuclear cells and human lymphoid tissue cultured ex vivo. Clin. Immunol. 111:275-285. [DOI] [PubMed] [Google Scholar]
- 49.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 50.Meng, G., X. Wei, X. Wu, M. T. Sellers, J. M. Decker, Z. Moldoveanu, J. M. Orenstein, M. F. Graham, J. C. Kappes, J. Mestecky, G. M. Shaw, and P. D. Smith. 2002. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 8:150-156. [DOI] [PubMed] [Google Scholar]
- 51.Moller-Larsen, A., and T. Christensen. 1998. Isolation of a retrovirus from multiple sclerosis patients in self-generated Iodixanol gradients. J. Virol. Methods 73:151-161. [DOI] [PubMed] [Google Scholar]
- 52.Muro, S., R. Wiewrodt, A. Thomas, L. Koniaris, S. M. Albelda, V. R. Muzykantov, and M. Koval. 2003. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J. Cell Sci. 116:1599-1609. [DOI] [PubMed] [Google Scholar]
- 53.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ott, D. E. 1997. Cellular proteins in HIV virions. Rev. Med. Virol. 7:167-180. [DOI] [PubMed] [Google Scholar]
- 55.Ott, D. E. 2002. Potential roles of cellular proteins in HIV-1. Rev. Med. Virol. 12:359-374. [DOI] [PubMed] [Google Scholar]
- 56.Pantaleo, G., L. Butini, C. Graziosi, G. Poli, S. M. Schnittman, J. J. Greenhouse, J. I. Gallin, and A. S. Fauci. 1991. Human immunodeficiency virus (HIV) infection in CD4+ T lymphocytes genetically deficient in LFA-1: LFA-1 is required for HIV-mediated cell fusion but not for viral transmission. J. Exp. Med. 173:511-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park, S. W., W. Royal III, R. D. Semba, G. W. Wiegand, and D. E. Griffin. 1998. Expression of adhesion molecules and CD28 on T lymphocytes during human immunodeficiency virus infection. Clin. Diagn. Lab. Immunol. 5:583-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patterson, S., and S. C. Knight. 1987. Susceptibility of human peripheral blood dendritic cells to infection by human immunodeficiency virus. J. Gen. Virol. 68:1177-1181. [DOI] [PubMed] [Google Scholar]
- 59.Patterson, S., A. Rae, N. Hockey, J. Gilmour, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75:6710-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pauza, C. D., and T. M. Price. 1988. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J. Cell Biol. 107:959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rescigno, M., and P. Borrow. 2001. The host-pathogen interaction: new themes from dendritic cell biology. Cell 106:267-270. [DOI] [PubMed] [Google Scholar]
- 62.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 63.Rothlein, R., M. L. Dustin, S. D. Marlin, and T. A. Springer. 1986. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J. Immunol. 137:1270-1274. [PubMed] [Google Scholar]
- 64.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76:7812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sim, A. T., and J. D. Scott. 1999. Targeting of PKA, PKC and protein phosphatases to cellular microdomains. Cell Calcium 26:209-217. [DOI] [PubMed] [Google Scholar]
- 66.Smed-Sorensen, A., K. Lore, J. Vasudevan, M. K. Louder, J. Andersson, J. R. Mascola, A. L. Spetz, and R. A. Koup. 2005. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 79:8861-8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strzelecka-Kiliszek, A., K. Kwiatkowska, and A. Sobota. 2002. Lyn and Syk kinases are sequentially engaged in phagocytosis mediated by FcγR. J. Immunol. 169:6787-6794. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki, Y., N. Misawa, C. Sato, H. Ebina, T. Masuda, N. Yamamoto, and Y. Koyanagi. 2003. Quantitative analysis of human immunodeficiency virus type 1 DNA dynamics by real-time PCR: integration efficiency in stimulated and unstimulated peripheral blood mononuclear cells. Virus Genes 27:177-188. [DOI] [PubMed] [Google Scholar]
- 69.Tardif, M. R., and M. J. Tremblay. 2003. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J. Virol. 77:12299-12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torgersen, K. M., T. Vang, H. Abrahamsen, S. Yaqub, and K. Tasken. 2002. Molecular mechanisms for protein kinase A-mediated modulation of immune function. Cell Signal 14:1-9. [DOI] [PubMed] [Google Scholar]
- 71.Tremblay, M. J., J. F. Fortin, and R. Cantin. 1998. The acquisition of host-encoded proteins by nascent HIV-1. Immunol. Today 19:346-351. [DOI] [PubMed] [Google Scholar]
- 72.Tsunetsugu-Yokota, Y., S. Yasuda, A. Sugimoto, T. Yagi, M. Azuma, H. Yagita, K. Akagawa, and T. Takemori. 1997. Efficient virus transmission from dendritic cells to CD4+ T cells in response to antigen depends on close contact through adhesion molecules. Virology 239:259-268. [DOI] [PubMed] [Google Scholar]
- 73.Turville, S. G., J. Arthos, K. M. Donald, G. Lynch, H. Naif, G. Clark, D. Hart, and A. L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood 98:2482-2488. [DOI] [PubMed] [Google Scholar]
- 74.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 75.Ugolini, S., I. Mondor, and Q. J. Sattentau. 1999. HIV-1 attachment: another look. Trends Microbiol. 7:144-149. [DOI] [PubMed] [Google Scholar]
- 76.Zolotukhin, S., B. J. Byrne, E. Mason, I. Zolotukhin, M. Potter, K. Chesnut, C. Summerford, R. J. Samulski, and N. Muzyczka. 1999. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6:973-985. [DOI] [PubMed] [Google Scholar]