Abstract
In long-term two-bottle tests, mice from the C57BL/6ByJ (B6) strain drink more monosodium L-glutamate (MSG) and inosine-5′-monophosphate (IMP) compared with mice from the 129P3/J (129) strain. The goal of this study was to assess the role of afferent gustatory input in these strain differences. We measured integrated responses of the mouse chorda tympani and glossopharyngeal nerves to lingual application of compounds that evoke umami taste in humans: MSG, monoammonium L-glutamate (NH4 glutamate), IMP and guanosine-5′-monophosphate (GMP), and also to other taste stimuli. Chorda tympani responses to MSG and NH4 glutamate were similar in B6 and 129 mice. Chorda tympani responses to IMP and GMP were lower in B6 than in 129 mice. Responses to umami stimuli in the glossopharyngeal nerve did not differ between the B6 and 129 strains. Responses to MSG, IMP and GMP were not affected by sodium present in these compounds because B6 and 129 mice had similar neural taste responses to NaCl. This study has demonstrated that the increased ingestive responses to the umami stimuli in B6 mice are accompanied by either unchanged or decreased neural responses to these stimuli. Lack of support for the role of the chorda tympani or glossopharyngeal nerves in the enhanced consumption of MSG and IMP by B6 mice suggests that it is due to some other factors. Although results of our previous study (Bachmanov et al., 2000) suggest that postingestive effects of MSG can affect its intake, contribution of other gustatory components (e.g., greater superficial petrosal nerve or central gustatory processing) to the strain differences in consumption of umami compounds also cannot be excluded. Strain differences in gustatory neural responses to nucleotides but not glutamate suggest that these compounds may activate distinct taste transduction mechanisms.
Keywords: glutamate, chorda tympani nerve, glossopharyngeal nerve, electrophysiology, genetics
Introduction
In humans, certain compounds naturally occurring in food evoke an umami taste sensation, which is distinct from the other four taste qualities: sweet, bitter, salty and sour. The umami compounds1 most commonly occurring in food and frequently used in experiments are salts of L-glutamic acid and purine 5′-nucleotides. Experiments using laboratory rodents have shown that these compounds possess a unique taste quality that probably corresponds to umami taste in humans (Ninomiya and Funakoshi, 1989a). At certain concentrations, solutions of these compounds are palatable to rodents (Hiji and Sato, 1967; Naim, 1979; Yamamoto et al., 1991). Our previous study demonstrated that consumption of umami-tasting compounds differs between mouse strains. In long-term two-bottle tests, mice from the C57BL/6ByJ (B6) strain drank more monosodium L-glutamate (MSG) and inosine-5′-monophosphate (IMP) than did mice from the 129P3/J (129) strain (Bachmanov et al., 2000).
Consumption of taste solutions in the long-term preference tests may depend on perception of their sensory attributes and/or on their postingestive effects. Because umami compounds often have nutritive value to mice (Brosnan, 2000; Reeds et al., 2000), both sensory and postingestive factors may contribute to the strain differences in their consumption. This study was conducted to assess the possible role of gustatory input from the chorda tympani and glossopharyngeal nerves in these strain differences. In the first experiment, we characterized responses in the chorda tympani and glossopharyngeal nerves to the main umami stimuli, MSG and its mixture with IMP. Because we did not find any strain differences in responses to the umami compounds in the glossopharyngeal nerve, in the second experiment we examined responses to a larger number of umami stimuli in the chorda tympani nerve only.
Materials and methods
Animals
Adult male mice of the C57BL/6ByJ (B6) and 129P3/J (129) strains were obtained from The Jackson Laboratory (Bar Harbor, ME) and weighed 24-38 g at the time of recording. The mice were kept at a Monell animal facility in a temperature-controlled room at 23°C on a 12-h light: 12-h dark cycle with lights on at 7:00 am and had free access to deionized water and Teklad Rodent Diet 8604 (Harlan Teklad, Madison, WI). In experiment 1, six B6 and nine 129 mice were used. In B6 mice, chorda tympani recordings were obtained from 5 mice, and glossopharyngeal nerve recordings were obtained from 5 mice (in 4 of these mice, recordings were obtained from both nerves, in the other two mice, recordings were obtained from only one nerve). In 129 mice, chorda tympani recordings were obtained from 6 mice, and glossopharyngeal nerve recordings were obtained from 6 mice (in 3 of these mice, recordings were obtained from both nerves, in the other 6 mice, recordings were obtained from only one nerve). In experiment 2, six B6 and seven 129 mice were used to obtain chorda tympani recordings.
Taste stimuli
In experiment 1, the following taste stimuli were used: 100 mM NH4Cl; 10 mM HCl; 20 mM quinine hydrochloride; 1000 mM sucrose; 10, 30, 100 and 300 mM NaCl; 10, 30, 100 and 300 mM CaCl2; 10, 30, 100, 300 and 1000 mM monosodium salt of L-glutamic acid (MSG), and a mixture of 0.3 mM inosine 5’-monophosphate disodium salt (IMP) with 10, 30, 100, 300 and 1000 mM MSG. This mixture was used to examine synergism between MSG and 5′-ribonucleotides (Ninomiya et al., 1992).
In experiment 2, the following taste stimuli were used: 100 mM NH4Cl; 100 mM KCl; 100 mM NaCl; 20 mM quinine hydrochloride; 300 mM sucrose; 0.1, 0.3, 1, 3, 10, 30, 100, 300 and 1000 mM MSG; 0.1, 0.3, 1, 3, 10, 30, 100 and 300 mM monoammonium salt of L-glutamic acid (NH4 glutamate); 0.1, 0.3, 1, 3, 10, 30, and 100 mM IMP; and 0.1, 0.3, 1, 3, 10 and 30 mM guanosine 5’-monophosphate disodium salt (GMP).
All taste compounds were purchased from Sigma (St. Louis, MO) and dissolved in deionized water.
Surgery and electrophysiological recording of taste responses in the chorda tympani and glossopharyngeal nerves
Techniques for surgery, taste stimulation and recordings have been described previously (Inoue et al., 2001b; Inoue et al., 2001a). Mice were anesthetized with sodium pentobarbital (40-50 mg/kg, with further doses as necessary). A cannula was inserted in the trachea, and the animal was placed supine in a non-traumatic headholder. The hypoglossal nerve was transected bilaterally to prevent inadvertent tongue movements. The right chorda tympani nerve was exposed at its exit from the lingual nerve by removal of the internal pterygoid muscle. The chorda tympani nerve was then dissected free from surrounding tissues and cut at the point of its entry to the bulla. The right glossopharyngeal nerve was exposed by removal of the diagastricus muscle and posterior horn of the hyoid bone. The glossopharyngeal nerve was then dissected free from underlying tissues and cut near its entrance to the posterior lacerated foramen. The entire chorda tympani or glossopharyngeal nerve was placed on a platinum wire electrode, and a few drops of mineral oil were placed in the wound site to prevent desiccation of the nerve. An indifferent electrode was positioned in nearby muscle tissue. The whole-nerve response was then amplified, integrated with a time constant of 1.0 sec, and displayed on chart recorder paper.
For chemical stimulation of the fungiform taste papillae (chorda tympani recording), the anterior one-half of the animal’s tongue was enclosed in a flow chamber. For chemical stimulation of the vallate and foliate papillae in the posterior tongue (glossopharyngeal nerve recording), an incision was made on each side of the animal’s face from the corner of the mouth to just above the angle of the jaw, and the papillae were exposed and their trenches opened via slight tension applied through a small suture sewn in the tip of the tongue. Solutions at room temperature (22 °C) were delivered into the flow chamber (for the anterior part) or directly to the tongue (for the posterior part) by gravity flow at a rate of 0.5 ml/sec for 30 sec. Between taste stimuli, the tongue was rinsed with deionized water for at least 1 min. Ammonium chloride (NH4Cl) at 100 mM was presented frequently throughout recording to serve as a reference stimulus. Concentration series of a given compound were applied in ascending order.
The magnitude of the integrated response was measured at a time point 20 sec after stimulus onset; it was expressed as a proportion of the average of the previous and following responses to 100 mM NH4Cl. This normalization procedure was necessary because the absolute magnitude of multifiber responses can vary over a recording session based on factors such as changing the distance between the firing nerve fiber and the electrode. We have chosen NH4Cl as a standard stimulus for data correction to be consistent with our previous studies (Bachmanov et al., 1997; Inoue et al., 2001b; Inoue et al., 2001a; Li et al., 2001; Inoue et al., 2004). Moreover, in our previous analyses (discussed in detail in Inoue et al., 2001a,b), we have shown that (a) NH4Cl responses normalized relative to responses to other taste stimuli are similar in the B6 and 129 strains, and (b) results of response normalization relative to NH4Cl are similar to normalization using an alternative approach proposed by Frank and Blizard (1999). An additional argument supporting the appropriateness of our standardization procedure is that in this study we observed strain differences in responses to only a subset of stimuli, rather than generalized differences that would be expected if the standardization procedure were inappropriate.
Data analyses
Separate analyses were conducted for responses to each taste compound in each nerve. Strain differences in responses to compounds tested at single concentrations were assessed using t-tests. Responses to compounds tested at multiple concentrations were analyzed using two-way ANOVA assessing effects of strain and concentration. If significant effects were detected using ANOVA, then planned comparison or post hoc t-tests were used to compare strain means for specific concentrations. For all tests, p < 0.05 was considered significant.
Results
For all solutions tested at several concentrations, there was a significant effect of concentration in ANOVA (F > 14, p < 0.015).
Experiment 1, Chorda tympani and glossopharyngeal nerves
Sample recordings of integrated responses of the chorda tympani and glossopharyngeal nerves of B6 and 129 mice to MSG and NH4Cl are shown in Figure 1. Mice from B6 and 129 strains had similar responses to MSG and a mixture of MSG and IMP in both nerves [effect of strain, F(1,7-9) < 1.8, p > 0.2; strain × concentration interaction, F(4,28-36) < 2.2, p > 0.1; Fig. 2]. Mice from both strains showed potentiation of responses to MSG by IMP in the chorda tympani but not glossopharyngeal nerve, which is consistent with results of previous studies (Ninomiya et al., 1992; Damak et al., 2003). The two strains also had similar responses in both nerves to control stimuli, NaCl and CaCl2 [effect of strain, F(1,6-9) < 0.6, p > 0.5; strain × concentration interaction, F(3,18-27) < 3.3, p > 0.1]. Compared with 129 mice, B6 mice had lower chorda tympani responses to HCl and quinine hydrochloride and higher chorda tympani responses to sucrose (p < 0.05, t-tests). Responses to HCl, quinine hydrochloride and sucrose in the glossopharyngeal nerve did not differ significantly between the two strains.
Figure 1.
Sample recordings of integrated responses of the chorda tympani (CT) and glossopharyngeal (GL) nerves of B6 and 129 mice to MSG and NH4Cl (Experiment 1). Horizontal bars under nerve recordings show 30-sec periods of taste stimulus application to the tongue. A scale a bar shows a 20-sec interval to indicate a time point when response magnitude was measured.
Figure 2.
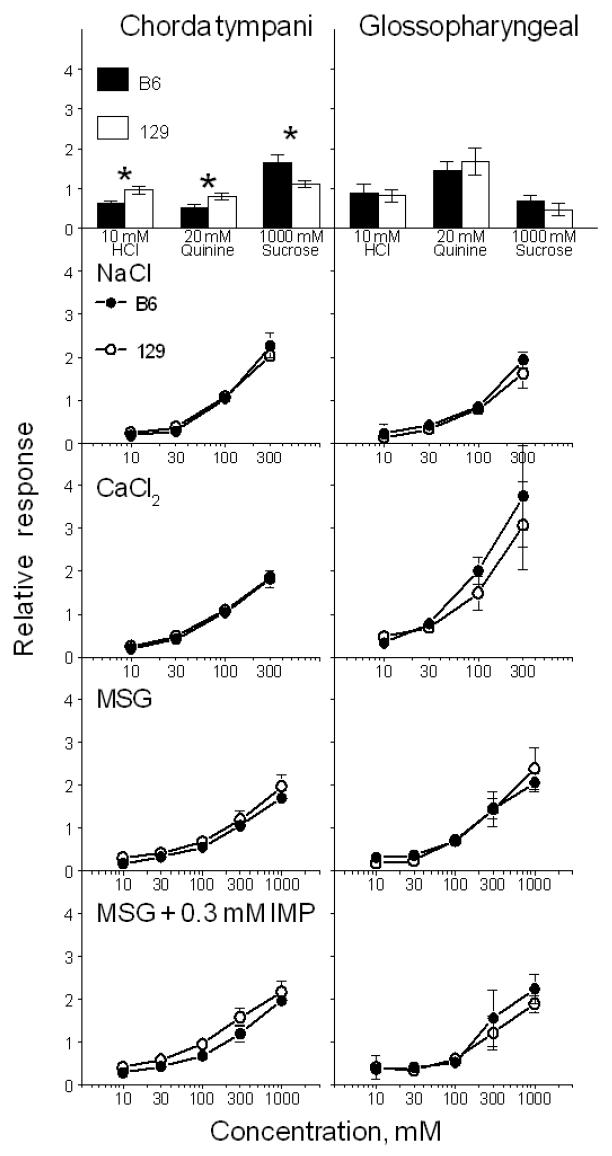
Chorda tympani (left) and glossopharyngeal (right) nerve responses (relative to 100 mM NH4Cl) in B6 (filled bars and circles) and 129 (open bars and circles) mice (Experiment 1). Means ± Standard Errors. *Significant difference between the B6 and 129 strains, p < 0.05, t-tests.
Experiment 2, Chorda tympani nerve
Mice from B6 and 129 strains did not differ in chorda tympani responses to MSG [effect of strain, F(1,11) = 3.2, p = 0.1; strain × concentration interaction, F(8,88) = 1.3, p = 0.2; Fig. 3]. Responses to NH4 glutamate were significantly affected by interaction between effects of strain and concentration [F(7,70) = 4.0, p = 0.001] indicating that relative to 129 mice, B6 mice tended to have smaller responses at low NH4 glutamate concentrations and larger responses at high NH4 glutamate concentrations. However, strain differences did not reach significant level at any concentration of this compound in post-hoc t-tests; the main effect of strain was also not significant [F(1,10) = 0.8, p = 0.4]. Responses to IMP and GMP were significantly lower in B6 than in 129 mice [effect of strain, F(1,10) > 8.7, p < 0.015], but interactions between effects of strain and concentration were not significant [F(5-6,50-60) < 1.9, p > 0.1]. Relative to 129 mice, B6 mice had lower responses to quinine hydrochloride (p = 0.004, t-test), tended to have higher responses to sucrose (p = 0.056), and they had similar chorda tympani responses to KCl and NaCl.
Figure 3.
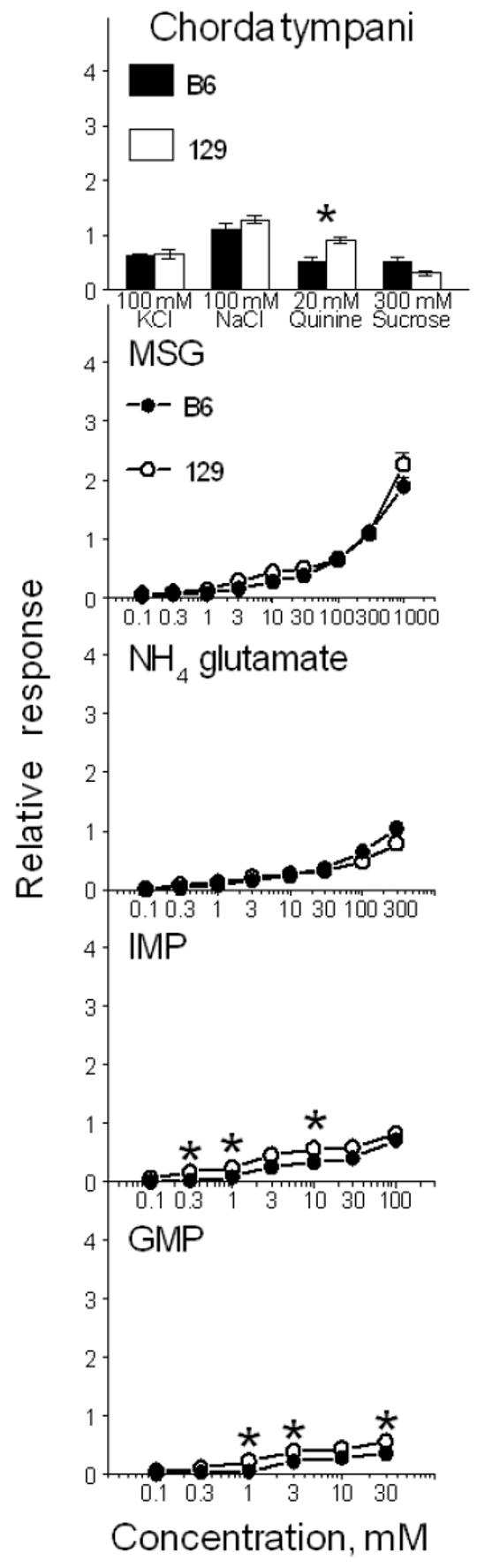
Chorda tympani responses (relative to 100 mM NH4Cl) in B6 (filled bars and circles) and 129 (open bars and circles) mice (Experiment 2). Means ± Standard Errors. *Significant difference between the B6 and 129 strains, p < 0.05, t-tests.
Discussion
In this study, we have found that magnitudes of integrated chorda tympani responses to umami taste stimuli of B6 mice were similar (for MSG and NH4 glutamate) or lower (for IMP and GMP) compared with 129 mice. While chorda tympani responses to some control non-umami taste stimuli (KCl, NaCl and CaCl2) were similar in mice from these two strains, B6 mice had higher responses to sucrose and lower responses to quinine hydrochloride and HCl than did 129 mice. All responses recorded from the glossopharyngeal nerve did not differ between B6 and 129 mice. Dependence of the strain differences on the stimuli suggests that these differences are due to mechanisms specific to each taste quality, rather than to non-specific mechanisms (e.g., nerve anatomy) or inadequate normalization of responses.
Three umami compounds that we have used in our experiments (MSG, IMP and GMP) contain sodium, which evokes salty taste sensation and activates receptors distinct from umami taste receptors (Gilbertson and Boughter, 2003). If there are strain differences in taste responses to Na+, this could affect responses to sodium salts of umami compounds. One possible approach to eliminate effect of sodium is to use a Na+ channel blocker, amiloride. However, this approach was not suitable for our study because B6 and 129 mice differ in sensitivity of gustatory neural responses to amiloride (Gannon and Contreras, 1993; Ninomiya et al., 1996). Thus, using amiloride in B6 and 129 mice would result in unequal suppression of Na+ component of responses to MSG, IMP and GMP. Instead, we assessed the role of Na+ component by measuring responses to series of NaCl concentrations. We have found no strain differences in magnitude of NaCl responses, which is consistent with results of previous studies using the B6 and 129 strains (Gannon and Contreras, 1993; Ninomiya et al., 1996) and implies that gustatory neural responses of B6 and 129 mice to sodium-containing MSG, IMP and GMP are not affected by differential responses to sodium. Similarity of responses to sodium-containing MSG and sodium-free NH4 glutamate further indicates that differential responses to sodium are not involved.
Another taste quality that can be involved in responses to umami compounds in rodents is sweetness. This is supported by several types of evidence: generalization of conditioned taste aversion between umami compounds and sweeteners (Yamamoto et al., 1991; Chaudhari et al., 1996; Stapleton et al., 1999; Heyer et al., 2003), suppression of taste responses to umami compounds by sweet taste blockers (Yamamoto et al., 1991; Sako and Yamamoto, 1999; Ninomiya et al., 2000), existence of sweetener-responsive neural units in the gustatory nerves that also respond to umami stimuli (Ninomiya and Funakoshi, 1989b; Sako et al., 2003; Formaker et al., 2004), and involvement of the T1R3 protein in reception of both sweet and umami compounds (Nelson et al., 2001; Li et al., 2002; Nelson et al., 2002). However, several experimental observations suggest that taste responses of B6 and 129 mice to umami compounds are not related to sweetness perception. First, results of this and our previous studies (Bachmanov et al., 1997; Inoue et al., 2001a; Li et al., 2001) show that B6 mice have higher chorda tympani responses to several sweeteners, while their responses to umami compounds are similar to, or lower than those of 129 mice. Second, although B6 mice have higher consumption of both sweeteners and umami compounds, these two traits do not correlate in the second generation (F2) of hybrids between B6 and 129 strains (Bachmanov et al., 2000) indicating that behavioral responses to sweeteners and umami compounds are genetically unrelated in these strains. Third, neither neural, nor behavioral responses to umami compounds are linked to the subtelomeric region of mouse chromosome 4 harboring the Tas1r genes and the Sac (saccharin preference) locus, a major determinant of behavioral and neural responses to sweeteners (Inoue et al., 2004).
The 5′-ribonucleotides are usually considered as enhancers of the taste of L-glutamate. Although synergism of these compounds has been studied intensively, little attention was paid to activation of afferent taste fibers by 5′-ribonucleotides independently from L-glutamate. However, large responses to higher concentrations of GMP alone were reported in the rat and mouse chorda tympani (Yoshii et al., 1986; Ninomiya et al., 1992). Yoshii et al. (1986) described an unique temporal dynamics during repeated stimulation with GMP, when GMP responses declined with time. Large synergism between 5′-ribonucleotides and amino acids does not necessarily mean that taste responses to both compounds are perceived via a single common mechanism. For example, the 5′-ribonucleotides could synergize with glutamate at the same receptor and in addition interact independently with a separate receptor. Consistent with this, strain differences in gustatory neural responses to IMP and GMP but not MSG or NH4 glutamate found in our study suggest that umami-tasting nucleic and amino acids may activate different taste transduction mechanisms.
The central goal of this study was to assess the role of afferent gustatory inputs from the chorda tympani and glossopharyngeal nerves in differential consumption of umami compounds by B6 and 129 mice. Our data demonstrate that although relative to 129 mice, B6 mice drink more MSG and IMP in the long-term two-bottle tests (Bachmanov et al., 2000), they have similar gustatory neural responses to MSG and lower responses to IMP. The possible role of lower neural responses to IMP in its higher consumption by B6 mice could be explained if gustatory information conveyed via the chorda tympani nerve is predominantly aversive (i.e., lower aversive sensation can result in lower suppression of intakes). However, this seems unlikely because IMP solutions used in our electrophysiological experiments were preferred by both B6 and 129 mice (Bachmanov et al., 2000). It seems more likely that the lower neural and higher ingestive behavioral responses to some umami stimuli in the B6 mice are unrelated mechanistically. In F2 hybrids between the B6 and 129 strains, there were no correlations between chorda tympani responses to umami stimuli and consumption of umami solutions (Inoue and Bachmanov, unpublished data) suggesting that these traits depend on different genes allelic between the B6 and 129 strains.
Lack of support for the role of the chorda tympani or glossopharyngeal nerves in the enhanced consumption of MSG and IMP by B6 mice suggests that it is due to some other factors. Although we have investigated the two major sources of gustatory peripheral input, we did not examine all aspects of gustatory processing of umami stimuli. For example, differences in patterns of single fiber activity, responses in the greater superficial petrosal nerve, or central gustatory processing could contribute to the strain differences in consumption of umami compounds. In our previous study (Bachmanov et al., 2000), we have found that the strain differences in MSG intake increase as a result of exposure to MSG. This suggests that strain-specific postingestive effects of umami compounds could also underlie strain differences in their intake. The postingestive effects of glutamate and nucleotides may be mediated by their metabolic (e.g., derived energy), physiological (e.g., stimulation of vagus activity or hormone release) or pharmacological (e.g., effects on glutamate and purinergic receptors) effects (Niijima et al., 1990; Niijima, 1991; Brosnan, 2000; Dingledine and Conn, 2000; Kondoh et al., 2000; Reeds et al., 2000; Young and Ajami, 2000; Fredholm et al., 2001; Vinade et al., 2004). Further studies are needed to find out whether sensory or postingestive factors, or both of them, underlie the strain differences in consumption of umami compounds.
In conclusion, our study has shown that B6 mice with higher MSG and IMP consumption have similar or lower responses to umami taste stimuli in the chorda tympani or glossopharyngeal nerves relative to 129 mice with lower MSG and IMP consumption. This suggests that the strain differences in consumption of umami compounds depend on some other factors, for example on different elements of gustatory processing, or on postingestive factors. Differences between B6 and 129 mice in gustatory neural responses to nucleotides but not glutamate suggest that these compounds may activate distinct taste transduction mechanisms. The goal of our ongoing genetic mapping studies is to identify genetic loci involved in behavioral and neural taste responses to umami compounds.
Acknowledgments
This work was supported by NIH grant DC00882 (GKB).
Footnotes
For brevity, we use expressions ′umami compounds′ and ′umami stimuli′ to define compounds that have umami taste to humans. This does not necessarily imply that they evoke equivalent taste sensation in rodents.
References
- Bachmanov AA, Tordoff MG, Beauchamp GK. Intake of umami-tasting solutions by mice: a genetic analysis. J. Nutr. 2000;130:935S–941S. doi: 10.1093/jn/130.4.935S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, Beauchamp GK. Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mamm. Genome. 1997;8:545–548. doi: 10.1007/s003359900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan JT. Glutamate, at the interface between amino acid and carbohydrate metabolism. J. Nutr. 2000;130:988S–990S. doi: 10.1093/jn/130.4.988S. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S. The taste of monosodium glutamate: membrane receptors in taste buds. J. Neurosci. 1996;16:3817–3826. doi: 10.1523/JNEUROSCI.16-12-03817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Conn PJ. Peripheral glutamate receptors: molecular biology and role in taste sensation. J. Nutr. 2000;130:1039S–1042S. doi: 10.1093/jn/130.4.1039S. [DOI] [PubMed] [Google Scholar]
- Formaker BK, Stapleton JR, Roper SD, Frank ME. Responses of the rat chorda tympani nerve to glutamate-sucrose mixtures. Chem. Senses. 2004;29:473–482. doi: 10.1093/chemse/bjh049. [DOI] [PubMed] [Google Scholar]
- Frank ME, Blizard DA. Chorda tympani responses in two inbred strains of mice with different taste preferences. Physiol. Behav. 1999;67:287–297. doi: 10.1016/s0031-9384(99)00071-2. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gannon K, Contreras RJ. Sodium intake linked to amiloride-sensitive gustatory transduction in C57BL/6J and 129/J mice. Physiol. Behav. 1993;57:231–239. doi: 10.1016/0031-9384(94)00279-e. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Boughter JD., Jr. Taste transduction: appetizing times in gustation. Neuroreport. 2003;14:905–911. doi: 10.1097/01.wnr.0000074346.81633.76. [DOI] [PubMed] [Google Scholar]
- Heyer BR, Taylor-Burds CC, Tran LH, Delay ER. Monosodium glutamate and sweet taste: generalization of conditioned taste aversion between glutamate and sweet stimuli in rats. Chem. Senses. 2003;28:631–641. doi: 10.1093/chemse/bjg056. [DOI] [PubMed] [Google Scholar]
- Hiji Y, Sato M. Preference-aversion function for sodium monoaminodicarboxylates in rats. Journal of Physiological Society Japan. 1967;29:168–169. [PubMed] [Google Scholar]
- Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole-nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem. Senses. 2001a;26:915–923. doi: 10.1093/chemse/26.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Li X, McCaughey SA, Beauchamp GK, Bachmanov AA. Soa genotype selectively affects mouse gustatory neural responses to sucrose octaacetate. Physiol. Genomics. 2001b;5:181–186. doi: 10.1152/physiolgenomics.2001.5.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects behavioral and neural taste responses to sweeteners in the F2 hybrids between C57BL/6ByJ and 129P3/J mice. J. Neurosci. 2004;24:2296–2303. doi: 10.1523/JNEUROSCI.4439-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh T, Mori M, Ono T, Torii K. Mechanisms of umami taste preference and aversion in rats. J. Nutr. 2000;130:966S–970S. doi: 10.1093/jn/130.4.966S. [DOI] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, Ninomiya Y, Beauchamp GK, Bachmanov AA. High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal Chromosome 4. Mamm. Genome. 2001;12:13–16. doi: 10.1007/s003350010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim M. Self-selection of food and water flavored with monosodium glutamate. In: Filer LJ, editor. Glutamic acid: Advances in Biochemistry and Physiology. Raven Press; New York, NY: 1979. pp. 11–23. [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An aminoacid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Niijima A. Effects of oral and intestinal stimulation with umami substance on gastric vagus activity. Physiol. Behav. 1991;49:1025–1028. doi: 10.1016/0031-9384(91)90218-d. [DOI] [PubMed] [Google Scholar]
- Niijima A, Togiyama T, Adachi A. Cephalic-phase insulin release induced by taste stimulus of monosodium glutamate (umami taste) Physiol. Behav. 1990;48:905–908. doi: 10.1016/0031-9384(90)90247-2. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Behavioural discrimination between glutamate and the four basic taste substances in mice. Comp. Biochem. Physiol. 1989a;92:365–370. doi: 10.1016/0300-9629(89)90577-x. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Peripheral neural basis for behavioural discrimination between glutamate and the four basic taste substances in mice. Comp. Biochem. Physiol. 1989b;92:371–376. doi: 10.1016/0300-9629(89)90578-1. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Fukami Y, Yamazaki K, Beauchamp GK. Amiloride inhibition of chorda tympani responses to NaCl and its temperature dependency in mice. Brain Res. 1996;708:153–158. doi: 10.1016/0006-8993(95)01218-4. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kurenuma S, Nomura T, Uebayashi H, Kawamura H. Taste synergism between monosodium glutamate and 5′-ribonucleotide in mice. Comp. Biochem. Physiol. A. 1992;101:97–102. doi: 10.1016/0300-9629(92)90634-3. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, Danilova V, Hellekant G. Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J. Nutr. 2000;130:950S–953S. doi: 10.1093/jn/130.4.950S. [DOI] [PubMed] [Google Scholar]
- Reeds PJ, Burrin DG, Stoll B, Jahoor F. Intestinal glutamate metabolism. J. Nutr. 2000;130:978S–982S. doi: 10.1093/jn/130.4.978S. [DOI] [PubMed] [Google Scholar]
- Sako N, Yamamoto T. Analyses of taste nerve responses with special reference to possible receptor mechanisms of umami taste in the rat. Neurosci. Lett. 1999;261:109–112. doi: 10.1016/s0304-3940(99)00019-1. [DOI] [PubMed] [Google Scholar]
- Sako N, Tokita K, Sugimura T, Yamamoto T. Synergistic responses of the chorda tympani to mixtures of umami and sweet substances in rats. Chem. Senses. 2003;28:261–266. doi: 10.1093/chemse/28.3.261. [DOI] [PubMed] [Google Scholar]
- Stapleton JR, Roper SD, Delay ER. The taste of monosodium glutamate (MSG), L-aspartic acid, and N-methyl-D-aspartate (NMDA) in rats: are NMDA receptors involved in MSG taste? Chem. Senses. 1999;24:449–457. doi: 10.1093/chemse/24.4.449. [DOI] [PubMed] [Google Scholar]
- Vinade ER, Izquierdo I, Lara DR, Schmidt AP, Souza DO. Oral administration of guanosine impairs inhibitory avoidance performance in rats and mice. Neurobiol. Learn. Mem. 2004;81:137–143. doi: 10.1016/j.nlm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Fujimoto Y, Fukanaga I, Miyasaka A, Imoto T. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol. Behav. 1991;49:919–925. doi: 10.1016/0031-9384(91)90204-2. [DOI] [PubMed] [Google Scholar]
- Yoshii K, Yokouchi C, Kurihara K. Synergistic effects of 5′-nucleotides on rat taste responses to various amino acids. Brain. Res. 1986;367:45–51. doi: 10.1016/0006-8993(86)91577-5. [DOI] [PubMed] [Google Scholar]
- Young VR, Ajami AM. Glutamate: an amino acid of particular distinction. J. Nutr. 2000;130:892S–900S. doi: 10.1093/jn/130.4.892S. [DOI] [PubMed] [Google Scholar]



