Abstract
Elastic fibers play the principal roles in providing elasticity and integrity to various types of human organs, such as the arteries, lung, and skin. However, the molecular mechanism of elastic fiber assembly that leads to deposition and crosslinking of elastin along microfibrils remains largely unknown. We have previously shown that developing arteries and neural crest EGF-like protein (DANCE) (also designated fibulin-5) is essential for elastogenesis by studying DANCE-deficient mice. Here, we report the identification of latent transforming growth factor-β-binding protein 2 (LTBP-2), an elastic fiber-associating protein whose function in elastogenesis is not clear, as a DANCE-binding protein. Elastogenesis assays using human skin fibroblasts reveal that fibrillar deposition of DANCE and elastin is largely dependent on fibrillin-1 microfibrils. However, downregulation of LTBP-2 induces fibrillin-1-independent fibrillar deposition of DANCE and elastin. Moreover, recombinant LTBP-2 promotes deposition of DANCE onto fibrillin-1 microfibrils. These results suggest a novel regulatory mechanism of elastic fiber assembly in which LTBP-2 regulates targeting of DANCE on suitable microfibrils to form elastic fibers.
Keywords: DANCE, elastic fiber, fibulin-5, LTBP-2, microfibril
Introduction
Elastic matrices impart resilience and structural integrity to various types of human organs, such as the large arteries, lungs, and skin (Rosenbloom et al, 1993). They play especially vital roles in the functions of the arteries and lungs, which undergo repeated cycles of extension and recoil to maintain the blood pressure or breathing process. Injury and degeneration of elastic matrices are profoundly associated with age-related symptoms and diseases, such as arteriosclerosis, lung emphysema, and wrinkled skin (Pasquali-Ronchetti and Baccarani-Contri, 1997; Bailey, 2001). Elucidating the mechanisms of elastogenesis might lead to new treatments for these age-related symptoms. However, little is known about the molecular mechanism of elastic fiber assembly. We and others reported that mice deficient in a newly identified protein, developing arteries and neural crest EGF-like (DANCE) (also designated fibulin-5 or EVEC), exhibit a severely disorganized elastic fiber system throughout the body (Nakamura et al, 2002; Yanagisawa et al, 2002). DANCE is a secreted 66-kDa molecule, which colocalizes with elastic fibers and is abundantly expressed in developing arteries (Nakamura et al, 1999). We have recently demonstrated that DANCE has an elastogenic organizer activity, by showing that recombinant DANCE can induce elastogenesis in cell culture (Hirai et al, 2007). DANCE deposits on microfibrils, then promotes coacervation and crosslinking of tropoelastin molecules along microfibrils. Thus, DANCE is not only necessary for elastogenesis, but also able to promote elastic fiber organization. Therefore, DANCE may provide important clues to the molecular basis of elastogenesis.
Elastic fibers are known to consist of two morphologically distinct components: microfibrils and polymerized elastin (Rosenbloom et al, 1993). Microfibrils are 10–12 nm filaments in the extracellular matrices, and mainly consist of large extended glycoproteins, fibrillin-1 and -2 (Sakai et al, 1986; Zhang et al, 1994), together with several kinds of proteins that are associated with them, such as latent transforming growth factor β-binding proteins (LTBPs) (Gibson et al, 1995; Taipale et al, 1995) and microfibril-associated glycoproteins (MAGPs) (Gibson et al, 1986, 1996). Microfibrils are considered to provide a scaffold for the orientated deposition of tropoelastin monomers and to play a central role in elastogenesis, although they can also form macroaggregates devoid of elastin (Ramirez et al, 2004). The expression of fibrillin-1 and -2 is differentially controlled, but overlaps in the development of elastic tissues (Mariencheck et al, 1995). Although fibrillin-1-mutated mice develop normal elastic matrices (Pereira et al, 1997) and fibrillin-2-mutated mice show syndactyly but normal elastogenesis (Arteaga-Solis et al, 2001; Chaudhry et al, 2001), mice deficient in both fibrillin-1 and -2 were recently reported to exhibit impaired elastogenesis (Carta et al, 2006). However, why only a part of microfibrils deposit elastin to form elastic fibers and how fibrillin-1 and -2 microfibrils are differentially utilized in elastogenesis remains unclear. These considerations prompted us to investigate whether there might be any microfibril molecules that regulate elastic fiber assembly. For example, LTBP proteins share a high degree of homology with fibrillins. There are four isoforms in the LTBP family (LTBP-1, -2, -3, and -4) (Kanzaki et al, 1990; Moren et al, 1994; Yin et al, 1995; Giltay et al, 1997; Saharinen et al, 1998; Hyytiainen et al, 2004). This family is named based on its ability to bind the latent form of transforming growth factor-β (TGF-β) and to modulate TGF-β bioactivity, but the family members also serve as structural components of extracellular matrices. Notably, LTBP-2 has the distinctive characteristic that it cannot interact with TGF-β, whereas LTBP-1, -3, and -4 can interact with TGF-β (Saharinen and Keski-Oja, 2000). In addition, LTBP-2 is reported to be specifically localized to the elastin-associated microfibrils (Gibson et al, 1995). These findings suggest that LTBP-2 may play an important role in elastogenesis unrelated to the regulatory function of TGF-β activity.
To elucidate the molecular mechanisms of elastic fiber assembly and the roles of DANCE in elastogenesis, we attempted here to identify DANCE-binding proteins. Several groups have previously identified some DANCE-binding proteins, such as elastin (Yanagisawa et al, 2002; Freeman et al, 2005), lysyl oxidase-like 1 (Liu et al, 2004), EMILIN (Zanetti et al, 2004), apolipoprotein(a) (Kapetanopoulos et al, 2002), and extracellular superoxide dismutase (Nguyen et al, 2004). However, it is still not possible to fully understand the role that DANCE plays in elastic fiber development. In the present study, we demonstrate the interaction of DANCE and LTBP-2. We show that the middle calcium-binding epidermal growth factor-like domain (cbEGF-like domain) of DANCE specifically interacts with the amino- (N-) terminal domain of LTBP-2. Knockdown experiments using small interfering RNA (siRNA) reveal that deposition of DANCE and elastin is dependent on fibrillin-1, not on either fibrillin-2 or LTBP-2. Intriguingly, downregulation of LTBP-2 rescues fibrillar deposition of DANCE and elastin onto fibrillin-2 or other potential microfibrils, even in the absence of fibrillin-1. Moreover, recombinant LTBP-2 protein promotes fibrillar deposition of DANCE onto fibrillin-1 microfibrils. These results imply that LTBP-2 may function not only as a structural protein, but also as a regulatory protein that determines which microfibrils DANCE should deposit on for subsequent assembly of elastic fiber components.
Results
Latent TGF-β-binding protein-2 as a DANCE-binding protein
To clarify the molecular role of DANCE in elastogenesis, we attempted to identify DANCE-binding proteins. For this purpose, we performed immunoprecipitation/SDS–PAGE. Bovine aortic smooth muscle cells were metabolically labeled with 35S-cysteine/methionine overnight, and the conditioned medium was harvested. An aliquot of the conditioned medium was combined with a purified FLAG-tagged DANCE recombinant protein or a mock control, followed by immunoprecipitation with a monoclonal anti-FLAG antibody. Other aliquots were subjected to immunoprecipitation with available antibodies against elastic fiber components, including elastin, fibrillin-1, fibrillin-2, and latent TGF-β-binding protein-2 (LTBP-2), to see whether there are these proteins in the conditioned medium. The immunoprecipitated complexes were separated by SDS–PAGE, and three major bands were detected as candidates for DANCE-binding proteins compared with the mock control (Figure 1, compare lanes 1 and 2, asterisks). The molecular weights of two of these bands are higher than 182 kDa, and that of the other is approximately 60 kDa (lane 2). We assumed that the larger molecules might be LTBP-2, because the molecular weight seems to be very similar to that of the protein immunoprecipitated with a monoclonal anti-LTBP-2 antibody (Figure 1, compare lanes 2 and 8). Fibrillin-1 and -2 were not detected, suggesting that these antibodies might not react with bovine fibrillin-1 and -2. In an analogous experiment using neonatal skin fibroblasts from wild-type mice, we found similar bands precipitated with the recombinant DANCE (data not shown). However, the 60-kDa band was not detected in the DANCE−/− neonatal fibroblast culture medium (data not shown); therefore, we infer that the 60-kDa molecule may be the DANCE itself, which is abundantly expressed in aorta (lane 2). To see whether these larger molecules are LTBP-2, we carried out double immunostaining of human skin fibroblasts with monoclonal antibodies against DANCE and LTBP-2. The result reveals that these two molecules colocalize, which suggests that they interact each other (Supplementary Figure S1).
Figure 1.
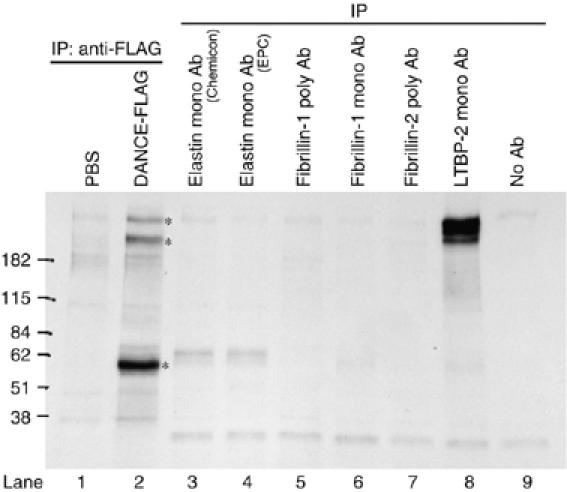
Latent TGF-β-binding protein-2 (LTBP-2) as a candidate DANCE-binding protein. Bovine smooth muscle cells were metabolically labeled with 35S-cysteine/methionine, and conditioned media were subjected to immunoprecipitation. Several DANCE-binding proteins are detected (lane 2, asterisks). The sizes of the bands larger than 182 kDa are similar to those of proteins precipitated with anti-LTBP-2 antibody (compare lanes 2 and 8). Another 60-kDa band is inferred to be DANCE, which is abundantly expressed in aortic smooth muscle cells (lane 2).
LTBP-2 specifically interacts with the sixth calcium-binding EGF-like domain of DANCE
To examine whether DANCE actually interacts with LTBP-2, we performed in vitro binding assays. Myc-tagged LTBP-2 proteins overexpressed by 293T cells were incubated with FLAG-tagged DANCE or a series of FLAG-tagged DANCE deletion mutant proteins (Figure 2A) also overexpressed by 293T cells. Each mixture was subjected to immunoprecipitation with anti-FLAG antibody, and then Myc-tagged proteins associated with the FLAG-tagged proteins were detected by Western blotting. As shown in Figure 2B, DANCE interacts with LTBP-2 (compare lane 1 with 11). Among the deletion mutant proteins, ΔM5-DANCE does not interact with LTBP-2 at all (Figure 2B, upper panel, lane 8). This result indicates that DANCE directly interacts with LTBP-2 through the sixth cbEGF-like domain. The carboxy- (C-) terminal domain of DANCE may also be involved in the interaction, as the binding of ΔC1- and ΔC2-DANCE with LTBP-2 was weaker than that of full-length DANCE with LTBP-2.
Figure 2.
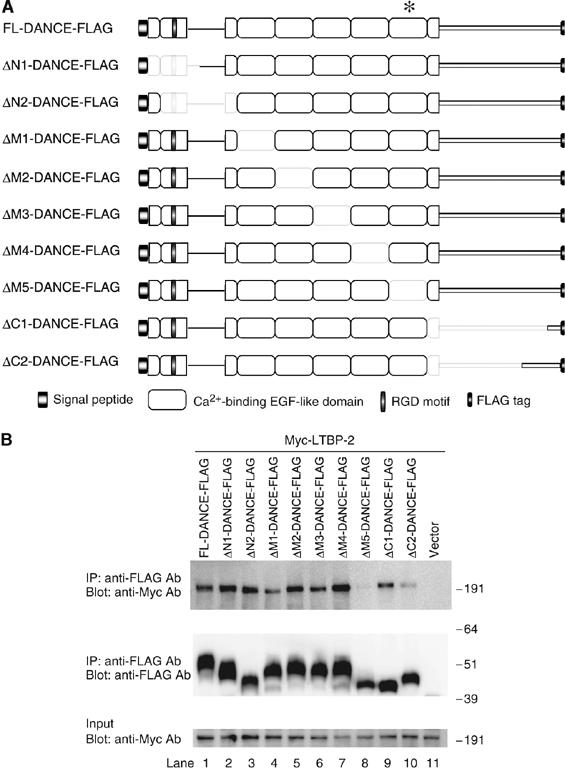
Schematic representation of the DANCE deletion mutants, and mapping of the binding domain of DANCE with LTBP-2. (A) Domain structure of the full-length DANCE and the DANCE deletion mutants used for the in vitro binding assay. These mutants were expressed as C-terminally FLAG-tagged proteins. We prepared expression vectors encoding FLAG-tagged DANCE (FL-DANCE-FLAG) or DANCE deletion mutants, including N-terminal domain deletion mutants (ΔN1- and ΔN2-DANCE-FLAG), central calcium-binding EGF (cbEGF)-like repeat domain deletion mutants (ΔM1-, ΔM2-, ΔM3-, ΔM4-, and ΔM5-DANCE-FLAG), and C-terminal domain deletion mutants (ΔC1- and ΔC2-DANCE-FLAG). The asterisk indicates the sixth cbEGF-like domain of DANCE, where LTBP-2 binds. (B) The sixth cbEGF-like domain of DANCE interacts with LTBP-2. 293T cells were transiently transfected with the vectors shown in A or mock vector. Expression vectors for Myc-tagged full-length LTBP-2 (LTBP-2-Myc) were also independently transfected into 293T cells. Transfected cells were cultured in serum-free medium for 48 h, and then the cell lysates and the conditioned media were harvested and mixed. After incubation of LTBP-2-Myc with a set of FLAG-tagged DANCE deletion mutants, each mixture was subjected to immunoprecipitation with anti-FLAG antibody. These immunoprecipitates were then separated by SDS–PAGE, and analyzed by Western blotting with a monoclonal anti-Myc antibody.
To see whether DANCE deletion mutants maintain their structural integrity, we performed a solid phase binding assay on recombinant tropoelastin. The result shows that ΔM5-DANCE binds tropoelastin as strongly as full-length DANCE (Supplementary Figure S2). We also carried out a deglycosylation assay using N-glycosidase F. Incubating ΔM5-DANCE with glycosidase did not change its molecular size, whereas the same glycosidase treatment caused size reduction of ΔM4-DANCE to a similar size of ΔM5-DANCE (Supplementary Figure S3). Taking into account that the sixth cbEGF-like domain contains putative N-glycosilation site (Nakamura et al, 1999) and that both mutants miss one cbEGF-like domain, this result indicates that not only ΔM4-DANCE, but also other DANCE mutants are properly processed after translation as well as the full-length DANCE.
DANCE interacts with the N-terminal domain of LTBP-2
To identify the DANCE-binding domain in the LTBP-2 molecule, we constructed expression vectors encoding five types of LTBP-2 truncation mutants with FLAG-tag (LTBP-2-A-FLAG through LTBP-2-E-FLAG, Figure 3A). FLAG-tagged LTBP-2 truncation mutants and Myc-tagged full-length DANCE recombinant proteins were transiently expressed in 293T cells, and were subjected to in vitro binding assays. As shown in Figure 3B (left panel), only LTBP-2-A fragment can interact with DANCE (lane 2). This result demonstrates that DANCE specifically interacts with the N-terminal domain of LTBP-2.
Figure 3.

Schematic representation of the LTBP-2 truncation mutants, and mapping of the binding domain of LTBP-2 with DANCE. (A) Domain structure of the full-length LTBP-2 and the LTBP-2 truncation mutants used for the in vitro binding assay. These mutants were expressed as N-terminally FLAG-tagged proteins flanked by the preprotrypsin signal sequence, except for the full-length LTBP-2. The LTBP-2-H fragment is prone to degradation, so we constructed C-terminal fusion proteins with the constant region of human IgG to prevent the degradation (LTBP-2-G-Ig, LTBP-2-H-Ig, and LTBP-2-I-Ig). The characteristics of each domain are described below the figure. The asterisk indicates the second four-cystein domain of LTBP-2, where DANCE binds. (B) Left panel, DANCE interacts with the N-terminal domain of LTBP-2 (LTBP-2-A). Right panel, DANCE interacts with the second four-cysteine domain of LTBP-2 (LTBP-2-I). The expression vector of each LTBP-2 truncation mutant was transfected into 293T cells. Mixtures of the media and cell lysates of FLAG-tagged LTBP-2 truncation mutants were incubated with DANCE-Myc, and then these reactants were subjected to immunoprecipitation with anti-FLAG antibody. The immunoprecipitates were separated by SDS–PAGE, and analyzed by Western blotting with anti-Myc antibody. (C) Calcium dependency of the LTBP-2–DANCE interaction. The expression vector for Myc-tagged full-length LTBP-2 was transfected into 293T cells. Mixtures of the conditioned media and cell lysates of Myc-LTBP-2 were incubated with the conditioned media of DANCE-FLAG in the presence of EDTA (0, 1, 2, 5, or 10 mM). These cocktails were subjected to immunoprecipitation with anti-FLAG antibody. The immunoprecipitates were separated by SDS–PAGE, and analyzed by Western blotting with anti-Myc antibody.
To more precisely identify the domain of LTBP-2 involved in this interaction, we constructed two expression vectors encoding FLAG-tagged LTBP-2-F and -G fragments by dividing the LTBP-2-A fragment (Figure 3A). As shown in Figure 3B (right panel), in vitro binding assays reveal that DANCE specifically interacts with LTBP-2-G fragment (lane 10), but not with LTBP-2-F fragment (lane 9). We further constructed expression vectors encoding FLAG-tagged LTBP-2-H and -I fragments by dividing the LTBP-2-G fragment (Figure 3A). Because the LTBP-2-H fragment is easily degraded, we constructed immunoglobulin fusion proteins to prevent degradation (LTBP-2-G-Ig, LTBP-2-H-Ig, and LTBP-2-I-Ig). DANCE interacted with the immunoglobulin-fused LTBP-2-G-Ig, as well as the intact LTBP-2-G fragment, indicating that the fused immunoglobulin does not affect the interaction with DANCE (Figure 3B, compare lanes 10 and 11). As shown in Figure 3B, in vitro binding assays revealed that DANCE specifically interacts with the LTBP-2-I-Ig, but not with the LTBP-2-H-Ig (Figure 3B, lanes 12 and 13). These results demonstrate that DANCE specifically interacts with the second four-cysteine repeat of LTBP-2.
Next, we examined the Ca2+ dependency of the interaction between DANCE and LTBP-2. Myc-tagged LTBP-2 and FLAG-tagged DANCE recombinant proteins were expressed in 293T cells, and subjected to in vitro binding assays in the presence or absence of EDTA (ethylenediaminetetraacetic acid). As shown in Figure 3C, the interaction between DANCE and LTBP-2 was markedly diminished in the presence of 1 mM EDTA, and almost abolished in the presence of 2 mM EDTA (Figure 3C, upper panel, lanes 1–3). These results indicate that the DANCE–LTBP-2 interaction is likely to be Ca2+ dependent, which is consistent with our finding that LTBP-2 interacts with the Ca2+-binding EGF (cbEGF)-like domain of DANCE, whereas the N-terminal domain of LTBP-2 does not contain any cbEGF domain.
DANCE interacts with LTBP-2 in vivo
To investigate whether DANCE colocalizes with LTBP-2 at the tissue level, we performed in situ hybridization using neonatal mice. As shown in Figure 4A and B, both the DANCE transcript and the LTBP-2 transcript are strongly detected in the cardiac outflow tract, cardiac valves, aorta, and lung. The expression patterns of these two transcripts are strikingly similar, except for the minor difference that DANCE is more strongly expressed in arteries than in lung distal airspace walls, whereas the expression of LTBP-2 is similar in these tissues. These expression patterns suggest that DANCE is localized in close proximity to LTBP-2 in vivo.
Figure 4.
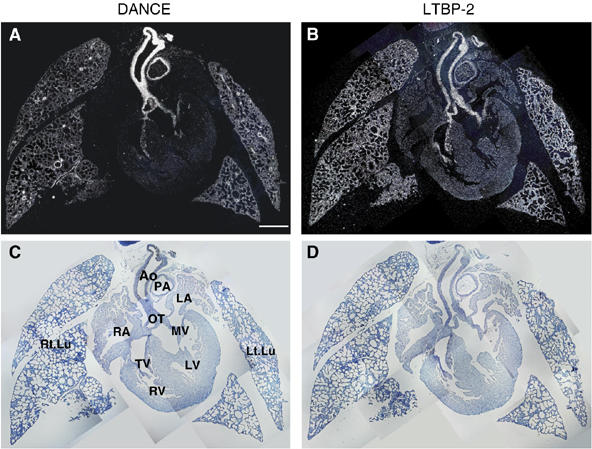
Expression patterns of DANCE (A) and LTBP-2 (B) transcripts in neonatal mice as shown by in situ hybridization with dark field (A, B) and bright field (C, D) views. Ao, aorta; PA, pulmonary artery; OT, cardiac outflow tract; MV, mitral valve; TV, tricupsid valve, LV, left ventricle; RV, right ventricle; LA, left atrium; RA, right atrium; Rt.Lu, right lung; Lt.Lu, left lung. Scale bar; 500 μm.
To examine the interaction of DANCE and LTBP-2 in vivo, we performed co-immunoprecipitation analysis using lung extracts from wild-type and DANCE−/− mice. Immunoreactive DANCE protein was precipitated from lung extracts with anti-DANCE antibody followed by Western blotting using an anti-LTBP-2 antibody. As shown in Figure 5, the interaction of DANCE and LTBP-2 was detected (upper panel, compare lanes 1, 2 and lanes 3, 4). This result indicates that endogenous DANCE interacts with endogenous LTBP-2 in vivo.
Figure 5.
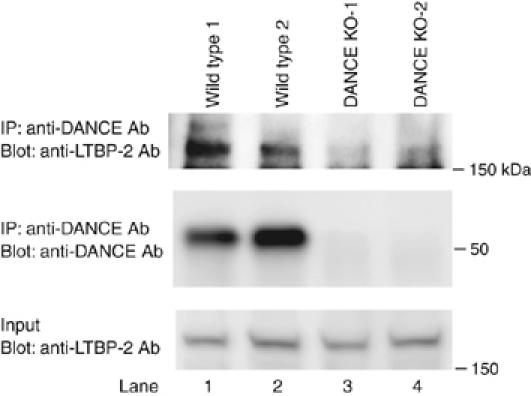
DANCE interacts with LTBP-2 in vivo. Whole lungs were dissected from two lines each of wild-type and DANCE-deficient mice. Proteins were extracted using a homogenizer on ice. Immunoreactive DANCE protein was precipitated from lung extracts with anti-DANCE antibody, followed by Western blotting with a polyclonal anti-LTBP-2 antibody.
BIAcore analysis of the interaction between DANCE and LTBP-2
To investigate the affinity of the binding of DANCE to LTBP-2, we performed kinetic analyses using surface plasmon resonance with a Biacore X instrument. The proteins used for the assay were purified from 293T cell lines stably overexpressing C-terminal histidine-tagged full-length DANCE and LTBP-2. Analysis by SDS–PAGE followed by Coomassie blue staining revealed that the purities of these proteins were more than 80% (Figure 6A). The purified DANCE recombinant protein was immobilized on a CM5 sensor chip, and LTBP-2 was used as the analyte. Kinetic analyses were performed at a range of concentrations of 15–360 μg/ml (75–1800 nM) of LTBP-2 on the DANCE-immobilized chip, and the dissociation constant (KD) of the binding of LTBP-2 to DANCE was determined to be 265 nM, which indicates that this interaction is direct and specific (Figure 6B).
Figure 6.
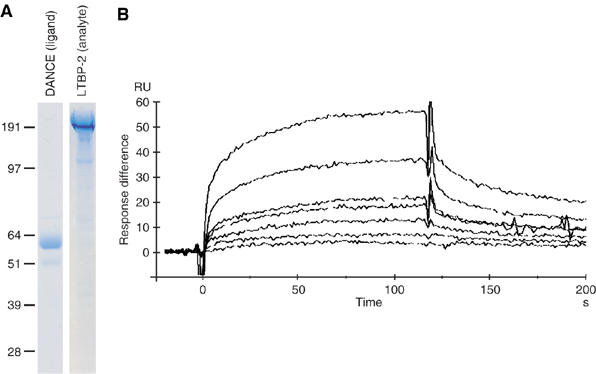
Quantification of the affinity of DANCE binding to LTBP-2 by surface plasmon resonance. (A) Recombinant full-length DANCE and full-length LTBP-2 proteins were purified by chelating chromatography, separated by SDS–PAGE, and stained with Coomassie Blue. (B) LTBP-2 was injected over a DANCE-immobilized sensor chip surface. Each sensorgram shows seven different analyte concentrations of 15, 30, 60, 90, 180, 240, and 360 μg/ml. Response difference; the difference between experimental and control flow cells in response units (RU). Time is shown in seconds (s).
Deposition of DANCE is dependent on fibrillin-1, not on either fibrillin-2 or LTBP-2
To investigate the physiological significance of the interaction of DANCE and LTBP-2, we used RNA interference (RNAi) to knock down target genes. For this purpose, we developed an in vitro culture system of human skin fibroblasts to assess the effects of gene knockdown on elastic fiber assembly. We reverse transfected each siRNA duplex into human skin fibroblasts, cultured the transfected cells in 10% serum-containing medium for more than 10 days, and subjected them to immunostaining with anti-elastin and anti-DANCE antibody. As controls, we used siRNA with a scrambled sequence of protein phosphatase PP2C gamma, a gene irrelevant to the extracellular matrix. Because LTBP-2 is a constituent of elastic microfibrils, we also examined the role of fibrillin-1 and -2, major constituents of elastic microfibrils, in addition to LTBP-2. We confirmed that treatment with siRNA results in more than a 90% decrease in the respective transcripts even in double or triple knockdown cells as detected by quantitative polymerase chain reaction (qPCR) (Figure 7A). No off-target knockdown was observed at least in mRNAs of fibrillin-1 and -2, LTBP-2, DANCE and elastin (data not shown). Fibrillin-1 and -2 knockdown specifically abolished each meshwork of fibrillin-1 and -2 microfibrils (Figure 7B–O). As shown in Figure 8B, fibrillin-1-knockdown cells develops only a faint meshwork of elastic fibers, whereas fibrillin-2-knockdown cells and LTBP-2-knockdown cells each develop abundant meshworks of elastic fibers like those in the control cells (Figure 8A, C and D). Moreover, DANCE is barely deposited on fibrillin-1-knockdown cells (Figure 8J), whereas DANCE is abundantly deposited and colocalizes with elastin on fibrillin-2- or LTBP-2-knockdown cells (Figure 8I, K and L). These results indicate that DANCE and elastin are deposited mainly on fibrillin-1 microfibrils in human skin fibroblast culture.
Figure 7.
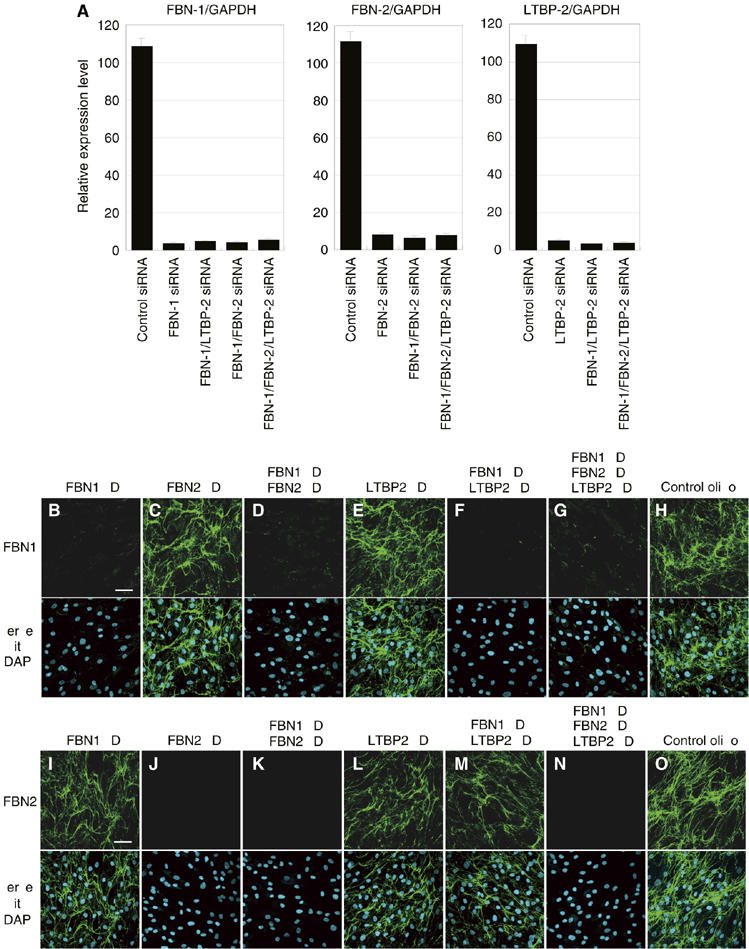
Quantitative PCR analysis of gene knockdown in skin fibroblasts (A), and fibrillin-1 and -2 knockdown specifically abolishes the meshwork of fibrillin-1 and -2 microfibrils, respectively (B–O). (A) Total RNA from siRNA-transfected skin fibroblasts was extracted 9 days after transfection. Complementary DNA was synthesized and was subjected to quantitative real-time PCR for the expression of fibrillin-1, fibrillin-2, LTBP-2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts. A graphic presentation of the results obtained by qPCR is shown. Levels of GAPDH transcript are used to normalize the cDNA levels. The relative amount of the PCR product amplified from control siRNA-treated fibroblasts is set at 100. Data are presented as the means±s.e. of three independent experiments, each performed in duplicate. (B–O) HSFs were transfected with each RNAi oligo as indicated and cultured in 10% serum containing media. Cells were stained with anti-fibrillin-1 (B–H) or anti-fibrillin-2 antibody (I–O). Lower panels are superimpositions of upper panels with DAPI (4,6-diamidino-2-phenylindole) nuclear staining. KD, knockdown; FBN-1, fibrillin-1; FBN-2, fibrillin-2. Scale bars; 60 μm.
Figure 8.
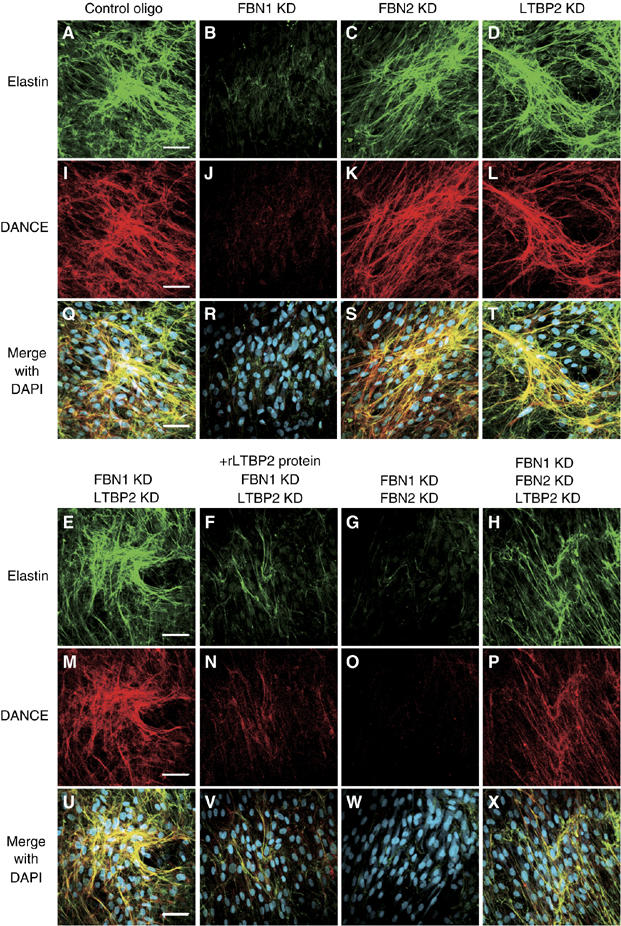
DANCE and elastin deposition is dependent on fibrillin-1 microfibril, but LTBP-2 knockdown induces fibrillin-1-independent deposition of DANCE and elastin. HSFs were transfected with each RNAi oligo as indicated, cultured in 10% serum containing media for 14 days and fixed. Cells were stained with anti-elastin (A–H) and anti-DANCE (I–P) antibodies. In (F) and (N), recombinant LTBP-2 (rLTBP2) was added to the culture medium to cancel the knockdown effect of LTBP-2. (Q–X) Superimpositions of (A–H) and (I–P) with DAPI nuclear staining, showing that DANCE colocalizes with elastic fibers. Scale bars; 60 μm.
LTBP-2 inhibits fibrillin-1-independent deposition of DANCE and elastin
Next, we investigated the role of LTBP-2 in the deposition of DANCE in the absence of fibrillin-1. For this purpose, we doubly knocked down LTBP-2 in addition to fibrillin-1. As shown in Figure 8E and M, the nearly abolished deposition of DANCE and elastin resulting from fibrillin-1 knockdown is unexpectedly rescued by additional LTBP-2 knockdown. The rescue effect of LTBP-2 knockdown is similar when each of three different siRNAs to LTBP-2 is independently transfected with fibrillin-1 siRNA (data not shown). To rule out off-target effects of LTBP-2 RNAi, we added recombinant LTBP-2 protein to these fibrillin-1–LTBP-2 double knockdown cells. As shown in Figure 8F and N, addition of recombinant LTBP-2 greatly reduces the deposition of DANCE and elastin to a similar level as observed in fibrillin-1 single knockdown cells. These results indicate that the rescue phenotype in double-knockdown cells is specifically due to depletion of LTBP-2, which implies that LTBP-2 inhibits fibrillin-1-independent deposition of DANCE and elastin.
Next, we asked whether DANCE is deposited on fibrillin-2 microfibrils instead of fibrillin-1 microfibrils, in the fibrillin-1–LTBP-2 double knockdown culture. To investigate this possibility, we examined the effect of fibrillin-1–fibrillin-2–LTBP-2 triple knockdown. The deposition of DANCE and elastin on fibrillin-1–fibrillin-2–LTBP-2 triple-knockdown cells is substantially less than that on fibrillin-1–LTBP-2 double-knockdown cells (Figure 8E, H, M and P). These results suggest that downregulation of LTBP-2 causes deposition of DANCE and elastin mainly on fibrillin-2 microfibrils. Moreover, it is possible that there might be potential microfibrils devoid of fibrillin-1 and -2 on which DANCE and elastin can be deposited in the absence of LTBP-2, because we detected weak, but more considerable fibrillar deposition of DANCE and elastin on fibrillin-1–fibrillin-2–LTBP-2 triple-knockdown cells than on fibrillin-1–fibrillin-2 double-knockdown cells (Figure 8G, H, O and P). We could not, however, rule out a possibility that even an undetectable amount of fibrillin-1 or -2 might support deposition of DANCE and elastin in the absense of LTBP-2. These data suggest that LTBP-2 inhibits fibrillin-1-independent deposition of DANCE and elastin onto fibrillin-2 or other potential microfibrils.
Recombinant LTBP-2 protein promotes deposition of DANCE onto fibrillin-1 microfibrils
Because LTBP-2 inhibits fibrillin-1-independent deposition of DANCE, we hypothesized that LTBP-2 may promote deposition of DANCE on fibrillin-1 microfibrils. To test this hypothesis, we added recombinant LTBP-2 into the medium of fibrillin-1, -2, or control-knockdown cells, cultured them in 5% serum-containing medium and subjected them to immunostaining with anti-DANCE and anti-fibrillin-1 antibody. As shown in Figure 9, recombinant LTBP-2 markedly increases the deposition of DANCE in fibrillin-2-knockdown cells (Figure 9C and D). On the other hand, we cannot detect deposition of DANCE at all on fibrillin-1-knockdown cells (Figure 9A and B). These results suggest that LTBP-2 not only inhibits deposition of DANCE onto fibrillin-2 or other potential microfibrils, but actively promotes deposition of DANCE onto fibrillin-1 microfibrils.
Figure 9.
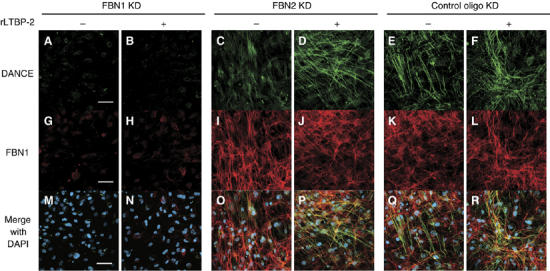
Recombinant LTBP-2 markedly increases deposition of DANCE onto fibrillin-1 microfibrils in fibrillin-2-knockdown cells. HSFs were transfected with each RNAi oligo as indicated, cultured in 5% serum containing media for 14 days and fixed. Two days after transfection, recombinant LTBP-2 protein (16 μg/ml) or mock was added to the culture medium. Cells were stained with anti-DANCE (A–F) and anti-fibrillin-1 (G–L) antibodies. (M–R) Superimpositions of (A–F) and (G–L) with DAPI nuclear staining. Scale bars; 60 μm.
To rule out the possibility that LTBP-2 might regulate deposition of tropoelastin through direct interaction with tropoelastin, we performed solid phase binding assay. As shown in Figure 10A, LTBP-2 does not interact with tropoelastin at all, whereas DANCE strongly interacts with tropoelastin.
Figure 10.
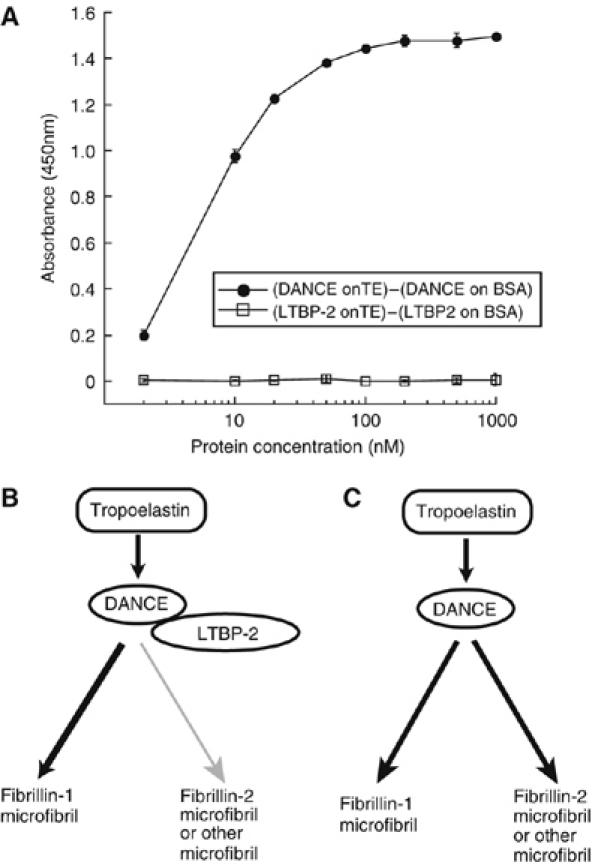
DANCE strongly interacts with tropoelastin, whereas LTBP-2 does not directly interact with tropoelastin (A), and working hypothesis on the role of LTBP-2 in elastic fiber assembly (B, C). (A) Solid phase binding assay on recombinant tropoelastin or bovine serum albumin (BSA) was performed using recombinant FLAG-tagged LTBP-2 or DANCE as a soluble ligand. LTBP-2 does not interact with tropoelastin at all, whereas DANCE definitely interacts with tropoelastin. Signals detected for binding on BSA are subtracted as nonspecific background. All measurements were performed in triplicate, and values shown are means±s.d. (B, C) Schematic illustration of our hypothesis. LTBP-2 promotes deposition of DANCE onto fibrillin-1 microfibrils, whereas inhibiting deposition of DANCE on fibrillin-2 or other potential microfibrils. LTBP-2 thus actively regulates the differential usage of microfibrils in elastic fiber assembly.
These data suggest a model on the role of DANCE–LTBP-2 interaction in elastic fiber assembly (Figure 10B and C): binding of LTBP-2 to DANCE promotes DANCE deposition on fibrillin-1 and inhibits DANCE deposition on fibrillin-2 or other potential microfibrils.
Discussion
The DANCE molecule has been shown to play an integral role in elastic fiber development. Identification of the roles of DANCE-binding proteins may lead to new understanding of the molecular mechanisms of elastic fiber assembly. In the present study, we newly demonstrate the interaction of DANCE and LTBP-2. LTBP-2 is known to associate with elastic tissue microfibrils and has been considered to perform a structural role within elastic fibers (Gibson et al, 1995). The distributions of DANCE and LTBP-2 transcripts are shown by in situ hybridization to strikingly coincide. The interaction of endogenous DANCE and LTBP-2 is demonstrated in mice organs by co-immunoprecipitation. Kinetic analysis reveals that the affinity of this interaction is potent and specific. We mapped the specific binding domain of these molecules and show that the sixth cbEGF-like domain of DANCE interacts with the N-terminal domain of LTBP-2. This N-terminal LTBP-2 domain has been suggested to be important for the interaction of LTBP-2 and extracellular matrices (Hyytiäinen et al, 1998). To investigate the functional roles of LTBP-2, we set up an in vitro culture system with which we can evaluate the effects of gene knockdown in elastic fiber development. Using this system, we demonstrate that deposition of DANCE and elastin is dependent on fibrillin-1 in human skin fibroblast cell culture. We also demonstrate that downregulation of LTBP-2 can direct fibrillin-1-independent deposition of DANCE and elastin, whereas recombinant LTBP-2 can promote deposition of DANCE on fibrillin-1 microfibrils. These results suggest that LTBP-2 might work as a molecular switch that regulates the differential usage of microfibrils in elastic fiber assembly.
Fibrillin-1 is the major structural component of elastic microfibrils. We have found that deposition of DANCE is dependent on fibrillin-1 in cell culture, which is consistent with a recent report showing that DANCE directly interacts with fibrillin-1 (Freeman et al, 2005). However, fibrillin-1-deficient mice are reported to develop normal elastic matrices, whereas DANCE-deficient mice show disorganized and fragmented elastic matrices (Pereira et al, 1997; Nakamura et al, 2002; Yanagisawa et al, 2002; Ramirez et al, 2004). Recent findings that fibrillin-1–fibrillin-2 double-deficient mice fail to complete fetal development with impaired elastogenesis suggest that fibrillin-2 can compensate for fibrillin-1 deficiency in elastic fiber development (Carta et al, 2006). In human skin fibroblast culture, however, we show that knockdown of fibrillin-1 is not compensated, even though there is abundant fibrillin-2 meshwork. It is often observed that more compensatory mechanisms work in the development than in cell culture. Further investigation is required to identify the compensatory mechanism between the fibrillins in embryogenesis, and to determine whether LTBP-2 is a part of the compensatory mechanism.
Although fibrillin-1 and -2 molecules are structurally similar glycoproteins, they are differentially expressed in terms of both developmental stages and tissue distribution. Fibrillin-2 is generally expressed in earlier gestational period than fibrillin-1, and the expression of fibrillin-2 coincides with the beginning of elastogenesis, leading to the idea that fibrillin-2 might play a role at early stage of elastogenesis, whereas fibrillin-1 might play a role at later stage (Zhang et al, 1995). Thus, fibrillins are differentially employed in the development of elastic matrices during embryogenesis. Here, we show that LTBP-2 may play an important role in regulating the differential usage of microfibrils from fibrillin-2 to fibrillin-1. Consistent with these findings, LTBP-2 transcripts start to express in elastic organs at a similar timing, when fibrillin-1 transcripts gradually increases on around 13.5 days postcoitum (Nunes et al, 1997; Shipley et al, 2000). On the other hand, microfibrils have been reported to be often composites of fibrillin-1 and -2 in skin fibroblasts culture (Charbonneau et al, 2003). LTBP-2 does not seem to either inhibit or promote deposition of DANCE onto these microfibrils (Figure 9E and F).
LTBP-2 has been shown to be specifically localized in elastin-containing microfibrils (Gibson et al, 1995). Moreover, the expression of LTBP-2 transcripts largely parallels that of tropoelastin transcripts (Shipley et al, 2000). On the other hand, fibrillin-1 is also observed in tissues devoid of elastin, and seems to play more redundant roles than elastic fiber matrices. In fact, immunostaining of elastin colocalizes with that of only a part of the fibrillin-1 microfibrils meshwork (Supplementary Figure S4). In contrast, the distributions of DANCE and elastin are quite similar (Figure 8A, I and Q). Therefore, among the meshwork of fibrillin-1 microfibrils, only microfibrils with DANCE deposition seem to develop mature elastic fibers. One intriguing possibility is that LTBP-2 can also be involved in the selection of fibrillin-1 microfibrils on which DANCE should be deposited. However, we cannot rule out the involvement of other molecules than LTBP-2, such as proteoglycans or other LTBP family members, in the process of DANCE deposition. LTBPs-1 and -2 have recently been shown to bind fibrillin-1 through their C-terminal region but not to fibrillin-2, which may account for the mechanism of LTBP-2 in the selection of fibrillins (Hirani et al, 2007). To clarify the precise role of LTBP-2 in elastic fiber assembly in vivo, further studies using conditional knockout mice will be needed, because LTBP-2-deficient mice are embryonic lethal at the implantation stage, due to a cause unrelated to elastic fibers (Shipley et al, 2000).
In summary, we have identified LTBP-2 as a DANCE-binding protein that can regulate DANCE deposition on microfibrils. We demonstrate that the DANCE and elastin deposition on microfibrils is dependent on fibrillin-1 in the presence of LTBP-2, and that downregulation of LTBP-2 causes fibrillin-1-independent deposition of DANCE and elastin on fibrillin-2 or other potential microfibrils. Moreover, recombinant LTBP-2 promotes deposition of DANCE on fibrillin-1 microfibrils. We propose that LTBP-2 might function as a molecular switch that determines which microfibrils DANCE should be deposited on, thereby regulating subsequent assembly of elastic fiber components. Further studies will be required to elucidate the role of LTBP-2 in elastic fiber assembly in vivo and the mechanism of the regulation of DANCE targeting.
Materials and methods
Cell culture
293T cells, bovine aortic smooth muscle cells (ASMCs), and human skin fibroblasts (HSFs) were maintained in DMEM (Sigma) supplemented with 2 mM glutamine, 10% penicillin/streptomycin, and 10% FBS at 37°C in 5% CO2. HSFs, which were taken from the facial skin of 3-month-old baby, were kindly provided by Dr M Naito (Kyoto University). These HSFs were passed for 8 to 10 times before following experiments.
Metabolic labeling and immunoprecipitation
The culture medium of bovine ASMCs was changed to Cys/Met-free DMEM (Invitrogen) supplemented with 0.5 mCi. of 35S-Cys/Met (Amersham) and 10% FBS, and the cultures were incubated overnight. An aliquot (5 ml) of the conditioned media was mixed with 20 μg of purified FLAG-tagged DANCE protein, and subjected to immunoprecipitation with anti-FLAG M2 affinity gel (Sigma). Other aliquots, 1.6 ml each, were subjected to immunoprecipitation with the following antibodies conjugated with protein-G/A Sepharose (Amersham): anti-human elastin monoclonal (Chemicon), anti-bovine tropoelastin monoclonal (Elastin Products Company (EPC), MM436), anti-human fibrillin-1 polyclonal (EPC, PR684), anti-human fibrillin-1 monoclonal (Neo Marker), anti-human fibrillin-2 polyclonal (EPC, PR225), or anti-bovine LTBP-2 monoclonal (EPC, MM425). Immune complexes were resolved by SDS–PAGE. The SDS–polyacrylamide gel was dried, and exposed to X-ray film.
Plasmid construction
Human full-length DANCE cDNA was cloned as described previously (Nakamura et al, 1999). pEF6/V5 (Invitrogen) was modified by incorporation of a C-terminal FLAG-tag or Myc-tag (pEF6/FLAG, pEF6/Myc). The DANCE ΔN1- (Δnucleotide(nt) 247-399) and ΔN2- (Δnt 247-504) DANCE cDNAs were amplified by PCR, and subcloned into pEF6/FLAG. ΔM1- (Δexon 5), ΔM2- (Δexon 6), ΔM3- (Δexon 7), ΔM4- (Δexon 8), ΔM5- (Δexon 9), ΔC1- (Δexons 10 and 11), and ΔC2-DANCE (Δexon 10) cDNA fragments were amplified by inverse PCR, followed by self-ligation. Human DANCE cDNA sequences are numbered according to GenBankTM accession number AF112152.
An expression vector encoding human full-length LTBP-2 was kindly provided by Dr J Keski-Oja (University of Helsinki). To prepare the FLAG-tagged LTBP-2 mutants, pEF6/V5 was modified by incorporation of an N-terminal FLAG-tag, following the preprotrypsin signal sequence (accatgtctgcacttctgatcctagctcttgttgg agctgcagttgct) (pEF6/ssFLAG). The following fragments of LTBP-2 cDNA were amplified by PCR, and subcloned into pEF6/ssFLAG: LTBP-2-A (exons 1–5), -B (exons 6–15), -C (exons 16–22), -D (exons 23–28), -E (exons 29–36), -F (exons 1–3), -G (exons 4–6), -H (exon 4), and -I (exon 5). LTBP-2-G-Ig, -H-Ig, and -I-Ig were C-terminal fusion proteins with the Fc portion of human IgG (nt 759–1457). Human LTBP-2 and Ig Fc portion cDNA sequences were obtained from GenBank accession number S82451 and Y14735, respectively. The coding region of each molecule was subcloned into pEF6/Myc. All constructs were confirmed by sequencing (ABI Prism 3100).
Transfection, in vitro binding assay, and Western blotting
293T cells were transfected using LipofectAMINE PLUS (Invitrogen). After transfection, they were cultured in serum-free DMEM/F12 (Sigma). The mixtures of conditioned media and cell lysates were subjected to immunoprecipitation with anti-FLAG M2 affinity gel followed by Western blotting as described previously (Hirai et al, 2007).
In situ Hybridization
We fixed neonatal mouse tissues in 4% PFA and embedded them in histoparaffin (Wako). The DANCE and LTBP-2 riboprobes were prepared as described previously (Shipley et al, 2000). Synthesis of in situ hybridization probes was carried out using the Riboprobe in vitro Transcription Systems (Promega). Paraffin sections were subjected to in situ hybridization as described previously (Nakamura et al, 1999). Counterstaining was performed with 0.02% toluidine blue. Samples were observed with an Axioplan 2 microscope (Zeiss) using a × 5 objective. Pictures were taken with an AxioCam HR digital camera (Zeiss) and AxioVision 3.1 software (Zeiss).
Mouse tissue extraction
Lung tissue samples were homogenized in PBS using a Polytron homogenizer (PT10-35, Kinematica AG). After centrifugation, the supernatants were subjected to immunoprecipitation with an anti-mouse DANCE polyclonal antibody (BSYN2473), followed by Western blotting. Either anti-mouse LTBP-2 polyclonal or anti-mouse DANCE polyclonal antibody was used as a primary antibody. HRP-conjugated anti-rabbit polyclonal antibody (Santa Cruz) or rabbit IgG TrueBlotTM (eBioscience), respectively, was used as a secondary antibody. Anti-DANCE antibody (BSYN2473) was generated by BioSynthesis Inc. as described previously (Yanagisawa et al, 2002). Anti-LTBP-2 antibodies were raised by Sigma Aldrich Japan against KLH-conjugated polypeptides, CEVIPEEEFDPQNAR and CASDLEEYDAEEGH, which correspond respectively to amino acids 234–247 and 1384–1397 of mouse LTBP-2 protein.
Expression and purification of DANCE and LTBP-2 proteins
Human DANCE and LTBP-2 cDNAs were subcloned into pEF6/FLAG-His to add C-terminal His6 and FLAG-tag. Recombinant proteins were purified, qualified, and quantified as described previously (McLaughlin et al, 2006).
BIAcore analysis
For kinetic binding studies, Biacore X was used. Purified recombinant DANCE protein was immobilized onto CM5 sensor chips by amine coupling in 10 mM sodium acetate (pH 4.0), giving 765.7 Response Units (RU). Subsequent binding experiments were performed in HBS-P buffer (BIACORE). The sensor chip was regenerated in 10 mM glycine-HCl, pH 2.0. Dissociation constants (KD) were determined by fitting all curves at once with the 1:1 Langmuir binding model using BIAevaluation software.
Gene knockdown experiments and reverse transfection
Duplexed RNA oligonucleotides (Stealth™ Select RNAi) were designed using BLOCK-iT™ RNAi Designer, and synthesized by Invitrogen. Three different sequences of RNAi duplexes were synthesized for each target gene and mixed for use to minimize off-target effects. Mixed RNAi duplexes (at final concentration 15 nM) were reverse transfected into HSFs using Lipofectamine 2000 (Invitrogen). Sequence information for the Stealth™ Select RNAi duplexes is provided in Supplementary Table I).
Reverse transcription–polymerase chain reaction and quantitative PCR
Total RNAs were extracted and transcribed to cDNA followed by qPCR as described previously (Hirai et al, 2007). Primers used for qPCR are provided in Supplementary Table II.
Immunocytochemical staining
HSFs were cultured on microscope cover glasses (Fisherbrand). The cells were fixed with 100% methanol at −20°C. The primary antibodies used were anti-human elastin polyclonal (PR533, EPC), anti-human DANCE monoclonal (10A), anti-human fibrillin-1 polyclonal (PR217, EPC), and anti-human fibrillin-2 monoclonal (mAb143) antibodies. The secondary antibodies used were Alexa 488, 546, or 647 anti-rabbit or mouse IgG (Invitrogen), followed by nuclear staining with Hoechst 33258. Stained cells were mounted with ProLong Gold antifade reagent (Invitrogen), and visualized using an Olympus confocal microscope (FV1000). Pictures were taken with FV10-ASW 1.4 software (Olympus). Anti-DANCE antibody (10A) was raised by Iwaki & Co Ltd, Japan by immunization of DANCE null mice with purified recombinant human DANCE protein. Anti-fibrillin-2 antibody (mAb143) was generously provided by Dr Lynn Y. Sakai (Shriners Hospital for Children, Oregon Health and Science University).
Solid phase binding assay
Various concentrations of recombinant FLAG-tagged LTBP-2 or DANCE were used as soluble ligands. Solid phase binding assays using purified tropoelastin were performed as described previously with the modification of 2 mM CaCl2 added in the reaction buffer (McLaughlin et al, 2006). The primary antibody used was anti-FLAG M2 antibody (Sigma). The secondary antibody used was HRP-conjugated anti-mouse IgG antibody (Santa Cruz). Signals were detected with Substrate Reagent Pack (R&D).
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Acknowledgments
We thank Ms N Tomikawa for excellent technical assistance. This work was supported in part by Japan Health and Labour Sciences Research Grants, Japan Society for the Promotion of Science, Japan Science and Technology Agency, Takeda Science Foundation, Sakakibara Memorial Foundation, and Japan Heart Foundation Research Grants to TN, by grants from the Ministry of Education, Science and Culture of Japan to TN and TK, and by grants from the Japan Society for the Promotion of Science, Japan Heart Foundation Research grant on Arteriosclerosis Update to MH.
References
- Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F (2001) Regulation of limb patterning by extracellular microfibrils. J Cell Biol 154: 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AJ (2001) Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 122: 735–755 [DOI] [PubMed] [Google Scholar]
- Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F (2006) Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem 281: 8016–8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau NL, Dzamba BJ, Ono RN, Keene DR, Corson GM, Reinhardt DP, Sakai LY (2003) Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J Biol Chem 278: 2740–2749 [DOI] [PubMed] [Google Scholar]
- Chaudhry SS, Gazzard J, Baldock C, Dixon J, Rock MJ, Skinner GC, Steel KP, Kielty CM, Dixon MJ (2001) Mutation of the gene encoding fibrillin-2 results in syndactyly in mice. Hum Mol Genet 10: 835–843 [DOI] [PubMed] [Google Scholar]
- Freeman LJ, Lomas A, Hodson N, Sherratt MJ, Mellody KT, Weiss AS, Shuttleworth A, Kielty CM (2005) Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem J 388: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MA, Hatzinikolas G, Davis EC, Baker E, Sutherland GR, Mecham RP (1995) Bovine latent transforming growth factor beta 1-binding protein 2: molecular cloning, identification of tissue isoforms, and immunolocalization to elastin-associated microfibrils. Mol Cell Biol 15: 6932–6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MA, Hatzinikolas G, Kumaratilake JS, Sandberg LB, Nicholl JK, Sutherland GR, Cleary EG (1996) Further characterization of proteins associated with elastic fiber microfibrils including the molecular cloning of MAGP-2 (MP25). J Biol Chem 271: 1096–1103 [DOI] [PubMed] [Google Scholar]
- Gibson MA, Hughes JL, Fanning JC, Cleary EG (1986) The major antigen of elastin-associated microfibrils is a 31-kDa glycoprotein. J Biol Chem 261: 11429–11436 [PubMed] [Google Scholar]
- Giltay R, Kostka G, Timpl R (1997) Sequence and expression of a novel member (LTBP-4) of the family of latent transforming growth factor-beta binding proteins. FEBS Lett 411: 164–168 [DOI] [PubMed] [Google Scholar]
- Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, Kita T, Nakamura T (2007) Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol 176: 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirani R, Hanssen E, Gibson MA (2007) LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol 26: 213–223 [DOI] [PubMed] [Google Scholar]
- Hyytiainen M, Penttinen C, Keski-Oja J (2004) Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci 41: 233–264 [DOI] [PubMed] [Google Scholar]
- Hyytiäinen M, Taipale J, Heldin CH, Keski-Oja J (1998) Recombinant latent transforming growth factor beta-binding protein 2 assembles to fibroblast extracellular matrix and is susceptible to proteolytic processing and release. J Biol Chem 273: 20669–20676 [DOI] [PubMed] [Google Scholar]
- Kanzaki T, Olofsson A, Moren A, Wernstedt C, Hellman U, Miyazono K, Claesson-Welsh L, Heldin CH (1990) TGF-beta 1 binding protein: a component of the large latent complex of TGF-beta 1 with multiple repeat sequences. Cell 61: 1051–1061 [DOI] [PubMed] [Google Scholar]
- Kapetanopoulos A, Fresser F, Millonig G, Shaul Y, Baier G, Utermann G (2002) Direct interaction of the extracellular matrix protein DANCE with apolipoprotein(a) mediated by the kringle IV-type 2 domain. Mol Genet Genomics 267: 440–446 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T (2004) Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36: 178–182 [DOI] [PubMed] [Google Scholar]
- Mariencheck MC, Davis EC, Zhang H, Ramirez F, Rosenbloom J, Gibson MA, Parks WC, Mecham RP (1995) Fibrillin-1 and fibrillin-2 show temporal and tissue-specific regulation of expression in developing elastic tissues. Connect Tissue Res 31: 87–97 [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY (2006) Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol 26: 1700–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moren A, Olofsson A, Stenman G, Sahlin P, Kanzaki T, Claesson-Welsh L, ten Dijke P, Miyazono K, Heldin CH (1994) Identification and characterization of LTBP-2, a novel latent transforming growth factor-beta-binding protein. J Biol Chem 269: 32469–32478 [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J Jr, Honjo T, Chien KR (2002) Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415: 171–175 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ruiz-Lozano P, Lindner V, Yabe D, Taniwaki M, Furukawa Y, Kobuke K, Tashiro K, Lu Z, Andon NL, Schaub R, Matsumori A, Sasayama S, Chien KR, Honjo T (1999) DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J Biol Chem 274: 22476–22483 [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Itoh S, Jeney V, Yanagisawa H, Fujimoto M, Ushio-Fukai M, Fukai T (2004) Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ Res 95: 1067–1074 [DOI] [PubMed] [Google Scholar]
- Nunes I, Gleizes PE, Metz CN, Rifkin DB (1997) Latent transforming growth factor-beta binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-beta. J Cell Biol 136: 1151–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali-Ronchetti I, Baccarani-Contri M (1997) Elastic fiber during development and aging. Microsc Res Tech 38: 428–435 [DOI] [PubMed] [Google Scholar]
- Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono R, Reinhardt DP, Sakai LY, Biery NJ, Bunton T, Dietz HC, Ramirez F (1997) Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet 17: 218–222 [DOI] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY, Dietz HC, Rifkin DB (2004) Fibrillin microfibrils: multipurpose extracellular networks in organismal physiology. Physiol Genomics 19: 151–154 [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, Abrams WR, Mecham R (1993) Extracellular matrix 4: the elastic fiber. FASEB J 7: 1208–1218 [PubMed] [Google Scholar]
- Saharinen J, Keski-Oja J (2000) Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell 11: 2691–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen J, Taipale J, Monni O, Keski-Oja J (1998) Identification and characterization of a new latent transforming growth factor-beta-binding protein, LTBP-4. J Biol Chem 273: 18459–18469 [DOI] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E (1986) Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol 103: 2499–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley JM, Mecham RP, Maus E, Bonadio J, Rosenbloom J, McCarthy RT, Baumann ML, Frankfater C, Segade F, Shapiro SD (2000) Developmental expression of latent transforming growth factor beta binding protein 2 and its requirement early in mouse development. Mol Cell Biol 20: 4879–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J (1995) Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem 270: 4689–4696 [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN (2002) Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415: 168–171 [DOI] [PubMed] [Google Scholar]
- Yin W, Smiley E, Germiller J, Mecham RP, Florer JB, Wenstrup RJ, Bonadio J (1995) Isolation of a novel latent transforming growth factor-beta binding protein gene (LTBP-3). J Biol Chem 270: 10147–10160 [DOI] [PubMed] [Google Scholar]
- Zanetti M, Braghetta P, Sabatelli P, Mura I, Doliana R, Colombatti A, Volpin D, Bonaldo P, Bressan GM (2004) EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol Cell Biol 24: 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Apfelroth SD, Hu W, Davis EC, Sanguineti C, Bonadio J, Mecham RP, Ramirez F (1994) Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol 124: 855–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hu W, Ramirez F (1995) Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol 129: 1165–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4


