Abstract
We studied the phenotype and distribution of “naturally” occurring CD4+ CD25+ T regulatory cells (CD4+ CD25+ nTreg cells) resident in rabbit conjunctiva, the main T-cell inductive site of the ocular mucosal immune system, and we investigated their suppressive capacities using herpes simplex virus type 1 (HSV-1)-specific effector T (Teff) cells induced during ocular infection. The expression of CD4, CD25, CTLA4, GITR, and Foxp3 was examined by reverse transcription-PCR, Western blotting, and fluorescence-activated cell sorter analysis in CD45+ pan-leukocytes isolated from conjunctiva, spleen, and peripheral blood monocyte cells (PBMC) of HSV-1-infected and uninfected rabbits. Normal conjunctiva showed a higher frequency of CD4+ CD25(Bright+) T cells than did spleen and PBMC. These cells expressed high levels of Foxp3, GITR, and CTLA4 molecules. CD4+ CD25(Bright+) T cells were localized continuously along the upper and lower palpebral and bulbar conjunctiva, throughout the epithelium and substantia propria. Conjunctiva-derived CD4+ CD25(Bright+) T cells, but not CD4+ CD25(low) T cells, efficiently suppressed HSV-specific CD4+ and CD8+ Teff cells. The CD4+ CD25(Bright+) T-cell-mediated suppression was effective on both peripheral blood and conjunctiva infiltrating Teff cells and was cell-cell contact dependent but independent of interleukin-10 and transforming growth factor β. Interestingly, during an ocular herpes infection, there was a selective increase in the frequency and suppressive capacity of Foxp3+ CD4+ CD25(Bright+) T cells in conjunctiva but not in the spleen or in peripheral blood. Altogether, these results provide the first evidence that functional Foxp3+ CD4+ CD25(Bright+) Treg cells accumulate in the conjunctiva. It remains to be determined whether conjunctiva CD4+ CD25+ nTreg cells affect the topical/mucosal delivery of subunit vaccines that stimulate the ocular mucosal immune system.
The immune system has evolved numerous mechanisms of peripheral T-cell immunoregulation, including a network of αβ and γδ CD4+ and CD8+ regulatory T (Treg) cells, that modulate primary and memory effector T (Teff) cell responses at various times and locations and in various inflammatory circumstances (32, 33, 37, 39, 46, 59, 77). Among the αβ Treg cells, several subpopulations of CD4+ Treg cells have been recently identified and shown to control Teff cell function in various animal species and in humans (reviewed in references 2, 61, and 68). Some are considered to be natural regulators, the so-called thymus-derived “natural” CD4+ CD25+ Treg cells (CD4+ CD25+ nTreg cells) (21), that constitutively express a specific set of “molecular markers” including CD25 (the interleukin-2 [IL-2] receptor α-chain), forkhead/winged-helix transcription factor (Foxp3), glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA4 or CD152) (reviewed in references 50 and 62). Other extrathymically generated CD4+ CD25+ Treg (iTreg) cells, designated “adaptive” antigen-specific Treg cells, produce and mediate their suppression via IL-10 and/or transforming growth factor β (TGF-β), such as Tr1 and T helper type 3 (Th3) cells (11, 69, 81, 86).
Converging evidence shows that CD4+ CD25+ Treg cells are important not only in the pathogenesis of herpes simplex virus type 1 (HSV-1) and HSV-2 infections but also in regulating herpes infection and immunity (4, 18, 75, 79). However, work in this area has been focused primarily on systemic CD4+ CD25+ Treg cells derived from either spleen (in mice) or peripheral blood (PB) (in humans) (4, 18, 75, 79). Despite the fact that CD4+ CD25+ Treg cells can be cultured from most common mucosa-associated lymphoid tissues, little is known of the presence and function of CD4+ CD25+ Treg cells that reside locally within mucosal sites of herpes infection (i.e., at ocular and genital mucosal tissues), where immunoregulation may be required. To our knowledge, the presence and function of CD4+ CD25+ Treg cells in the ocular mucosal immune system (OMIS), the immune barrier that protects the surface of the eye, have not been reported (13, 42, 43, 83). It is recognized that conjunctiva-associated lymphoid tissue (CALT), the major OMIS T-cell inductive site, remains in a state of controlled inflammation (13, 28, 42, 43), suggesting that active suppression by conjunctiva resident Treg cells plays a role in normal ocular mucosal homeostasis (24, 26, 27, 44, 83). This mechanism may also be involved in controlling local mucosal immunity against the many pathogens that incessantly reach the surface of the eye (6, 10, 56, 87, 88). However, to date, the presence of dedicated conjunctival mucosa Treg cells, the extent of their repertoire, and their influence on ocular mucosal virus-specific CD4+ and CD8+ Teff cells have not been examined.
Because of the obvious ethical and practical considerations involved in studying conjunctiva in humans, a critical question has been which species would be the most appropriate animal model to investigate conjunctiva Treg cells (13, 29). Instead of the common laboratory species, mice, rats, and guinea pigs, we elected to use rabbits as the working animal model, bearing in mind that (i) numerous similarities between rabbit and human OMIS exist (13, 66, 67); (ii) several T-cell-mediated ocular surface diseases, including herpetic conjunctivitis and recurrent corneal herpetic stromal keratitis (HSK), have been reported to be similar in rabbits and humans, making it the most relevant animal model for exploring these human eye disorders (53, 55, 80); (iii) compared to mice and rats, rabbit CALT more closely resembles human CALT (23, 25, 28, 44); (iv) microanatomy and immunohistological studies indicate that rabbit conjunctival mucosa is comparable to that of humans and has a typical follicular ultrastructure with an abundance of “conjunctival lymphoid follicles,” whereas no lymphoid tissue was identified in mice and rats (28, 42, 43, 47, 84); (v) from a practical standpoint, unlike mice, rats, and guinea pigs, rabbits possess a relatively large conjunctival surface, offering abundant mucosa-associated lymphoid tissue for in vitro studies; and (vi) the recent availability of many monoclonal and polyclonal antibodies specific to rabbit immune cell CD markers, cytokines, and growth factors provide useful immunological tools for an unprecedented phenotypic and functional analysis of rabbit T-cell repertoire and function.
The present study aimed mainly to (i) assess CD4+ CD25+ Treg cells that reside in normal rabbit conjunctiva and those induced in immune conjunctiva following an ocular HSV-1 infection, (ii) investigate their phenotype and distribution, and (iii) evaluate their suppressive effect on HSV-specific CD4+ and CD8+ Teff cells. Unexpectedly, we found that under physiological conditions, rabbit conjunctiva contained a large number of CD4+ T cells that naturally express high levels of CD25 T cells [CD4+ CD25(Bright+) T cells] and express Foxp3, GITR, and CTLA4 molecules. Normal conjunctiva CD4+ CD25+ T cells were distributed along the epithelium and the substantia propria in both upper and lower lids. Naïve and immune conjunctiva-derived CD4+ CD25(Bright+) T cells, but not CD4+ CD25(low) T cells, suppressed ocular mucosal (conjunctiva) and systemic (PB) HSV-specific CD4+ and CD8+ Teff cell responses in vitro. The suppressive activity was cell-cell contact dependent but independent of anti-inflammatory cytokines IL-10 and TGF-β. Our data provide the first evidence that natural-like Foxp3+ CD4+ CD25(Bright+) T cells with phenotypic and functional similarities to human CD4+ CD25+ nTreg cells accumulate in rabbit conjunctiva. The cellular and molecular mechanisms that lead to the selective retention of Foxp3+ CD4+ CD25+ Treg cells in the conjunctiva and their potential role in ocular mucosal immunity and infection are discussed.
MATERIALS AND METHODS
Animals.
New Zealand White female rabbits (Harlan, Indianapolis, IN) were used for all experiments. All experimental procedures used in this study conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and University of California Irvine Animal Care and Use Committee guidelines.
Virus and infection.
HSV-1 strain McKrae was used for in vitro and in vivo studies. Virus was grown and titers were determined using rabbit skin cell monolayers in minimal essential medium containing 5% fetal calf serum as previously described (8, 56, 87). Rabbits were infected ocularly in both eyes without corneal scarification with 25 μl of a virus suspension containing 2 × 105 PFU.
Antibodies.
Anti-human IL-10 monoclonal antibody (mAb), GITR mAb, and TGF-β1 mAb were purchased from R&D Systems (Minneapolis, MN). Anti-rabbit CD8 and CD45 mAbs were purchased from Chemicon International (Temecula, CA). Anti-Rabbit CD25 mAb was purchased from Antigenix (Huntington Station, NY). Anti-rabbit CD4 mAb was purchased from Chemicon (Temecula, CA). Anti-human CTLA4 mAb was purchased from GeneTex (San Antonio, TX). Purified anti-human FOXP3 mAb was purchased from Bio-Legend (San Diego, CA). Anti-β-actin-horseradish peroxidase (HRP) mAb was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-rabbit anti-mouse immunoglobulin G2b (IgG2b), HRP-goat anti-rabbit IgG, and HRP-rabbit anti-mouse IgG1 were purchased from Chemicon (Temecula, CA).
Preparation of single-cell suspension from normal rabbit conjunctiva.
The bulbar and palpebral conjunctiva tissue from normal rabbit eyes was excised and collected in Hanks balanced salt solution (1×). Conjunctiva tissues were spun down at 1,600 rpm for 5 min at 4°C and digested with collagenase type I (GIBCO, Carlsbad, CA) at 3 mg/ml for 3 h at 37°C with occasional vortexing every 15 min. The digested tissue suspension was passed through a 70-μm nylon cell strainer and spun down at 1,600 rpm for 5 min at 4°C. This process was repeated two times. The final pellet was resuspended in Hanks balanced salt solution (1×), passed through a 40-μm cell strainer, and spun down at 1,600 rpm for 5 min at 4°C. This process was repeated two times. The final pellet was resuspended in complete RPMI 1640 medium and kept on ice. The live cells were counted using a hemocytometer.
Depletion of CD8+ cells.
Single-cell suspensions from rabbit conjunctiva were labeled with phycoerythrin-conjugated anti-rabbit CD8 antibody (10 μl antibody/7.5 × 107 cells) followed by incubation with anti-phycoerythrin microbeads (30 μl bead solution/7.5 × 107 cells) according to the manufacturer's instructions. CD8+ cells were depleted from single-cell suspensions by using a MACS column ((Miltenyi Biotec, Bergisch Gladbach, Germany).
Purification of CD4+ CD25+ cells from CD8+-depleted conjunctival cells.
CD4+ CD25+ cells were purified from CD8+-depleted conjunctival cells by using biotin-conjugated anti-rabbit CD25 antibody (30 μl antibody/7.5 × 107 cells) followed by incubation with microbeads conjugated to anti-biotin mAb (40 μl anti-beads/7.5 × 107 cells) according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). The CD4+ CD25+ cells were purified through a MACS column. The CD4+ CD25− cells were further purified by anti-rabbit CD4-fluorescein isothiocyanate (FITC) antibody. The CD4+ CD25− cells from the column were incubated with anti-rabbit CD4-FITC antibody (30 μl antibody/7.5 × 107 cells) followed by incubation with anti-FITC microbeads (40 μl anti-beads/7.5 × 107cells) according to the manufacturer's instructions.
Preparation of whole-cell lysate from purified CD4+ CD25+ cells.
The cell pellet was thawed on ice and washed two times with 500 μl of cold 1× phosphate-buffered saline (PBS) and spun down at 1,600 rpm for 5 min at 4°C. Cell pellets were resuspended in 500 μl of radioimmunoprecipitation assay buffer containing protease inhibitors and lysed for 10 min on ice with occasional vortexing during incubation. The lysed cells were spun down at 1,600 rpm for 5 min at 4°C, and the supernatant was collected as whole-cell lysate. The total protein concentration of the whole-cell lysate was measured by Bio-Rad protein assay reagent using bovine serum albumin as a standard.
Western blot.
Precast NuPAGE gel (catalog no. NP0322BOX), MES (morpholineethanesulfonic acid) running buffer (catalog no. NP0002), NuPAGE-LDS sample buffer (catalog no. NP0007), NuPAGE sample reducing agent (catalog no. NP0004), and SeeBlue Plus2 prestained standard were purchased from Invitrogen (Carlsbad, CA). Tween 20 was purchased from Sigma (St Louis, MO). An Immobilon-P polyvinylidene difluoride membrane was purchased from Millipore (Billerica, MA). An ECL plus Western blot detection kit was purchased from Amersham Biosciences (Quebec, Canada). Proteins in the whole-cell lysate were separated in 4 to 12% Tris gradient gels at a constant voltage of 200 V. MES (1×) was used as a running buffer, and rainbow molecular mass markers were used as running controls. At the end of electrophoresis, the gel was soaked in Western blot transfer buffer (25 mM Tris, 192 mM glycine, 20% [vol/vol] methanol [pH 8.3]) for 5 min with mild shaking. The gel was transferred onto an Immobilon-P filter membrane (0.45-μm pore size) using a Bio-Rad Mini Gel transfer unit at room temperature for 2.5 h at 30 V using cold Western blot transfer buffer. After transfer, the polyvinylidene difluoride membrane was soaked in TBS-T blocking buffer (Tris-buffered saline with 0.05% Tween 20) containing 5% nonfat dry milk for 1 h at room temperature with mild shaking. For immunodetection, the membrane was washed three times (10 min/wash) with TBS-T and incubated with primary antibody diluted in TBS-T containing 1% dry milk overnight at 4°C with mild shaking. The membrane was washed three times (10 min/wash) with TBS-T and probed with the respective secondary antibody conjugated with HRP in TBS-T containing 1% dry milk for 1 h at room temperature under mild shaking conditions. The membrane was washed three times (10 min/wash) with TBS-T and developed using an ECL Plus Western blot detection kit for 5 min. For the detection of protein bands, the developed membrane was exposed to Kodak X-ray film. For the detection of β-actin, the membrane was stripped with stripping solution (25 mM glycine [pH 2.4] and 1% sodium dodecyl sulfate) for at least 1 h at room temperature with vigorous shaking.
RNA extraction, cDNA synthesis, and PCR.
The cells were lysed directly in RLT buffer (QIAGEN) by scraping, immediately frozen in liquid nitrogen, and then thawed on ice. This lysate was then processed over RNeasy columns according to the manufacturer's instructions (QIAGEN). The RNA was eluted from the silica column with 50 μl water. One microliter of the product was analyzed using an Agilent 2100 Bioanalyzer using the nano-RNA protocol to verify both the quantity and quality of the RNA. The RNA obtained from cells was converted to cDNA using the Sensiscript synthesis protocol (QIAGEN). For these samples, 500 ng of RNA was reverse transcribed in a 20-μl reaction volume. Reactions were carried out for 30 min at 42°C, followed by 5 min at 95°C and cooling to 4°C. After synthesis, the cDNA from all sources was diluted to equivalent input RNA levels with water (10 ng input RNA/μl) and stored at −20°C until use. cDNA samples were subjected to PCR using specific primers. Primers were designed using the Primer 3 Internet software program (The Whitehead Institute, Cambridge, MA), and their specificities were confirmed by a BLAST Internet software-assisted search of the nonredundant nucleotide sequence database (National Library of Medicine, Bethesda, MD).
PCRs were carried out with 1 to 3 ng reverse-transcribed RNA, 200 nM forward and reverse primers, and SYBR green master mix (Applied Biosystems) in a final volume of 25 μl. Cycling parameters were 50°C for 5 min and 95°C 3 min, followed by 45 cycles of 95°C for 30 s and 60°C 90 s. Plates were read after each cycle, and a melting curve was generated after amplification. For quantitative real-time PCR, samples were normalized by beta-2-microglobulin amplification and were amplified in triplicate using an MJ Research Opticon thermal cycler. PCR controls without reverse transcriptase (water control) or with normal human genomic DNA as a template were routinely negative.
Flow cytometry analysis.
Lymphocyte T-cell suspensions obtained from the rabbit conjunctiva, spleen, and PB monocyte cells (PBMC) were stained for different cell surface molecules. Cells (1 × 106) were incubated with antibodies for 1 h at 4°C. The cells were washed two times, fixed with 2% paraformaldehyde, and acquired using FACScan (BD Biosciences, San Jose, CA). The data were analyzed using Cellquest software (BD Biosciences, San Jose, CA).
Immunohistochemistry and confocal microscopy.
Rabbits were euthanized, and the conjunctivas were fixed with 2% paraformaldehyde. Superior and inferior conjunctivas were collected as an en bloc sample from the eyelid to the cornea. After overnight fixation, the conjunctiva samples were cut into small longitudinal bands. Then samples were blocked with anti-FcRγ antibody (US biological, MA) at a dilution of 1:100 and in goat serum-PBS overnight. The anti-CD4 antibody conjugated to FITC at a dilution of 1:100 and 14.3 μM DAPI (4′,6′-diamidino-2-phenylindole) (Molecular Probes, CA) were applied overnight at 4°C. Samples were then mounted in 50% glycerol-PBS. The primary antibody for CD25 at a dilution of 1:100 was also applied overnight at 4°C. After washing, the anti-CD25 antibody labeled with donkey anti-mouse IgG antibody conjugated to FITC (Jackson Immunoresearch Laboratories, PA) at a dilution of 1:250 was mixed with 14.3 μM DAPI (Molecular Probes, CA) overnight at 4°C. Samples were then washed and mounted in 50% glycerol-PBS. Confocal microscopy was performed with a laser confocal and multiphoton microscope system with a conventional laser confocal microscope (LSM 510 META; Zeiss, Jena, Germany) equipped with a Chameleon titanium laser (Chameleon, Coherent, CA). Scanning was performed from the surface of the conjunctival epithelium to a depth of 80 to 100 μM. The optical slices obtained were reconstructed as a maximum-intensity projection or a cross-sectional image.
T-cell proliferation assay.
Single cells were suspended in RPMI 1640 supplemented with 5 × 105 M 2-mercaptoethanol, 10% fetal calf serum, Glutamax I (Gibco BRL, Life Technologies, United Kingdom), 100 U/ml penicillin, and 100 μg/ml streptomycin (complete RPMI 1640 medium). Teff cells from rabbit conjunctiva were incubated with RPMI 1640 medium containing 5% bovine serum albumin and plated at 5 × 105 cells/well in the absence or presence of increasing numbers of CD4+ CD25+ T cells from rabbit eyes in a 96-well plate. Cells were stimulated with heat-inactivated HSV-1 virus in duplicate cultures and incubated for 96 h. T-cell proliferation was measured by [3H]thymidine uptake for 18 h. Results were expressed as the difference between signal and background values. The T-cell proliferation response was considered to be positive when the stimulation index was >2 and the Δcpm was >10,00. Purified Treg cells (0.5 × 106 cells/ml) were activated in RPMI medium supplemented with l-glutamine (2 mM), 2-mercaptoethanol (5 × 10−5 M), sodium pyruvate (1 mM), penicillin (100 IU/ml), streptomycin (100 μg/ml), nonessential amino acids (1 ml/100 ml), and 2% (vol/vol) autologous serum in the presence of human recombinant IL-2 (5 ng/ml) and soluble anti-CD3 antibodies (1 ng/ml). Irradiated (2,500 rad) splenocytes (1.5 × 106 cells/ml) were added to the culture. Cells were activated for 24 or 96 h. Cells were harvested onto glass fiber filters using an LKB 96-well harvester (Wallac, Turku, Finland) after an additional 24 h. Uptake of [3H]thymidine was measured on an LKB Betaplate counter. The results are expressed as mean counts per minute for triplicate cultures (standard errors were routinely <10%).
CD3 stimulation.
CD3-coated Dynabeads (Dynal, Bromborough, United Kingdom) were prepared by conjugating 5 μg CD3 antibodies (both from Pharmingen) to 107 beads according to the manufacturer's instructions. For proliferation, 1 × 105 to 5 × 105 responder T cells were incubated with 1 × 104 to 5 × 104 CD3-coated Dynabeads/well. The cells were cultivated in a total volume of 200 μl in flat-bottomed 96-well plates and assessed for proliferation 3 days later. [3H]thymidine (1 μCi) was added for the last 16 h of culture. After the cells were harvested, their thymidine content was analyzed by the use of a gamma counter as previously described (5-7).
Transwell assay and cytokine-neutralizing assays.
HSV-specific CD4+ CD25− Teff cells were cultured with or without conjunctival CD4+ CD25+ nTreg cells together in one well or separated through a microporous membrane with a 0.4-μm pore size (Transwell; Corning Inc., Acton, MA). In the transwell assay, the CD4+ CD25− T cells were put into the outer compartment of a 24-well plate, and the CD4+ CD25+ nTreg cells were put into the inner compartment. For neutralizing assays, CD4+ CD25− T cells were cultured with CD4+ CD25+ nTreg cells in the presence of 0.5 μg/ml anti-rabbit IL-10 and 10 μg/ml of anti-rabbit TGF-β (R&D Systems, Minneapolis, MN) or isotype controls. Culture, stimulation, and staining procedures were performed as described above.
Statistical analysis.
Results are expressed as means ± standard deviations. Groups of data were compared by Mann-Whitney U test. Differences were considered to be statistically significant when the P value was <0.05.
RESULTS
Normal conjunctiva contains a predominant CD4+ CD25+ T-cell subpopulation.
Fresh conjunctiva, spleens, and PB were collected from normal rabbits with no apparent ocular inflammatory disease. A protocol was developed for the isolation of intraconjunctival CD45+ leukocytes (ICLs) and of CD4+ CD25+ T cells with high yield, purity, and viability. This comprises three main steps: (i) dissecting conjunctiva from the upper and lower lids and bulbar surface, (ii) digesting conjunctival tissue and releasing immune and nonimmune cells following a 2-h collagenase treatment, and (c) using immunomagnetic microbead positive selection of CD45+ ICLs or CD4+ CD25+ T cells.
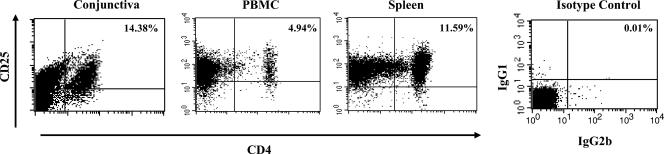
We first isolated conjunctiva resident CD45+ leukocytes and assessed their frequency among total conjunctival cells. About 14.38% ± 2% CD45+ ICLs were detected from 9.5 million total conjunctival cells/eye, with 92 to 98% purity and over 95% viability (Table 1). Using flow cytometry (fluorescence-activated cell sorter [FACS]) and an established panel of mAbs specific for rabbit CD3, CD4, and CD25 molecules, we determined the percentage of conjunctiva resident CD4+ CD25+ and CD4+ CD25− T cells among total conjunctival cells (Table 1) and within the CD45+ ICL population (Table 2 and Fig. 1). These results were then compared to those for spleens and PB from the same rabbits. In normal conjunctiva, ∼9.7% to ∼12.8% of CD45+ ICLs were CD4+ CD25+ T cells, compared to less then 5.5% in PB (Table 1). Within the CD45+ leukocyte population, CD4+ CD25+ T cells were therefore two to three times more abundant in conjunctiva than in PB (P < 0.05). Surprisingly, spleen and conjunctiva had similar percentages of CD4+ CD25+ T cells, even though the spleen is a primary lymphoid organ. In normal conjunctiva, ∼1.2% to 2.7% of CD45+ ICLs were CD4+ CD25− T cells, compared to ∼2.8% to 4.3% in PB and ∼56.1% to 66.7% in the spleen. As expected, very little staining for CD4 or CD25 markers was detected using isotype control mAbs. Thus, compared to spleen and PB, CD4+ CD25+ T cells form a predominant subpopulation within normal rabbit ICLs.
TABLE 1.
Number of immune cells isolated from individual conjunctivas of rabbits with no ocular inflammatory disease
| Immune cell phenotypea | No. of cells/eyeb
|
Yield (%) ± SD | ||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | ||
| Unsorted conjunctival cells | 8.26 × 106 | 9.5 × 106 | 9.5 × 106 | |
| CD45+ leukocytes | 1.31 × 106 | 1.65 × 106 | 0.95 × 106 | 23.32 ± 2 |
| CD4+ CD25+ T cells | 2.70 × 105 | 2.33 × 105 | 1.9 × 105 | 4.66 ± 1 |
| CD4+ CD25− T cells | 0.98 × 105 | 1.21 × 105 | 1.13 × 105 | 2.4 ± 2 |
Fresh upper and lower conjunctivas (n = 60 eyes) were harvested from normal rabbits with no apparent ocular inflammatory disease. CD4+ CD25+ and CD4+ CD25− T cells were isolated from sorted CD45+ cells.
The average numbers of total CD45+, CD4+ CD25+, and CD4+ CD25− cells recovered per eye. Results are the averages of three independent experiments.
TABLE 2.
Percentages of CD4+ CD25+ and CD4+ CD25− T cells detected within CD45+ ICL populationsa
| Cell phenotype among CD45+ leukocytes | % of cells recovered
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Conjunctiva
|
Spleen
|
PB
|
|||||||
| Expt 1 | Expt 2 | Expt 3 | Expt 1 | Expt 2 | Expt 3 | Expt 1 | Expt 2 | Expt 3 | |
| CD4+ CD25+ T cells | 11.2 | 9.7 | 12.8 | 12.3 | 10.6 | 11.8 | 5.1 | 5.5 | 5.2 |
| CD4+ CD25− T cells | 1.2 | 2.7 | 2.2 | 56.1 | 66.7 | 64.9 | 3.1 | 2.8 | 4.3 |
| IgG1 isotype control | 0.2 | 0.4 | 0.1 | 0.2 | 0.3 | 0.1 | 0.2 | 0.1 | 0.1 |
| IgG2b isotype control | 1.1 | 0.4 | 0.1 | 1.2 | 0.4 | 0.2 | 0.1 | 0.8 | 0.2 |
Fresh conjunctivas (n = 60 eyes), spleens (n = 10) and PBMC (n = 10) were harvested from normal rabbits in three different experiments (experiments 1, 2, and 3). Conjunctiva cell suspensions were stained with CD3, CD4, and CD25 and acquired by FACS. The percentages of CD4+ CD25+ and CD4+ CD25− T cells were determined by gating CD45+ cells and acquiring 10,000 events per sample.
FIG. 1.
CD4+ CD25+ T cells are a predominant subpopulation of rabbit intraconjunctival CD45+ leukocytes. Fresh conjunctiva, PBMC, and spleen were harvested from normal rabbits. Immunomagnetic enrichment was used to sort out CD45+ leukocytes. The sorted CD45+ leukocyte population was stained with rabbit-specific antibody to CD4 and CD25 molecules (left three graphs) or with isotype control mAbs. Shown are representative experiments from a total of five experiments.
Normal conjunctiva-derived CD4+ CD25+ T cells exhibit phenotypic characteristics of “natural” regulatory T cells.
To further characterize the conjunctiva CD4+ CD25+ T-cell subpopulation, we used FACS, Western blotting, and semiquantitative reverse transcription (RT)-PCR to assess the expression of CD4, CD25, CTLA4, GITR, and Foxp3, a set of specific markers previously described as being naturally expressed by murine and human CD4+ CD25+ Treg cells (4). CD4+ CD25+ T cells sorted from fresh conjunctiva samples were ∼98% pure. The yield of CD4+ CD25+ T cells was ∼4.57% of the total conjunctival cells and ∼13.9% of the CD45+ ICL population. CD4+ CD25− T cells were also purified from fresh conjunctiva samples and used in parallel (control).
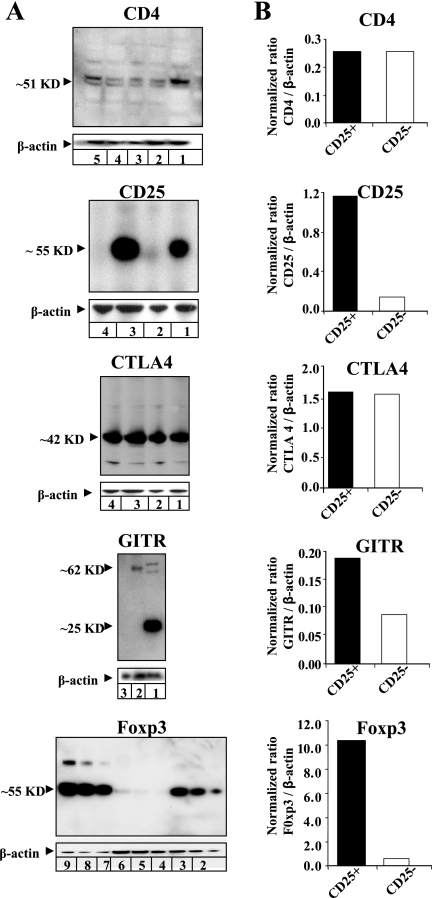
As expected, CD4 protein was detected in both conjunctival CD4+ CD25+ and CD4+ CD25− T cells by Western blotting (Fig. 2). The molecular mass was ∼51 kDa, similar to that of human CD4. Control normal mouse sera did not recognize rabbit CD4 (not shown). Using a mouse mAb specific for rabbit CD25, high levels of CD25 protein with the expected molecular mass of ∼55 kDa were detected in extracts of conjunctival CD4+ CD25+ T cells. In contrast, conjunctival CD4+ CD25− T cells had low levels of CD25 protein. Unexpectedly, both CD4+ CD25+ and CD4+ CD25− T cells had significant CTLA4 protein. The rabbit CTLA4 protein had a molecular mass of ∼42 kDa, similar to that of human CTLA4. The GITR protein was expressed at high levels by CD4+ CD25+ T cells compared to CD4+ CD25− T cells. Rabbit GITR protein had a molecular mass of ∼62 kDa, similar to the molecular mass of GITR in human CCRF-CEM cells. All protein-loading controls were confirmed by stripping and reprobing the same blots with a mAb specific for the rabbit housekeeping protein β-actin.
FIG. 2.
Western blot analysis of CD4, CD25, CTLA4, GITR, and Foxp3 proteins from CD4+ CD25+ T cells versus CD4+ CD25− T cells isolated from normal rabbit conjunctiva. (A) CD4 (lanes: 1, positive control CCRF-CEM cell extract; 2, 13 μg CD4+ CD25+ cell lysate; 3, 13 μg CD4+ CD25− cell lysates; 4, 27 μg CD4+ CD25+; 5, 27 μg CD4+ CD25−), CD25 (lanes: 1, 7 μg CD4+ CD25+; 2, 7 μg CD4+ CD25−; 3, 14 μg CD4+ CD25+; 4, 14 μg CD4− CD25−), CTLA4 (lanes: 1, 7 μg CD4+ CD25+; 2, 7 μg CD4+ CD25−; 3, 14 μg CD4+ CD25+; 4, 14 μg CD4+ CD25−), GITR (lanes: 1, CCRF-CEM; 2, 7 μg CD4+ CD25+; 3, 7 μg CD4+ CD25−) (arrowheads indicate GITR monomer and dimer), and Foxp3 (lanes: 1, 2.5 μg human PBMC; 2, 5 μg human PBMC; 3, 7.5 μg human PBMC; 4, 6 μg CD4+ CD25−; 5, 12 μg CD4+ CD25−; 6, 18 μg CD4+ CD25−; 7, 6 μg CD4+ CD25+; 8, 12 μg CD4+ CD25+; 9, 18 μg CD4+ CD25+). (B) Results from three experiments normalized to β-actin.
Transcription factor Foxp3 protein, the most specific marker of CD4+ CD25+ nTreg cells (21), was abundant in conjunctival CD4+ CD25+ T cells but not in CD4+ CD25− T cells (Fig. 2). Rabbit Foxp3 protein had a molecular mass of 55 kDa, similar to the molecular mass of Foxp3 detected in human PBMC-derived CD4+ CD25+ T cells. Consistent with Western blot results, RT-PCR revealed abundant Foxp3 mRNA in freshly isolated conjunctival CD4+ CD25+ T cells, whereas the CD4+ CD25− T cells had no detectable Foxp3 mRNA (not shown).
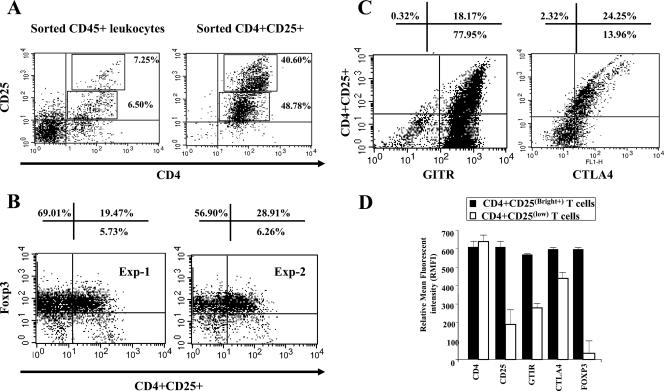
The above-described results were confirmed by FACS analysis of the expression of Foxp3, GITR, and CTLA4 molecules on conjunctival CD4+ CD25+ T cells. A total of 15.75% (7.25% CD25+ low, 6.50% high) of sorted CD45+ ICLs constitutively coexpressed both CD4 and CD25 cell surface markers (Fig. 3A, left). Furthermore, within sorted CD4+ CD25+ T cells, we detected two cell subsets that expressed cell surface CD25 molecules at high [CD4+ CD25(Bright+) T cells] and low [CD4+ CD25(low) T cells] levels (Fig. 3A, right). The percentages of these two CD4+ CD25+ T-cell subsets were similar: 40.60% ± 2% expressed high levels of the CD25 molecule, while 48.78% ± 2% expressed moderate to low levels of the CD25 molecule. Foxp3 (Fig. 3B), GITR, and CTLA4 (Fig. 3C) were detected in the CD4+ CD25+ conjunctival population. By gating on either CD4+ CD25(Bright+) T cells or CD4+ CD25(low) T cells, we determined that staining for Foxp3, GITR, and CTLA4 protein markers was more intense in CD4+ CD25(Bright+) T cells than in CD4+ CD25(low) T cells (Fig. 3D). Together, results from Western blotting, RT-PCR, and FACS (i) indicated that CD4+ CD25+ T cells are heterogeneous and can be divided into two subsets based on the level of CD25 molecule expression, CD4+ CD25(Bright+) T cells and CD4+ CD25(low) T cells, and (ii) showed that compared to CD4+ CD25(low) T cells, CD4+ CD25(Bright+) T cells expressed higher levels of Foxp3 and GITR markers, thus exhibiting a “natural” Foxp3+ CD4+ CD25+ nTreg cell-like phenotype.
FIG. 3.
Normal conjunctiva CD4+ CD25+ T cells exhibit phenotypic characteristics of nTreg cells. Upper and lower fresh conjunctivas were collected from normal rabbits, and CD4+ CD25+ T cells were analyzed by FACS either as unsorted conjunctival cells or as sorted CD45+ leukocytes. (A) The percentage of CD4+ CD25(Bright+) and CD4+ CD25(low) T-cell subsets before sorting of cells (left) and within the CD45+ T-cell-enriched population (right). (B) Expression of transcription factor Foxp3 in conjunctiva CD4+ CD25(Bright+) T cells in two independent experiments. (C) Detection of cell surface GITR and intracytoplasmic CTLA4 proteins in CD4+ CD25(Bright+) T cells by FACS. CTLA4 staining was performed after cell membrane permeabilization. Shown is a representative experiment of a total of five experiments. (D) Relative mean florescent intensities in CD4+ CD25(Bright+) versus CD4+ CD25(low) cells.
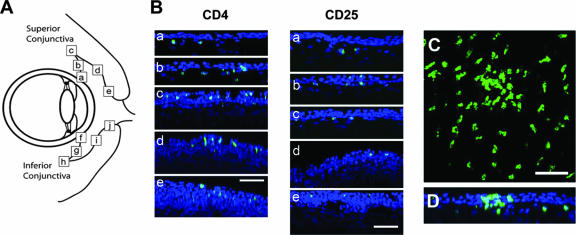
Distribution of CD4+ and CD25+ T cells in rabbit conjunctiva.
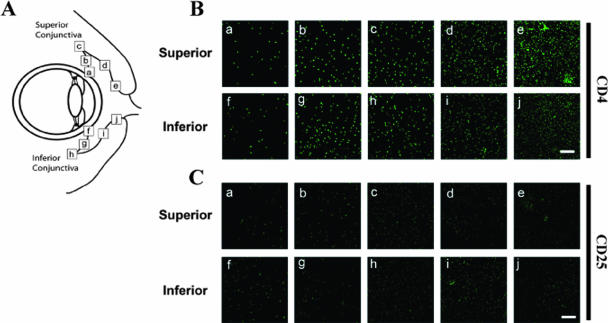
Immunohistological confocal microscopy analysis was carried out on upper and lower bulbar and palpebral conjunctival tissues to examine the in vivo distribution of CD4+ CD25+ T cells. Maximum-intensity projections from a typical conjunctival sample stained with anti-CD4 and anti-CD25 are shown in Fig. 4. Cell density was sampled at defined locations along the respective superior and inferior conjunctiva (Fig. 4A), including the limbus (a and f), bulbar area (b and g), fornix (c and h), palpebral area (d and i), and lid margin (e and j). Overall, the density of CD4+ cells (Fig. 4B) was much higher than the density of CD25+ cells (Fig. 4C). Furthermore, stained CD4 cells appeared with a higher density at the lid margin (e and j) than at the limbus (a and f). CD4+ CD25+ T cells were observed mainly in the palpebral conjunctiva, more pronounced in the upper than in the lower lid (Fig. 4B and C).
FIG. 4.
CD4+ CD25+ cell distribution in superior and inferior conjunctiva. (A) Schematic location of samples tested in this study. a and f, limbal conjunctiva; b and g, bulbar conjunctiva, c and h, fornix conjunctiva; d and i, palpebral conjunctiva; e and j, conjunctiva near the lid margin. Sections from different regions of superior and inferior conjunctiva, indicated by the letters a to j shown in A, were stained with mAbs specific for rabbit CD4 or anti-CD25 molecules. (B) Maximum-intensity projection image of scanning of conjunctiva stained for anti-CD4 (upper two lines) or anti-CD25 (lower two lines). Labels a thorough j are matched to Fig. 2A. The scale bar shows 200 mm. (C) Image reconstructed from scanned images of (a) limbal conjunctiva, (b) fornix conjunctiva, and (c) lid margin conjunctiva.
Interestingly, x-z slices through the three-dimensional data sets showed a preferred retention of CD4+ CD25+ T cells within the conjunctival epithelium or substantia propria, dependent on the conjunctival compartment (Fig. 5). At the limbus, CD4+ CD25+ T cells were retained exclusively within the substantia propria below the conjunctival epithelium (Fig. 5B, a). However, in the bulbar conjunctiva and fornix (Fig. 5B, panels b and c, respectively), CD4+ CD25+ T cells were present both in the conjunctival epithelium and in the substantia propria. CD4+ CD25+ T cells were localized almost exclusively to the conjunctival epithelium in the palpebral and lid margin (Fig. 5B, panels d and e, respectively). Some cells appeared to reside within the superficial epithelial cell layer. Additionally, clusters of CD4+ T cells at the lid margin (Fig. 5C) were almost completely intraepithelial (Fig. 5D). Together, these results indicated that CD4+ CD25+ T cells were localized within both conjunctival epithelium and the stroma. The rabbit conjunctiva exhibited clusters of T cells that are organized in follicle-like structures, thus sharing a typical ultrastructure with human conjunctiva.
FIG. 5.
CD4+ CD25+ cell distribution in superior and inferior conjunctivas. (A) Schematic location of samples tested in this study. Images were reconstructed from scanned images of (a) limbal conjunctiva, (b) fornix conjunctiva, and (c) lid margin conjunctiva. Blue and green indicate nuclei of conjunctival cells and CD4+ cells, respectively. (B) accumulation of the CD4+ CD25+ cells at the superior palpebral conjunctiva. (C) Maximum-intensity projection image of x-y scanning of CD4+ cells at the lid margin (region e). (D) x-z reconstructed image from x-y scanning image. CD4+ cells accumulate at the superficial conjunctiva. Scale bar, 200 mm.
Conjunctiva-derived CD4+ CD25(Bright+) T cells exhibit functional characteristics of “natural” Treg cells.
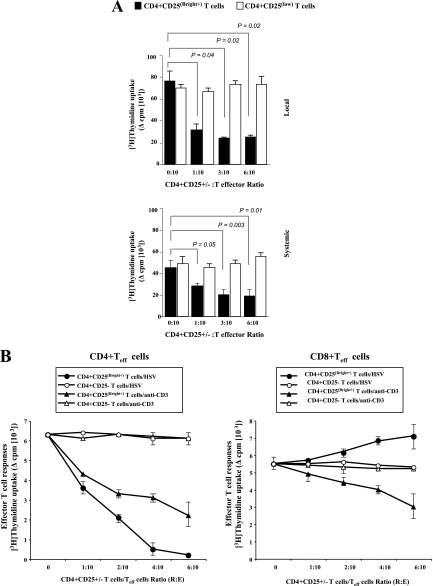
Normal conjunctiva CD4+ CD25(Bright+) and CD4+ CD25(low) T cells were sorted, and their suppressive effect on HSV-specific CD4+ and CD8+ Teff cells was evaluated in vitro. Rabbits were ocularly infected with HSV-1 (McKrae), and 11 days postinfection, the CD4+ CD25− and CD8+ CD25− Teff cells were sorted from immune conjunctiva. Teff cells were also isolated from immune PBMC. As shown in Fig. 6A, conjunctiva-derived CD4+ CD25(Bright+) T cells but not CD4+ CD25(low) T cells induced a significant decrease in the proliferation of HSV-specific CD4+ Teff cells (P < 0.05). The suppressive effect was observed against HSV-specific Teff cells derived either locally from the conjunctiva (Fig. 6A, top) or systemically from the PBMC (Fig. 6A, bottom). The suppression was efficient on CD4+ Teff cells with a ratio as low as 1 CD4+ CD25(Bright+) cell to 10 Teff cells. In the presence of CD4+ CD25(Bright+) T cells, CD4+ Teff cell proliferation was inhibited 70.6% ± 3.6% (P = 0.04) in conjunctiva and 45.5% ± 2.4% (P = 0.05) in PB. The CD4+ CD25(Bright+) T-cell subpopulation used in this study was ∼97% pure, was positive for Foxp3+, and was obtained from animals with no apparent ocular infection or inflammation; therefore, it is unlikely that they contained a significant number of activated Teff cells.
FIG. 6.
Normal conjunctiva CD4+ CD25(Bright+) T cells exhibit functional properties of nTreg cells. (A) Effect of addition of naïve conjunctiva-derived CD4+ CD25(Bright+) T cells on proliferative responses of CD4+ Teff cells from ocularly infected rabbits to whole HSV-1 recall antigens. Proliferation assays of HSV-1-specific CD4+ CD25− Teff cells were performed by stimulating a constant number (1 × 105 cells) of immune HSV-specific CD4+ CD25− Teff cells from either the conjunctiva (top) or PBMC (bottom) of HSV-1-infected rabbits in the absence or presence of increasing numbers of conjunctiva-derived CD4+ CD25(Bright+) T cells. As a control, the open bars show CD4+ CD25+ instead of CD4+ CD25− cells used as responder cells. Data are the differences between the mean counts per minute for CD4+ CD25−-stimulated Teff cells minus the mean counts per minute for unstimulated CD4+ CD25− Teff cells. (B) T-cell proliferation assays were performed by stimulating CD4+ CD25− Teff cells (left panels) or CD8+ Teff cells (right panels) derived from immune conjunctiva (5 × 104 cells) with either heat-inactivated HSV (multiplicity of infection of 0.3) (circles) or an anti-rabbit CD3 mAb (triangles) in the absence or in the presence of increasing numbers of conjunctiva-derived CD4+ CD25(Bright+) T cells (closed symbols) or CD4+ CD25− T cells (open symbols). The ratio of CD4+ CD25+ T cells to CD4+ CD25− and CD8+ CD25− Teff cells is indicated on the x axis in each graph. These panels are representative data from a total of three separate experiments. Values are means ± standard deviations of triplicate experiments per condition (*, P < 0.05).
Normal conjunctiva CD4+ CD25(Bright+) T cells markedly impaired the expansion of CD8-depleted HSV-specific CD4+ CD25− Teff cells (Fig. 6B, left). By contrast, titration of the same dose of normal conjunctiva CD4+ CD25(Bright+) T cells into the cultures of CD8+ CD25− T cells enhanced HSV-specific CD8+ Teff proliferation (Fig. 6B, right), suggesting the presence of helper functions (e.g., due to cytokine secretion or antigen presentation) within this CD4+ T-cell subpopulation. As a control, titration of the same dose of normal conjunctiva CD4+ CD25− T cells into the cultures of HSV-specific CD4+ or CD8+ Teff cells did not affect the degree of proliferation, thereby excluding the possibility that an increase in the total responder cell number was responsible for the observed suppression. Finally, unlike their CD4+ CD25− T-cell counterparts, conjunctiva CD4+ CD25(Bright+) T cells were hyporesponsive in vitro to phytohemagglutinin stimulation (not shown).
In subsequent experiments, the CD4+ CD25(Bright+) T cells derived from normal conjunctiva significantly suppressed the anti-CD3-induced proliferative T-cell responses of allogeneic PBMC-derived CD4+ and CD8+ Teff cells at a ratio of 1 CD4+ CD25(Bright+) T cell to 10 allogeneic Teff cells (Fig. 6B, left and right, respectively). The results indicate that conjunctiva CD4+ CD25(Bright+) T cells exhibit “natural” Foxp3+ CD4+ CD25+ nTreg cell-like function.
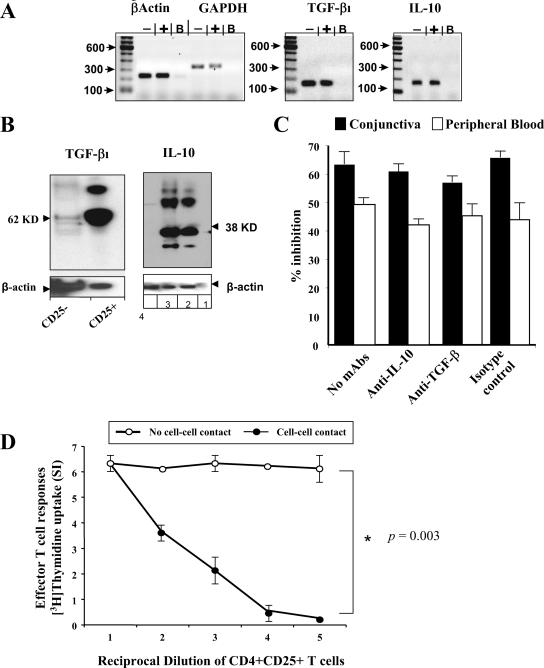
Suppressive activity of conjunctiva CD4+ CD25+ Treg cells is cell-cell contact dependent but independent of IL-10 and TGF-β.
Using Western blotting, we detected IL-10 in conjunctival CD4+ CD25+ T cells but not in CD4+ CD25− T cells (Fig. 7B). The apparent molecular mass of rabbit IL-10 was ∼39 kDa, similar to that of human recombinant IL-10, which was used as a control. As expected, normal mouse serum did not recognize rabbit IL-10 in CD4+ CD25+ T-cell lysates (not shown). Using a mouse mAb specific for rabbit TGF-β1, we showed that conjunctival CD4+ CD25+ T cells expressed high levels of TGF-β1 with the expected molecular mass of ∼62 kDa. In contrast, conjunctival CD4+ CD25− T cells did not express TGF-β1. All protein-loading controls were confirmed by stripping and reprobing with a mAb specific for rabbit β-actin. In addition, using RT-PCR, we identified transcripts for both IL-10 and TGF-β1 in CD4+ CD25+ T cells but not in CD4+ CD25− T cells (Fig. 7A and Tables 3 and 4). As expected, RT-PCR detected similar levels of housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin mRNAs in both CD4+ CD25+ and CD4+ CD25− T cells.
FIG. 7.
Suppressive activity of conjunctival CD4+ CD25+ Treg cells is cell-cell contact dependent but independent of IL-10 and TGF-β1. (A) CD4+ CD25+ and CD4 CD25− T cells were isolated from naïve rabbit conjunctiva. The cells were lysed in RLT buffer by scraping, immediately frozen in liquid nitrogen, and then thawed on ice. The RNAs were eluted from the silica column, and the product was analyzed to verify both the quantity and quality of the RNA as described in Materials and Methods. The RNA was obtained from CD4+ CD25+ and CD4 CD25 rabbit T cells and was subjected to PCR using primers specific for IL-10, TGF-β1, GAPDH, and β-actin. Lanes: −, CD4+ CD25 rabbit cell RNAs; +, CD4+ CD25+ cell RNAs; B, control buffer. (B) Western blot analysis of IL-10 and TGF-β1 proteins from CD4+ CD25+ T cells versus CD4+ CD25− T cells isolated from normal rabbit conjunctiva. Data for TGF-β1 (lanes: 1, 13 μg CD4+ CD25+ cell lysate; 2, 13 μg CD4+ CD25− cell lysates) and IL-10 (lanes: 1, positive control CCRF-CEM cell extract; 2, 13 μg CD4+ CD25+ cell lysate; 3, 13 μg CD4+ CD25− cell lysates) are shown. (C) Immune PBMC (white columns) or immune conjunctiva (black columns)-infiltrating CD4 CD25− T cells induced during an ocular infection with HSV-1 (McKrae) were depleted of CD8+ cells and cultured in the presence of normal conjunctiva CD4+ CD25+ regulatory T cells alone (no mAbs) or with anti-IL-10, anti-TGF-β1, or isotype control IgG. (D) Immune conjunctiva-infiltrating CD4 CD25− Teff cells induced during an ocular infection with HSV-1 (McKrae) were cultured in the presence of normal conjunctiva CD4+ CD25+ T cells but separated through a microporous membrane (no cell-cell contact) and not separated together in one well (cell-cell contact). Data are the means of data from triplicate wells.
TABLE 3.
Relative quantitation of IL-10 from rabbit conjunctiva CD4+ CD25+ and CD4+ CD25− T cells by real-time PCRa
| RNA source | Avg CT ± SD
|
ΔCT ± SDb | ΔΔCT ± SDc | IL-10 level relative to CD25+ (range)d | |
|---|---|---|---|---|---|
| IL-10 | GAPDH | ||||
| CD4+CD25+ T cells | 20.21 ± 6.43 | 20.73 ± 4.45 | −0.52 ± 7.82 | −5.95 ± 7.82 | 61.66 (0.27-13,950) |
| CD4+CD25- T cells | 25.34 ± 0.64 | 19.91 ± 2.17 | 5.43 ± 2.26 | 0.00 ± 2.26 | 1.00 (0.21-4.81) |
The values represent the mean threshold cycles (CT) for target amplification ± standard deviations. Testing of each sample was performed in triplicate.
The DCT was determined by subtracting the target gene CT (IL-10) from the GAPDH CT. The standard deviation of the difference was calculated from the standard deviations of the target gene and GAPDH values.
The DDCT was calculated by subtracting the DCT of the CD4+ CD25− T cells from the DCT of the compared CD4+ CD25+ cells. As this was an arbitrary constant, the standard deviation is equivalent to the standard deviation of the DCT.
The range given for the target genes relative to CD4+ CD25− T cells was determined by the following expression: 2(ΔDCT ± standard deviation).
TABLE 4.
Relative quantitation of TGF-β1 from rabbit conjunctiva CD4+ CD25+ and CD4+ CD25− T cells by real-time PCRa
| RNA source | Avg CT ± SD
|
ΔCT ± SDb | ΔΔCT ± SDc | TGF-β1 relative to CD25+ (range)d | |
|---|---|---|---|---|---|
| TGF-β1 | GAPDH | ||||
| CD4+ CD25+ T cells | 21.52 ± 5.21 | 20.73 ± 4.45 | 0.79 ± 6.86 | −2.42 ± 6.86 | 5.34 (0.05-619.13) |
| CD4+ CD25− T cells | 23.12 ± 1.60 | 19.91 ± 2.17 | 3.21 ± 2.70 | 0.00 ± 2.70 | 1.00 (0.15-6.48) |
The values represent the mean CT values for target amplification ± standard deviations. Testing of each sample was performed in triplicate.
The DCT was determined by subtracting the target gene CT (TGF-β1) from the GAPDH CT. The standard deviation of the difference is calculated from the standard deviations of the target gene and GAPDH values.
The DDCT was calculated by subtracting the DCT of the CD4+ CD25− T cells from the DCT of the compared CD4+ CD25+ cells. As this was an arbitrary constant, the standard deviation is equivalent to the standard deviation of the DCT.
The range given for the target genes relative to CD4+ CD25− T cells was determined by the following expression: 2(ΔDCT ± standard deviation).
Since CD4+ CD25+ Treg cells appeared to express both IL-10 and TGF-β, we next used anti-cytokine-blocking and transwell experiments to assess whether the down-regulation effect on HSV-specific Teff cells occurred through these cytokines or via a cell-cell contact. Following ocular herpes infection, we sorted immune PBMC and immune conjunctiva infiltrating CD4+ CD25− T cells and cocultured them with normal conjunctiva CD4+ CD25+ Treg cells in the presence of neutralizing antibodies to either IL-10 or TGF-β. Coculture of normal conjunctiva CD4+ CD25+ Treg cells with either immune PBMC or immune conjunctiva infiltrating CD4+ CD25− T cells resulted in a significant decrease in their proliferation. Neutralization of either IL-10 or TGF-β had no effect on the suppressive activity of normal conjunctiva CD4+ CD25+ Treg cells (Fig. 7C). Transwell data shown in Fig. 7D indicate that CD4+ CD25+ Treg cells cultured in the inner wells did not inhibit the proliferative activity of CD4+ CD25− Teff cells cultured in the outer wells. By contrast, the coculture of CD4+ CD25+ Treg cells and immune CD4+ CD25− Teff cells in the outer wells led to a strong suppression of HSV-specific Teff cell expansion.
These results show that cell-cell contact is required for the suppressive activity of conjunctiva CD4+ CD25+ Treg cells. In addition, although conjunctiva CD4+ CD25+ Treg cells expressed IL-10 and TGF-β, neutralization of these cytokines did not abrogate the suppressive function. This result suggests that rabbit conjunctiva CD4+ CD25+ Treg cells are bona fide endogenous naturally occurring CD4+ CD25+ nTreg cells.
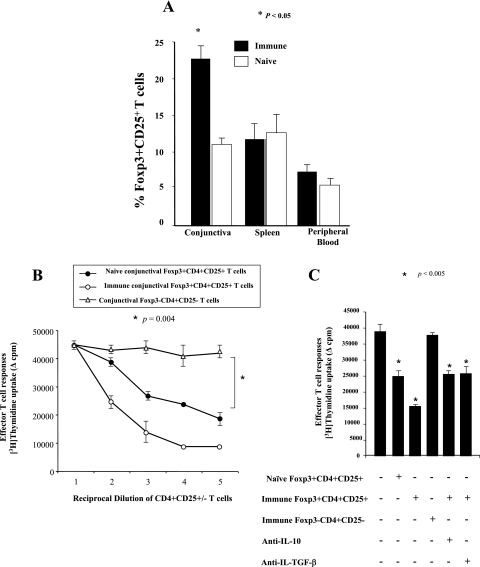
Increased Foxp3+ CD4+ CD25+ Treg cells and their suppressive activities in immune conjunctiva during ocular herpes infection.
Groups of 10 rabbits each were either ocularly infected with HSV-1 (McKrae) or left uninfected. Eleven days postinfection, conjunctivas, spleens, and blood were collected, and the phenotype and function of residual CD4+ CD25+ T cells were examined. As shown in Fig. 8 (summary of 10 rabbits), there was approximately a twofold increase in the frequency of Foxp3+ CD4+ CD25+ T cells in immune conjunctiva of infected rabbits compared with naïve conjunctiva (P < 0.005). However, no significant increase was detected in immune PBMC or spleen. Consistent with this, immune conjunctiva Foxp3+ CD4+ CD25+ T cells exhibited a significantly higher (P < 0.005) suppressive activity (62.3 versus 37.5% of inhibition of allogeneic Teff cell proliferation) than nonimmune Foxp3+ CD4+ CD25+ T cells (Fig. 8B). The suppressive activity was Treg/Teff ratio dependent. As a control, immune conjunctiva Foxp3− CD4+ CD25− T cells did not affect the degree of Teff cell proliferation (Fig. 8B and C). The suppressive activity of immune conjunctiva Foxp3+ CD4+ CD25+ Treg cells was cell-cell contact dependent but independent of IL-10 and TGF-β (Fig. 8C). This result suggests that ocular infection may contribute to a recruitment, accumulation, and/or expansion of Foxp3+ CD4+ CD25+ Treg cells within conjunctiva, close to the site of herpes infection. Ocular infection may also contribute to enhancing the suppressive function of Foxp3+ CD4+ CD25+ Treg cells locally at sites of infection.
FIG. 8.
Increased CD4+ CD25+ nTreg cells and their suppressor activity in immune conjunctiva of rabbits with ocular herpes infection. (A) Relative frequency of CD4+ CD25+ T cells isolated from the immune conjunctiva, spleen, and PB of HSV-1-infected rabbits and uninfected controls. Rabbits were either infected ocularly with 2 × 105 PFU of HSV-1 (left panels) or left uninfected (right panels). Eleven days postinfection, conjunctiva, spleen, and blood were collected. The percentages of Foxp3+ CD4+ CD25+ T cells in total CD4+ cells isolated from the conjunctivas of normal rabbits (n = 15) and infected rabbits (n = 17) were determined by using FACS. *, P < 0.05. (B) Conjunctiva CD4+ CD25+ T cells from HSV-1-infected rabbits possess higher regulatory activity. A constant number of immune conjunctiva CD4+ CD25− Teff cells (2 × 105 cells/well) were cocultured with an increasing number of CD4+ CD25+ T cells derived from either normal or immune conjunctiva. As control, a constant number of immune conjunctiva CD4+ CD25− Teff cells (2 × 105 cells/well) were cocultured with an increasing number of CD4+ CD25− T cells derived from normal conjunctiva. After 4 days of culture, T-cell proliferation was measured using an additional overnight [3H]thymidine (1 μCi/well) uptake assay. Results from a representative experiment of three are shown. (C) CD4+ CD25+ Treg cells sorted from immune conjunctiva-mediated suppression were cell-cell contact dependent but independent of IL-10 and TGF-β1. Immune conjunctiva-infiltrating CD4+ CD25− Teff cells induced during an ocular HSV-1 infection were depleted of CD8+ cells and cultured alone or in the presence of immune or normal conjunctiva-derived CD4+ CD25+ regulatory T cells. In some experiments, immune conjunctiva-infiltrating CD4+ CD25− Teff cells were incubated with CD4+ CD25+ T cells that were also from immune conjunctiva and separated through a microporous membrane (no cell-cell contact), and neutralizing antibodies against either IL-10 or TGF-β1 were added to the culture wells. Data are the means of duplicate wells.
DISCUSSION
Regulatory T cells have been identified in many species during the last decade (reviewed in reference 82), but information on the rabbit Treg cell repertoire, phenotype, and function is particularly lacking. To our knowledge, this study is the first report on the identification and phenotypic and functional characterization of rabbit Treg cells. Rabbits harbor Foxp3+ CD4+ CD25(Bright+) nTreg cells that exhibited phenotypic and functional characteristics similar to those of murine and human thymus-derived Foxp3+ CD4+ CD25+ nTreg cells. During herpes infection, CD4+ CD25+ Treg cells have become major culprits in immunosuppression, and at the same time, their deficiency has been invoked as a cause of immunopathogenic hyperreactivity and inflammation (70). Evidence in both murine and human models of herpes infection and disease indicate that CD4+ CD25+ Treg cells regulate herpes infection, disease, and immunity. In the HSV-1 murine system, CD4+ CD25+ Treg cells affect both viral clearance and immunoinflammatory HSK disease, while in humans, they modulate CD4+ Teff cell memory responses to HSV-2 (4, 18, 75, 79). However, most of these data were obtained using systemic CD4+ CD25+ Treg cells either derived from mouse spleen or derived from human PB (4, 18, 75, 79). The present study demonstrates that during an ocular infection with HSV-1, an increase in the number of CD4+ CD25+ Treg cells and their suppressor activity occurred in immune conjunctiva but not in the spleen or in circulating PB. These findings stress the importance of mucosal CD4+ CD25+ Treg cells that reside locally close to sites of herpes infection (i.e., at ocular and genital mucosal surfaces), where regulation is required (13, 28, 42, 43). They also indicate that spleen and PB may not always be the most appropriate compartments in which to investigate the repertoire and function of Treg cells (4, 21).
The chief functional units of OMIS, also known as the eye-associated lymphoid tissue, are the conjunctiva, lacrimal gland, and lacrimal drainage system (42). The major T-cell inductive site of the OMIS is the conjunctiva mucosal surface (13, 28, 42, 43), which forms a continuous lymphoid tissue from the eyelid margin to the cornea (13, 14, 23, 42, 43). Beside the conjunctiva itself, the extent of ocular compartments at which conjunctival CD4+ CD25+ nTreg cells exert suppressive functions in vivo remains to be fully elucidated. The cornea, in contrast to the conjunctiva, is considered to be an “immune-privileged” compartment of the eye, with a minimum of lymphoid cells (36). Under physiologic conditions, the cornea maintains its transparency, allowing the passage of light to the retina (16, 43). Under inflammatory conditions, such as during HSK, the cornea must rely on the support of neighboring lymphoid tissues and local lymphoid cell follicles for immune protection and immune modulation (16, 43). When the topographical distribution of conjunctiva lymphoid tissue is projected onto the ocular surface, it overlies the surface of the cornea during eye closure and is hence in a suitable position to assist corneal immunity and immunoregulation (43). On one hand, corneal inflammation often induces the development of organized conjunctival leukocytic aggregates (12, 63). On the other hand, conjunctival immune cells have been shown to influence the corneal immune response and the course of HSK after corneal HSV-1 infection (1). Likewise, it is clinically known that long-term eye closure alleviates corneal inflammation (A. B. Nesburn, unpublished data).
It may be hypothesized that conjunctiva resident CD4+ CD25(Bright+) Treg cells can suppress “aggressor” CD4+ Teff cells in the cornea and resolve corneal HSK inflammation, a leading cause of human visual impairment and blindness worldwide (54, 56, 58, 60). This does not imply excluding the involvement of CD4+ CD25(Bright+) Treg cells from draining lymphoid organs, such as preauricular and cervical lymph nodes, in corneal immunity and inflammation (34, 48). Recurrent corneal HSK has been reported to be similar in rabbits and humans. Hence, our future plans will be to investigate, using a rabbit model, whether conjunctiva CD4+ CD25+ Treg cells play a crucial role in HSK and to determine the HSV genes and antigens that might be involved in the induction and accumulation of CD4+ CD25+ Treg cells locally in the conjunctiva. This investigation will use the already available HSV mutant knockout from specific genes.
Recent studies showed that immigration and appropriate localization into mucosal sites is a prerequisite for the in vivo activity of CD4+ CD25+ nTreg cells, the balance of the ratio of Treg cells to Teff cells, and the resolution of inflammatory diseases (2, 19, 74). Results from the present study may also suggest that among the circulating CD4+ T cells, a dominant portion that naturally expresses the CD25 molecule is retained and survives in the conjunctival tissue by yet-to-be-defined mechanisms. It has also been shown that human conjunctival epithelial cells can induce the expression of human mucosal lymphocyte antigen 1 on CD4+ T cells (31). These cells are retained in the intraepithelial compartment of the conjunctiva by virtue of the interaction between human mucosal lymphocyte antigen 1 and its natural ligand, E-cadherin, expressed on epithelial cells (31). Suffia and coworkers recently showed a critical role for the αE chain (CD103) of αEβ7 in the retention of CD4+ CD25+ Treg cells at mucocutaneous sites of inflammation (73). The antigen VLA-4 was also shown to be involved in the migration of CD4+ CD25+ nTreg cells to the eye (75). Similar mechanisms may allow the retention of CD4+ CD25+ nTreg cells within the epithelial compartment of the conjunctiva.
As Treg cells reside in lymphoid organs, early studies suggested that they inhibit predominantly naïve T-cell priming in the draining lymph nodes (17). Later studies of various infectious diseases showed that Treg cells infiltrate and traffic into inflammatory peripheral mucocutaneous sites and suppress Teff cell function (71). Interestingly, we found that ocular herpes infection triggered an increased accumulation of Foxp3+ CD4+ CD25+ Treg cells and their suppressive activity in the conjunctiva and to a lesser extent in spleen and PB. During ocular infection, there was a slight decrease in Foxp3+ CD4+ CD25+ Treg cells in the spleen compared to those in the conjunctiva, which could reflect a redistribution of Treg cells, similar to what has been reported during human immunodeficiency virus and hepatitis C virus infections (41). The expansion of Foxp3+ CD4+ CD25+ Treg cells close to the site of herpes infection is consistent with the notion that they might be triggered by viral antigens (4, 41, 52). Whether Foxp3+ CD4+ CD25+ Treg cells are thymus originated or converted from conventional CD4+ CD425− T cells, either locally or at the periphery, is unknown. It remains to be defined how and why Foxp3+ CD4+ CD25+ Treg cells predominate in conjunctiva and what their antigenic specificity is. Although conjunctiva CD4+ CD25+ Treg cells express Foxp3 and exhibit functional characteristics of nTreg cells, we cannot rule out that HSV-specific CD4+ CD25+ iTreg cells that secrete IL-10 and TGF-β are produced locally during ocular infection (4, 11, 86). Indeed, during ocular HSV infection, high levels of both TGF-β and IL-10 were detected in immune conjunctiva CD4+ CD25+ nTreg cells (data not shown). Conjunctiva CD4+ CD25+ nTreg cells are more likely to contribute to the early events occurring during an ocular infection, whereas both CD4+ CD25+ nTreg and inducible CD4+ CD25+ iTreg cells control later stage of the local immune response (9). Regardless of conjunctiva Treg cell origin, migratory molecular patterns, and antigen specificity, our results suggest compartmentalization of a relatively high number of Foxp3+ CD4+ CD25+ Treg cells in conjunctiva close to effector sites of infection where they can possibly modulate local ocular immunity and inflammation. Whether the human conjunctiva is also endowed with a large subpopulation of Foxp3+ CD4+ CD25+ Treg cells and whether they function during ocular herpes infection and HSK disease remain to be determined.
Human CD4+ T cells have a more variable expression of CD25 than do mouse CD4+ T cells, and only those that express CD25 with the highest intensities [CD4+ CD25(Bright+)] are suppressive (3, 4). CD4+ T cells expressing low levels of CD25 [CD4+ CD25(low)] are instead activated effector or memory T cells and lack regulatory function (35). During the last few years, we have been selecting and characterizing mAbs that specifically recognize rabbit CD3, CD4, CD25, Foxp3, GITR, and CTLA4 molecules using FACS, Western blotting, and confocal microscopy. Using that panel of mAbs, we fully characterize, for the first time, the phenotype of a rabbit Treg cell population. In addition, we showed that conjunctival CD4+ CD25(Bright+) T cells, but not CD4+ CD25(low) T cells, exhibited functional characteristics of endogenous CD4+ CD25+ nTreg cells, as they suppressed Teff cells via a cell-cell contact-dependent but cytokine-independent mechanism. However, whether a direct Treg-Teff cell contact or an indirect mechanism of suppression via other immune cells such as dendritic cells occurs in vivo remains to be investigated (21). Nevertheless, the rabbit conjunctival CD4+ CD25(Bright+) T cells described in this study expressed CTLA4, GITR, and transcription factor Foxp3, key regulatory molecules in the suppressive activity of CD4+ CD25+ Treg cells (20, 38, 40, 85). Rabbit conjunctiva Foxp3+ CD4+ CD25(Bright+) cell subsets are therefore phenotypically and functionally indistinguishable from human Foxp3+ CD4+ CD25+ nTreg cells in that they are homogenously positive for Foxp3 and display powerful T-cell-suppressive functions.
It has become evident that Foxp3+ CD4+ CD25+ nTreg cells not only influence self-antigen and pathogen-specific Teff cells but also dampen vaccine-mediated pathogen-specific immunity (49, 78, 79). It is speculated that rabbit OMIS is part of a generalized mucosal immune system capable of taking up conjunctival applied antigens and then disseminating the Teff cell response to other mucosal sites (22). Here, we demonstrate that CD4+ CD25+ nTreg cells derived from conjunctiva limited Teff cell function both mucosally and systemically. This result supports the idea that OMIS is part of the generalized mucosal immune system. Conceivably, the abundance and suppressive effect of conjunctiva Foxp3+ CD4+ CD25+ Treg cells could explain the difficulty of many vaccines in achieving effective ocular mucosal immunity when applied topically to the eye or injected subconjunctivally (30, 45, 56, 65). Therefore, strategies to interfere with conjunctiva Foxp3+ CD4+ CD25+ Treg cell function or to manipulate their number could be promising approaches for ocular vaccines and immunotherapies in T-cell-mediated ocular diseases (51). Current strategies for improving vaccine and pathogen-specific immunotherapy have tried to reduce the immunosuppressive effects of CD4+ CD25+ Treg cells and of negatively acting factors such suppressor of cytokine signaling 1 and programmed cell death 1 using neutralizing antibodies and small interfering RNA (49). From the ocular mucosal immunization standpoint, knowledge of nonpathological conjunctival CD4+ CD25+ Treg cells is therefore a vital basis for the development of successful topical ocular immunoprophylactic or immunotherapeutic vaccines. Likely, a strategy to manipulate conjunctiva CD4+ CD25+ Treg cells would mean applying a compound (or a molecular adjuvant) in a single dose followed shortly by topical ocular administration or subconjunctival injection of a vaccine or a therapeutic compound (64). We are currently assessing the effect of depletion/manipulation of rabbit conjunctiva CD4+ CD25+ Treg cells on the immunogenicity of herpes peptide and lipopeptide epitope vaccines topically applied to the eye (A. B. Nesburn and A. A. Chentoufi, unpublished data).
Of note, it is critical to understand what other consequences may occur from manipulating conjunctiva CD4+ CD25+ Treg cells. This is mainly because, beside their role in maintaining regional tolerance, physiological CD4+ CD25+ Treg cells might also be beneficial in reducing collateral immunopathological damage that might be caused by vigorous immune responses, such as those induced by exogenous antigens and pathogens that constantly attack the ocular surfaces (8, 54, 56, 58, 87). Rouse and coworkers recently reported that in the mouse model of HSV-1 ocular infection, CD4+ CD25+ Treg cells dampened spleen-derived HSV-specific CD8+ Teff cell responses (68). Intraperitoneal injection of anti-CD25 mAbs (PC61) before parenteral peptide immunization improves the magnitude of the spleen CD8+ T-cell response to the immunodominant peptide SSIEFARL (69, 76). However, there is no indication whether such manipulation resulted in the depletion of mouse conjunctival CD4+ CD25+ Treg cells, if there is any (14, 15, 72). Also unknown is whether the suppressive action of CD4+ CD25+ Treg cells is executed in secondary lymphoid organs or at the site of inflammation or both. Thus, investigating this “double-edged sword” question in the rabbit model will help delineate how to manipulate such vital cells so as to benefit ocular mucosal vaccination without exacerbating ocular inflammatory diseases. The challenge is to understand how to tailor conjunctiva CD4+ CD25+ Treg cell function to achieve the proper balance between the protection of the ocular mucosal surface and ocular immunopathological disease. This is especially important in a delicate organ such as the eye (12, 57).
In conclusion, this study provides the first evidence that normal rabbit conjunctivae contain a large subpopulation of functional Foxp3+ CD4+ CD25+ Treg cells that expressed high levels of CD25, CTLA4, and GITR markers and exhibited a cell-cell contact-dependent suppressive activity with no obvious role of immunoregulatory cytokines such as IL-10 and TGF-β. During HSV-1 ocular infection, there was a selective increase in Foxp3+ CD4+ CD25+ Treg cell frequency and function within immune conjunctiva, where they could efficiently limit HSV-specific CD4+ and CD8+ Teff cell function.
Acknowledgments
This work was supported by NIH grants EY15225, EY16663, EY14900, and EY13191 and by a Research to Prevent Blindness Unrestricted Challenge grant, the Skirball Program in Molecular Ophthalmology, and the Discovery Eye Foundation. S.L.W. is an RPB Senior Scientific Investigator. L.B is an RPB Special Award Investigator.
We have no financial conflict of interest.
Footnotes
Published ahead of print on 2 May 2007.
REFERENCES
- 1.Bauer, D., A. Schmitz, N. Van Rooijen, K. P. Steuhl, and A. Heiligenhaus. 2002. Conjunctival macrophage-mediated influence of the local and systemic immune response after corneal herpes simplex virus-1 infection. Immunology 107:118-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid, Y., R. B. Blank, and I. Suffia. 2006. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol. Rev. 212:287-300. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y., and B. T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353-360. [DOI] [PubMed] [Google Scholar]
- 5.BenMohamed, L., Y. Belkaid, E. Loing, K. Brahimi, H. Gras-Masse, and P. Druilhe. 2002. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur. J. Immunol. 32:2274-2281. [DOI] [PubMed] [Google Scholar]
- 6.BenMohamed, L., G. Bertrand, C. D. McNamara, H. Gras-Masse, J. Hammer, S. L. Wechsler, and A. B. Nesburn. 2003. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 77:9463-9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BenMohamed, L., A. Thomas, and P. Druilhe. 2004. Long-term multiepitopic cytotoxic-T-lymphocyte responses induced in chimpanzees by combinations of Plasmodium falciparum liver-stage peptides and lipopeptides. Infect. Immun. 72:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettahi, I., X. Zhang, R. E. Afifi, and L. BenMohamed. 2006. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol. 19:220-236. [DOI] [PubMed] [Google Scholar]
- 9.Cavassani, K. A., A. P. Campanelli, A. P. Moreira, J. O. Vancim, L. H. Vitali, R. C. Mamede, R. Martinez, and J. S. Silva. 2006. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J. Immunol. 177:5811-5818. [DOI] [PubMed] [Google Scholar]
- 10.Cespedes, I. S., F. N. Toka, A. Schollenberger, M. Gierynska, and M. Niemialtowski. 2001. Pathogenesis of mousepox in H-2(d) mice: evidence for MHC class I-restricted CD8(+) and MHC class II-restricted CD4(+) CTL antiviral activity in the lymph nodes, spleen and skin, but not in the conjunctivae. Microbes Infect. 3:1063-1072. [DOI] [PubMed] [Google Scholar]
- 11.Chatenoud, L., and J. F. Bach. 2006. Adaptive human regulatory T cells: myth or reality? J. Clin. Investig. 116:2325-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, L., C. Cursiefen, S. Barabino, Q. Zhang, and M. R. Dana. 2005. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Investig. Ophthalmol. Vis. Sci. 46:4536-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chodosh, J., and R. C. Kennedy. 2002. The conjunctival lymphoid follicle in mucosal immunology. DNA Cell Biol. 21:421-433. [DOI] [PubMed] [Google Scholar]
- 14.Chodosh, J., R. E. Nordquist, and R. C. Kennedy. 1998. Anatomy of mammalian conjunctival lymphoepithelium. Adv. Exp. Med. Biol. 438:557-565. [DOI] [PubMed] [Google Scholar]
- 15.Chodosh, J., R. E. Nordquist, and R. C. Kennedy. 1998. Comparative anatomy of mammalian conjunctival lymphoid tissue: a putative mucosal immune site. Dev. Comp. Immunol. 22:621-630. [DOI] [PubMed] [Google Scholar]
- 16.Dana, M. R., and P. Hamrah. 2002. Role of immunity and inflammation in corneal and ocular surface disease associated with dry eye. Adv. Exp. Med. Biol. 506:729-738. [DOI] [PubMed] [Google Scholar]
- 17.de Lafaille, M. A. C., A. C. Lino, N. Kutchukhidze, and J. J. Lafaille. 2004. CD25− T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J. Immunol. 173:7259-7268. [DOI] [PubMed] [Google Scholar]
- 18.Diaz, G. A., and D. M. Koelle. 2006. Human CD4+ CD25high cells suppress proliferative memory lymphocyte responses to herpes simplex virus type 2. J. Virol. 80:8271-8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faal, N., R. L. Bailey, D. Jeffries, H. Joof, I. Sarr, M. Laye, D. C. Mabey, and M. J. Holland. 2006. Conjunctival FOXP3 expression in trachoma: do regulatory T cells have a role in human ocular Chlamydia trachomatis infection? PLoS Med. 3:e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontenot, J. D., J. P. Rasmussen, M. A. Gavin, and A. Y. Rudensky. 2005. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6:1142-1151. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot, J. D., and A. Y. Rudensky. 2005. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 6:331-337. [DOI] [PubMed] [Google Scholar]
- 22.Franklin, R. M., D. W. McGee, and K. F. Shepard. 1985. Lacrimal gland-directed B cell responses. J. Immunol. 135:95-99. [PubMed] [Google Scholar]
- 23.Fukushima, A., T. Sumi, K. Fukuda, N. Kumagai, T. Nishida, H. Yagita, and H. Ueno. 2006. Interleukin 10 and transforming growth factor beta contribute to the development of experimentally induced allergic conjunctivitis in mice during the effector phase. Br. J. Ophthalmol. 90:1535-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukushima, A., T. Yamaguchi, M. Azuma, H. Yagita, and H. Ueno. 2006. Involvement of programmed death-ligand 2 (PD-L2) in the development of experimental allergic conjunctivitis in mice. Br. J. Ophthalmol. 90:1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukushima, A., T. Yamaguchi, K. Fukuda, T. Sumi, N. Kumagai, T. Nishida, S. Imai, and H. Ueno. 2006. CD8+ T cells play disparate roles in the induction and the effector phases of murine experimental allergic conjunctivitis. Microbiol. Immunol. 50:719-728. [DOI] [PubMed] [Google Scholar]
- 26.Fukushima, A., T. Yamaguchi, W. Ishida, K. Fukata, and H. Ueno. 2006. Role of VLA-4 in the development of allergic conjunctivitis in mice. Mol. Vis. 12:310-317. [PubMed] [Google Scholar]
- 27.Fukushima, A., T. Yamaguchi, W. Ishida, K. Fukata, H. Yagita, and H. Ueno. 2006. Roles of OX40 in the development of murine experimental allergic conjunctivitis: exacerbation and attenuation by stimulation and blocking of OX40. Investig. Ophthalmol. Vis. Sci. 47:657-663. [DOI] [PubMed] [Google Scholar]
- 28.Gamache, D. A., S. D. Dimitrijevich, L. K. Weimer, L. S. Lang, J. M. Spellman, G. Graff, and J. M. Yanni. 1997. Secretion of proinflammatory cytokines by human conjunctival epithelial cells. Ocul. Immunol. Inflamm. 5:117-128. [DOI] [PubMed] [Google Scholar]
- 29.Gebert, A., and R. Pabst. 1999. M cells at locations outside the gut. Semin. Immunol. 11:165-170. [DOI] [PubMed] [Google Scholar]
- 30.Ghaem-Maghami, S., G. Ratti, M. Ghaem-Maghami, M. Comanducci, P. E. Hay, R. L. Bailey, D. C. Mabey, H. C. Whittle, M. E. Ward, and D. J. Lewis. 2003. Mucosal and systemic immune responses to plasmid protein pgp3 in patients with genital and ocular Chlamydia trachomatis infection. Clin. Exp. Immunol. 132:436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes, J., H. Dua, L. Rizzo, M. Nishi, A. Joseph, and L. Donoso. 2004. Ocular surface epithelium induces expression of human mucosal lymphocyte antigen (HML-1) on peripheral blood lymphocytes. Br. J. Ophthalmol. 88:280-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graca, L., B. Silva-Santos, and A. Coutinho. 2006. The blind-spot of regulatory T cells. Eur. J. Immunol. 36:802-805. [DOI] [PubMed] [Google Scholar]
- 33.Grajewski, R. S., P. B. Silver, R. K. Agarwal, S. B. Su, C. C. Chan, G. I. Liou, and R. R. Caspi. 2006. Endogenous IRBP can be dispensable for generation of natural CD4+CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. J. Exp. Med. 203:851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamrah, P., S. O. Huq, Y. Liu, Q. Zhang, and M. R. Dana. 2003. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 74:172-178. [DOI] [PubMed] [Google Scholar]
- 35.Hirahara, K., L. Liu, R. A. Clark, K. Yamanaka, R. C. Fuhlbrigge, and T. S. Kupper. 2006. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J. Immunol. 177:4488-4494. [DOI] [PubMed] [Google Scholar]
- 36.Jester, J. V., A. Budge, S. Fisher, and J. Huang. 2005. Corneal keratocytes: phenotypic and species differences in abundant protein expression and in vitro light-scattering. Investig. Ophthalmol. Vis. Sci. 46:2369-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karim, M., C. I. Kingsley, A. R. Bushell, B. S. Sawitzki, and K. J. Wood. 2004. Alloantigen-induced CD25+CD4+ regulatory T cells can develop in vivo from CD25−CD4+ precursors in a thymus-independent process. J. Immunol. 172:923-928. [DOI] [PubMed] [Google Scholar]
- 38.Kashiwada, M., G. Cattoretti, L. McKeag, T. Rouse, B. M. Showalter, U. Al-Alem, M. Niki, P. P. Pandolfi, E. H. Field, and P. B. Rothman. 2006. Downstream of tyrosine kinases-1 and Src homology 2-containing inositol 5′-phosphatase are required for regulation of CD4+CD25+ T cell development. J. Immunol. 176:3958-3965. [DOI] [PubMed] [Google Scholar]
- 39.Keino, H., M. Takeuchi, T. Kezuka, T. Hattori, M. Usui, O. Taguchi, J. W. Streilein, and J. Stein-Streilein. 2006. Induction of eye-derived tolerance does not depend on naturally occurring CD4+CD25+ T regulatory cells. Investig. Ophthalmol. Vis Sci. 47:1047-1055. [DOI] [PubMed] [Google Scholar]
- 40.Khattri, R., T. Cox, S. A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337-342. [DOI] [PubMed] [Google Scholar]
- 41.Kinter, A. L., M. Hennessey, A. Bell, S. Kern, Y. Lin, M. Daucher, M. Planta, M. McGlaughlin, R. Jackson, S. F. Ziegler, and A. S. Fauci. 2004. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 200:331-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knop, E., and N. Knop. 2003. Eye-associated lymphoid tissue (EALT) is continuously spread throughout the ocular surface from the lacrimal gland to the lacrimal drainage system. Ophthalmologe 100:929-942. (In German.) [DOI] [PubMed] [Google Scholar]
- 43.Knop, E., and N. Knop. 2005. The role of eye-associated lymphoid tissue in corneal immune protection. J. Anat. 206:271-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knop, N., and E. Knop. 2005. Ultrastructural anatomy of CALT follicles in the rabbit reveals characteristics of M-cells, germinal centres and high endothelial venules. J. Anat. 207:409-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuklin, N. A., M. Daheshia, S. Chun, and B. T. Rouse. 1998. Role of mucosal immunity in herpes simplex virus infection. J. Immunol. 160:5998-6003. [PubMed] [Google Scholar]
- 46.Lewis, J. M., M. Girardi, S. J. Roberts, S. D. Barbee, A. C. Hayday, and R. E. Tigelaar. 2006. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat. Immunol. 7:843-850. [DOI] [PubMed] [Google Scholar]
- 47.Liu, H., C. K. Meagher, C. P. Moore, and T. E. Phillips. 2005. M cells in the follicle-associated epithelium of the rabbit conjunctiva preferentially bind and translocate latex beads. Investig. Ophthalmol. Vis. Sci. 46:4217-4223. [DOI] [PubMed] [Google Scholar]
- 48.Liu, Y., P. Hamrah, Q. Zhang, A. W. Taylor, and M. R. Dana. 2002. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J. Exp. Med. 195:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez, M., R. Aguilera, C. Perez, A. Mendoza-Naranjo, C. Pereda, M. Ramirez, C. Ferrada, J. C. Aguillon, and F. Salazar-Onfray. 2006. The role of regulatory T lymphocytes in the induced immune response mediated by biological vaccines. Immunobiology 211:127-136. [DOI] [PubMed] [Google Scholar]
- 50.Maloy, K. J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816-822. [DOI] [PubMed] [Google Scholar]
- 51.McGuirk, P., and K. H. Mills. 2002. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23:450-455. [DOI] [PubMed] [Google Scholar]
- 52.McKee, A. S., and E. J. Pearce. 2004. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J. Immunol. 173:1224-1231. [DOI] [PubMed] [Google Scholar]
- 53.Metcalf, M. F., J. I. McNeill, and H. E. Kaufman. 1976. Experimental disciform edema and necrotizing keratitis in the rabbit. Investig. Ophthalmol. 15:979-985. [PubMed] [Google Scholar]
- 54.Nesburn, A., T. Ramos, X. Zhu, H. Asgarzadeh, V. Nguyen, and L. BenMohamed. 2005. Local and systemic B-cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein d together with cytosine-phosphate-guanine adjuvant. Vaccine 23:873-883. [DOI] [PubMed] [Google Scholar]
- 55.Nesburn, A. B. 1980. Common viral eye diseases and latent infections. Ophthalmology 87:1202-1207. [DOI] [PubMed] [Google Scholar]
- 56.Nesburn, A. B., I. Bettahi, X. Zhang, X. Zhu, W. Chamberlain, R. E. Afifi, S. L. Wechsler, and L. BenMohamed. 2006. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul. Surf. 4:178-187. [DOI] [PubMed] [Google Scholar]
- 57.Ozaki, A., W. Ishida, K. Fukata, A. Fukushima, and H. Ueno. 2004. Detection of antigen-specific T cells in experimental immune-mediated blepharoconjunctivitis in DO11.10 T cell receptor transgenic mice. Microbiol. Immunol. 48:39-48. [DOI] [PubMed] [Google Scholar]
- 58.Peng, W., G. Henderson, M. Inman, L. BenMohamed, G. C. Perng, S. L. Wechsler, and C. Jones. 2005. The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal ganglia of acutely infected mice. J. Virol. 79:6162-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pennington, D. J., B. Silva-Santos, T. Silberzahn, M. Escorcio-Correia, M. J. Woodward, S. J. Roberts, A. L. Smith, P. J. Dyson, and A. C. Hayday. 2006. Early events in the thymus affect the balance of effector and regulatory T cells. Nature 444:1073-1077. [DOI] [PubMed] [Google Scholar]
- 60.Perng, G. C., B. Maguen, L. Jin, K. R. Mott, J. Kurylo, L. BenMohamed, A. Yukht, N. Osorio, A. B. Nesburn, G. Henderson, M. Inman, C. Jones, and S. L. Wechsler. 2002. A novel herpes simplex virus type 1 transcript (AL-RNA) antisense to the 5′ end of the latency-associated transcript produces a protein in infected rabbits. J. Virol. 76:8003-8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Powrie, F. 2004. Immune regulation in the intestine: a balancing act between effector and regulatory T cell responses. Ann. N. Y. Acad. Sci. 1029:132-141. [DOI] [PubMed] [Google Scholar]
- 62.Powrie, F., and K. J. Maloy. 2003. Immunology. Regulating the regulators. Science 299:1030-1031. [DOI] [PubMed] [Google Scholar]
- 63.Qian, Y., F. L. Leong, A. Kazlauskas, and M. R. Dana. 2004. Ex vivo adenovirus-mediated gene transfer to corneal graft endothelial cells in mice. Investig. Ophthalmol. Vis. Sci. 45:2187-2193. [DOI] [PubMed] [Google Scholar]
- 64.Rajagopalan, G., M. K. Smart, R. Patel, and C. S. David. 2006. Acute systemic immune activation following conjunctival exposure to staphylococcal enterotoxin B. Infect. Immun. 74:6016-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richards, C. M., A. T. Aman, T. R. Hirst, T. J. Hill, and N. A. Williams. 2001. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J. Virol. 75:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romanowski, E. G., T. Araullo-Cruz, and Y. J. Gordon. 1997. Topical corticosteroids reverse the antiviral effect of topical cidofovir in the Ad5-inoculated New Zealand rabbit ocular model. Investig. Ophthalmol. Vis. Sci. 38:253-257. [PubMed] [Google Scholar]
- 67.Romanowski, E. G., Y. J. Gordon, T. Araullo-Cruz, K. A. Yates, and P. R. Kinchington. 2001. The antiviral resistance and replication of cidofovir-resistant adenovirus variants in the New Zealand White rabbit ocular model. Investig. Ophthalmol. Vis. Sci. 42:1812-1815. [PubMed] [Google Scholar]
- 68.Rouse, B. T., P. P. Sarangi, and S. Suvas. 2006. Regulatory T cells in virus infections. Immunol. Rev. 212:272-286. [DOI] [PubMed] [Google Scholar]
- 69.Rouse, B. T., and S. Suvas. 2004. Regulatory cells and infectious agents: detentes cordiale and contraire. J. Immunol. 173:2211-2215. [DOI] [PubMed] [Google Scholar]
- 70.Rudensky, A. Y., and D. J. Campbell. 2006. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J. Exp. Med. 203:489-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siegmund, K., M. Feuerer, C. Siewert, S. Ghani, U. Haubold, A. Dankof, V. Krenn, M. P. Schon, A. Scheffold, J. B. Lowe, A. Hamann, U. Syrbe, and J. Huehn. 2005. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood 106:3097-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stern, M. E., R. W. Beuerman, R. I. Fox, J. Gao, A. K. Mircheff, and S. C. Pflugfelder. 1998. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 17:584-589. [DOI] [PubMed] [Google Scholar]
- 73.Suffia, I., S. K. Reckling, G. Salay, and Y. Belkaid. 2005. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J. Immunol. 174:5444-5455. [DOI] [PubMed] [Google Scholar]
- 74.Suffia, I. J., S. K. Reckling, C. A. Piccirillo, R. S. Goldszmid, and Y. Belkaid. 2006. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J. Exp. Med. 203:777-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suvas, S., A. K. Azkur, B. S. Kim, U. Kumaraguru, and B. T. Rouse. 2004. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 172:4123-4132. [DOI] [PubMed] [Google Scholar]
- 76.Suvas, S., and B. T. Rouse. 2005. Regulation of microbial immunity: the suppressor cell renaissance. Viral Immunol. 18:411-418. [DOI] [PubMed] [Google Scholar]
- 77.Toda, A., and C. A. Piccirillo. 2006. Development and function of naturally occurring CD4+CD25+ regulatory T cells. J. Leukoc. Biol. 80:458-470. [DOI] [PubMed] [Google Scholar]
- 78.Toka, F. N., M. Gierynska, S. Suvas, S. P. Schoenberger, and B. T. Rouse. 2005. Rescue of memory CD8(+) T cell reactivity in peptide/TLR9 ligand immunization by codelivery of cytokines or CD40 ligation. Virology 331:151-158. [DOI] [PubMed] [Google Scholar]
- 79.Toka, F. N., S. Suvas, and B. T. Rouse. 2004. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J. Virol. 78:13082-13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trousdale, M. D., E. C. Dunkel, and A. B. Nesburn. 1980. Effect of acyclovir on acute and latent herpes simplex virus infections in the rabbit. Investig. Ophthalmol. Vis. Sci. 19:1336-1341. [PubMed] [Google Scholar]
- 81.Wang, R. F. 2006. Functional control of regulatory T cells and cancer immunotherapy. Semin. Cancer Biol. 16:106-114. [DOI] [PubMed] [Google Scholar]
- 82.Wei, S., I. Kryczek, and W. Zou. 2006. Regulatory T-cell compartmentalization and trafficking. Blood 108:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wirostko, E., P. Bhattacherjee, K. E. Eakins, and W. Manski. 1978. Cellular aspects of conjunctival inflammation induced by the synergistic action of histamine and prostaglandins. Immunopharmacology 1:49-56. [DOI] [PubMed] [Google Scholar]
- 84.Wotherspoon, A. C., S. Hardman-Lea, and P. G. Isaacson. 1994. Mucosa-associated lymphoid tissue (MALT) in the human conjunctiva. J. Pathol. 174:33-37. [DOI] [PubMed] [Google Scholar]
- 85.Yagi, H., T. Nomura, K. Nakamura, S. Yamazaki, T. Kitawaki, S. Hori, M. Maeda, M. Onodera, T. Uchiyama, S. Fujii, and S. Sakaguchi. 2004. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int. Immunol. 16:1643-1656. [DOI] [PubMed] [Google Scholar]
- 86.You, S., N. Thieblemont, M. A. Alyanakian, J. F. Bach, and L. Chatenoud. 2006. Transforming growth factor-beta and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol. Rev. 212:185-202. [DOI] [PubMed] [Google Scholar]
- 87.Zhang, X., A. Issagholian, E. A. Berg, J. B. Fishman, A. B. Nesburn, and L. BenMohamed. 2005. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nɛ-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J. Virol. 79:15289-15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu, X., T. V. Ramos, H. Gras-Masse, B. E. Kaplan, and L. BenMohamed. 2004. Lipopeptide epitopes extended by Ne-palmitoyl lysine moiety increases uptake and maturation of dendritic cell through a Toll-like receptor 2 pathway and triggers a Th1-dependent protective immunity. Eur. J. Immunol. 34:1142-1149. [DOI] [PubMed] [Google Scholar]