Abstract
Purpose
To determine the effect of corneal drying on the outcome of optical coherence tomography (OCT).
Design
Cohort study
Participants
Seventeen normal participants (mean age, 39±12 years)
Methods
Subjects underwent a series of peripapillary circular StratusOCT scans (version 3.0; Carl Zeiss Meditec, Inc., Dublin, CA) in a randomly selected eye. Baseline scan sets were acquired, and thereafter, blinking was prevented by taping the eyelid. Eyelid taping was immediately followed by 6 to 8 serial scan sets, each separated by 20 seconds. After removing the eyelid tape, 3 additional scans were acquired at 1, 2, and 4 minutes of blinking freely.
Main Outcome Measures
The analyzed outcome measures were scan quality as defined by signal‐to‐noise ratio (SNR) and signal strength (SS) provided by the built‐in OCT software and mean nerve fiber layer (NFL) thickness.
Results
Significant reductions in SNR, SS, and NFL were noted at each scanning point in the drying phase (for each, P<0.015, paired t test) except for NFL thickness measurements acquired at 140 and 160 seconds. The reduction in NFL thickness exceeded the 95% confidence limit of the reported reproducibility error of StratusOCT after 15 seconds of corneal drying. After 1 and 2 minutes of blinking freely, there was still a significant reduction in NFL thickness compared with the baseline value, which was no longer evident at the 4‐minute scan.
Conclusions
Corneal dryness affects OCT scan quality and measured NFL thickness after a short exposure time. It is recommended to instruct those who are scanned to blink frequently or to instill artificial tears.
Optical coherence tomography (OCT) is an imaging technology that can be used to produce high‐resolution images of ocular structures. It uses light to create 2‐dimensional images demonstrating the layers of the retina.1–3 Optical coherence tomography is used for the detection and evaluation of a variety of pathological processes such as glaucoma, macular edema, and macular hole.4–10
Our clinical experience indicates that the longer the duration of OCT scanning sessions, the more likely it is that the images acquired will be of poorer quality. We also have observed that when the patient is instructed to blink, the scan quality improves visibly. The corneal tear film serves a key role in the maintenance of a smooth, regular optical surface.11,12 While obtaining an OCT scan, the patient is required to stare at a target on a video display for several minutes and at certain times is instructed to hold the examined eye open while the images are acquired. Previous studies have shown that looking intently at a target, such as a book or a video display terminal, reduces the rate of blinking with an increase in the rate of tear evaporation.13,14 It therefore is likely that OCT scanning sessions result in increased tear evaporation secondary to prolonged staring and occasional voluntary cessation of blinking. Optical coherence tomography is a light‐based imaging method, and measurements are dependent on the ability of the light source to enter and exit the eye. A smooth optical surface maintained by the tear film would allow for minimal scattering of the illuminating and subsequently reflected light, whereas the relatively rough surface of the eye with a degraded tear film could scatter the measurement beam and degrade image quality. The aim of this study was to investigate the effect of ocular dryness on the quality of OCT images and the resulting measurements.
Patients and Methods
Healthy volunteers were enrolled in this prospective study. Institutional review board and ethics committee approval was obtained for the study, and all participants gave their informed consent to participate in the study. The study followed the principles of the Declaration of Helsinki.
Each participant underwent a full medical and ocular history, and a detailed ocular examination was performed, including visual acuity and slit‐lamp and fundus examinations.
The healthy volunteers were recruited from the faculty and staff of an academic ophthalmology facility. Criteria for inclusion were age >20 years, best‐corrected visual acuity equal to 20/30 or better, refraction <6.5 diopters (D) spherical equivalent, no history of significant systemic or ocular disease including dry eye disease, no previous ocular surgeries, and no abnormal findings on ophthalmic examination. If contact lens wearers were recruited, they were required to cease contact lens use for at least 24 hours before the experiment was conducted. The eye used in the experiment was assigned by a coin flip if both eyes were eligible.
Study Protocol
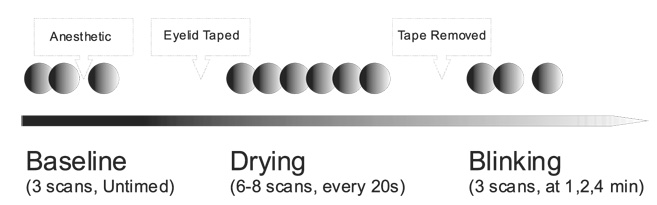
Each participant underwent a baseline fluorescein staining examination and tear break up time (BUT) assessment in the selected eye using a sterile ophthalmic strip with 0.6 mg fluorescein sodium and a sterile balanced salt solution. The examination was performed by one of the authors of this study (RJN), and care was taken to ensure that the fluorescein strip did not touch the corneal epithelium during application. Any irregularities of corneal staining were noted and tear BUTs were recorded. The corneal evaluation was conducted immediately before the instillation of 1% tropicamide and 2.5% phenylephrine for pupillary dilation before recording OCT images. The participants were imaged using StratusOCT (version 3.0; Carl Zeiss Meditec, Inc., Dublin, CA) using the Fast Retinal Nerve Fiber Layer scanning protocol. This scanning protocol consists of 3 consecutive, circumpapillary nerve fiber layer (NFL) images at a scanning radius of 3.4 mm centered on the optic nerve head. All OCT scans in this study were acquired by the same experienced OCT operator (DMS). Each participant underwent a series of up to 14 OCT scans in the selected eye (Fig 1); 2 baseline scans were acquired before any intervention. Administration of an anesthetic drop (proparacaine hydrochloride 0.5%) was followed by a postanesthetic scan. The upper eyelid was taped to the forehead to prevent blinking of the selected, anesthetized eye. A stopwatch timer was started at the moment the eye was taped open, and up to 8 serial scans, 1 every 20 seconds, were acquired (total exposure time, up to 2 minutes and 40 seconds). The scanning during this drying time was stopped before all 8 scans were acquired only if the operator determined that the scan signal had completely degraded, as indicated on the live scanning image, to ensure that excessive drying of the cornea did not occur. After these drying scans, the tape was removed and 3 more scans were acquired at 1, 2, and 4 minutes after the participant was allowed to blink freely. Each participant then underwent an additional fluorescein staining corneal examination to evaluate the cornea after the scanning and drying procedures.
Figure 1.
Experimental scanning sequence.
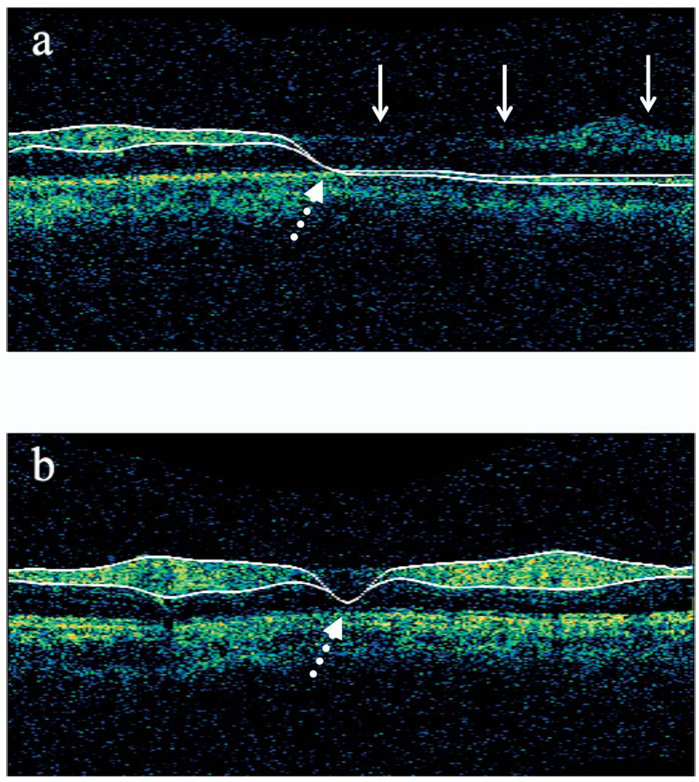
Images were evaluated for accuracy of the automated NFL border‐detection algorithm used by the device to measure NFL thickness. Scans were excluded if the images demonstrated one or both of the following: (1) obviously inaccurate border detection for more than a consecutive 15% or additive 20% of the total image or (2) if the automatically detected borders of the NFL collapsed, meaning that a string of 0s was recorded for at least 10 consecutive points in the scan for NFL thickness (Fig 2). The mean of at least 2 qualified scans for each scanning point was used for the analysis.
Figure 2.
Examples of failed nerve fiber layer (NFL) detection algorithm. a, Inaccurate border detection (continuous arrows). b, Collapse of the automatically detected NFL borders resulting in a false 0 value for NFL thickness (dotted arrows).
The OCT scan data were exported using the built‐in data exporting software of the StratusOCT, and the following were collected from the exported data for each scan: scan time, signal‐to‐noise ratio (SNR; a general indication of overall scan quality), signal strength (SS; an image quality parameter), and global peripapillary retinal NFL thickness.
Statistical Analysis
The data were summarized for each scanning time point and the mean of the 3 scans was used for the analysis. Paired t test analysis was used for comparison of the qualified scans with the baseline values. The primary analysis used generalized estimating equations (GEEs) to test for linear trends in the change variables over time, within each phase (drying, blinking). This analysis makes the most efficient use of the data, while taking into account the high level of correlation among repeated measures in the same eye. The GEE model used identity as the link function with a normal distribution and compound symmetry as the working correlation matrix.
Results
Seventeen healthy participants were enrolled in the study. The mean age of the group was 39±12 years. Six of the participants were male, and 6 of the 17 eyes (35%) were right eyes. All participants underwent a fluorescein staining examination, with no surface abnormalities or staining irregularities. Mean BUTs for all participants was 13.9±5.5 seconds. All participants were scanned to at least 80 seconds of drying time. The number of scans of sufficient quality to calculate NFL thickness decreased with increasing drying time (Fig 3).
Figure 3.
Graph showing the relationship between drying time and nerve fiber layer (NFL) border detection success. Drying time 1 through drying time 8 signifies the drying scans during which the eyelid was taped to prevent blinking; each was taken 20 seconds apart. Blink 1, blink 2, and blink 3 are scans after the tape was removed and the participant was allowed to blink freely, taken at 1, 2, and 4 minutes after tape removal, respectively.
Paired t test analysis of the mean of the 2 baseline scans versus the scans just after administration of the anesthetic drop revealed no significant difference in measured SNR (36.5±3.2 dB vs. 36.6±3.4 dB; P = 0.81), SS (8.2±1.5 vs. 8.2±1.5; P = 0.71), or average NFL thickness (106.1±13.7 µm vs. 106.0±13.2 µm; P = 0.89). For the rest of the analyses, the SNR, SS, and NFL thickness of the scans taken after administration of the anesthetic drop were used as the baseline values, because these scans were closest in time to the actual drying scans. The mean SNR, SS, and retinal nerve fiber layer thickness values are reported for each scan of the experimental sequence in Table 1.
Table 1.
Mean (Standard Deviation) Optical Coherence Tomography Parameters at Each Drying and Free Blinking Phase
| Scan Sequence | n1* | Time | Signal‐to‐Noise Ratio (dB) | Signal Strength | n2* | Nerve Fiber Layer Thickness (µm) |
|---|---|---|---|---|---|---|
| Baseline | 17 | 0.0 | 36.6 (3.4) | 8.2 (1.6) | 17 | 106.0 (13.2) |
| Drying 1 | 17 | 22.0 (3.1) | 31.4 (5.1) | 6.3 (2.2) | 16 | 102.0 (13.0) |
| Drying 2 | 17 | 40.9 (1.9) | 29.6 (6.1) | 5.4 (2.3) | 14 | 100.1 (12.3) |
| Drying 3 | 16 | 61.2 (2.2) | 29.0 (6.4) | 5.2 (2.3) | 12 | 102.5 (8.9) |
| Drying 4 | 17 | 80.9 (2.5) | 26.9 (7.1) | 4.5 (2.4) | 10 | 101.0 (9.6) |
| Drying 5 | 16 | 101.0 (2.3) | 26.6 (6.8) | 4.3 (2.5) | 9 | 98.3 (11.7) |
| Drying 6 | 14 | 120.4 (1.7) | 27.2 (6.5) | 4.4 (2.4) | 9 | 97.3 (12.6) |
| Drying 7 | 10 | 139.7 (1.6) | 28.6 (6.6) | 4.9 (2.5) | 7 | 99.7 (10.7) |
| Drying 8 | 8 | 161.3 (3.3) | 29.2 (6.1) | 5.3 (2.4) | 6 | 101.7 (9.5) |
| Blinking 1 | 17 | 64.3 (5.3) | 32.6 (5.8) | 6.7 (2.2) | 17 | 102.0 (11.4) |
| Blinking 2 | 17 | 122.5 (10.4) | 34.1 (4.4) | 6.9 (2.0) | 17 | 101.5 (12.0) |
| Blinking 3 | 16 | 244.3 (10.9) | 34.9 (3.9) | 7.6 (1.7) | 16 | 104.7 (12.0) |
n1 = no. of qualified scans for time, signal‐to‐noise ratio, and signal strength; n2 = no. of qualified scans for nerve fiber layer thickness.
The number of measurements varied because of missed scans, premature cessation of scanning because of excessive signal loss, or nonqualified scans.
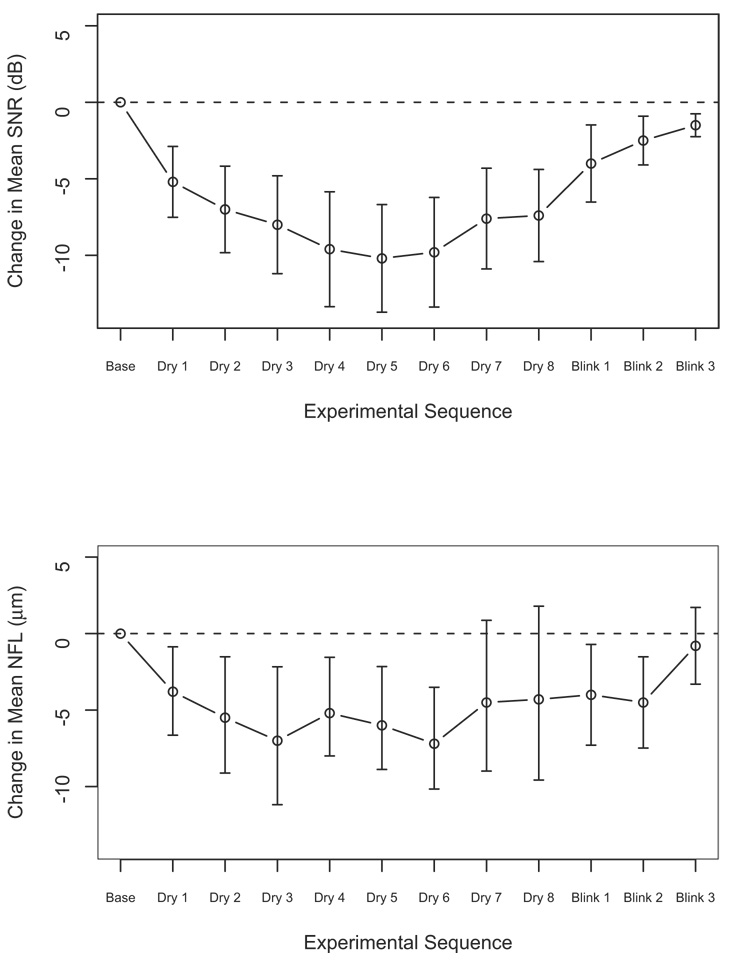
The SNR and SS decreased with time during the drying phase (−3.07 dB/minute and −1.24/minute, respectively; P<0.0001 for both, GEE analysis) and increased during the blinking phase (0.63 dB/minute and 0.25/minute; P = 0.05 and 0.03, respectively). The NFL also decreased during the drying phase (−1.97 µm/minute; P = 0.0001) and increased during the blinking phase (1.12 µm/minute; P = 0.01). Figure 4 demonstrates the differences between mean SNR and NFL measurements at each scanning point and the baseline measurement. At each drying point, there was a significant decrease in SNR, SS, and NFL compared with the baseline values (for all, P<0.015, paired t test) except for the NFL thickness change in drying time 7 and 8. After blinking, the difference remained significant for SNR and SS (P<0.004). For NFL measurements, the difference was significant at the first 2 measurements (P<0.02), but was not significant at the last scan.
Figure 4.
Change in (top) mean signal‐to‐noise ratio (SNR) and (bottom) nerve fiber layer (NFL) thickness from baseline. All measurements during drying were significantly lower than the baseline measurements. dB = decibels.
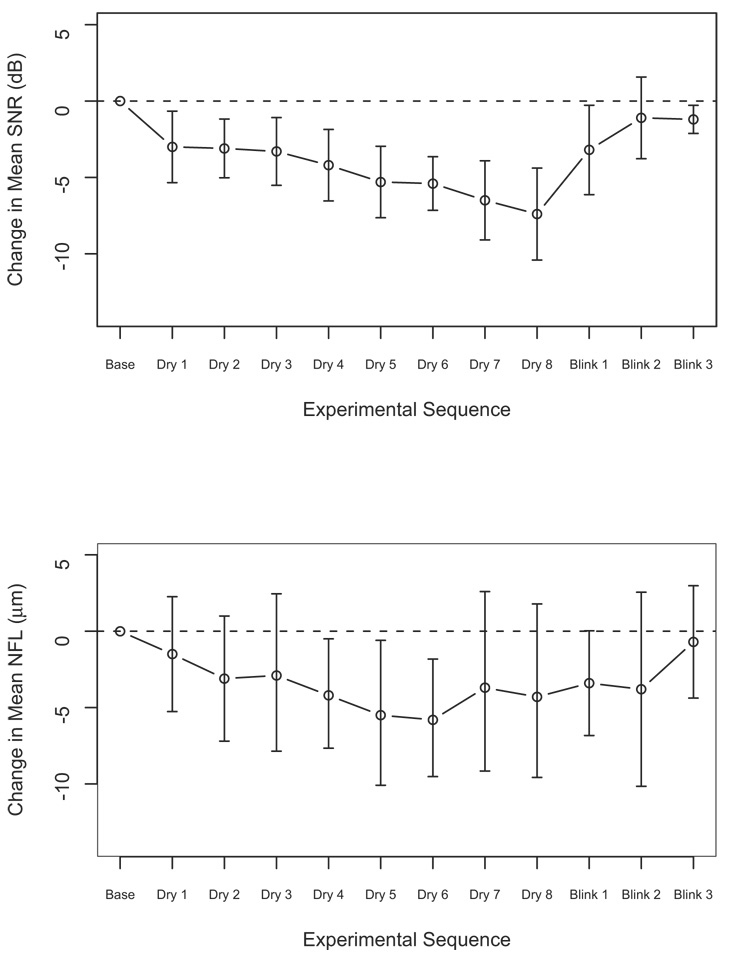
Assessment of the subgroup with qualified measurements throughout the dryness period (160 seconds; 8 eyes) showed a consistent reduction in the SNR and NFL thickness measurements as compared with baseline (Fig 5). The difference from baseline was significant throughout the drying time for the quality parameters (P<0.025, paired t test). For NFL, the difference was significant only at drying times 4 through 6 (P<0.014).
Figure 5.
Changes in (top) mean signal‐to‐noise ratio (SNR) and (bottom) nerve fiber layer (NFL) thickness from baseline for those with qualified scans at 160 seconds (n = 8). dB = decibels.
When the rate of NFL changes in the early phase of corneal drying was modeled (GEE analysis), there was a reduction of 2.0 µm at 15 seconds, which exceeds the 95% confidence limit of the intravisit reproducibility error for this parameter as published in a previous study.4
Baseline BUT was not a significant confounder (P>0.05) in any of the tested models for SNR, SS, or NFL thickness, either in the entire group or in the subgroup with extended qualified scans.
Discussion
The study evaluated the effect of extended corneal exposure, with resulting corneal drying, on the quality of OCT scans and the quantitative measurements obtained by those scans. Prolonged corneal exposure led to significant reduction both in scan quality and NFL thickness measurements (Table 1; Fig 4, Fig 5). The reduction in scan quality led to a substantial decrease in the percentage of scans that qualified for analysis (Fig 3).
The SNR sharply declined at the early stages of the drying phase, reaching a plateau at 80 seconds (drying time 4) and an incline at 140 seconds (drying time 7; Fig 4). However, eyes with qualified scans beyond 130 seconds of corneal dryness showed a continuous reduction in scan quality throughout the drying period (Fig 5). It is apparent that the upswing in the scan quality in the entire group was to the result of the switch in the composition of the studied population. A near constant number of eyes were qualified until drying time 4, and thereafter a gradual reduction was noted. At drying time 7, a substantial number of scans were not qualified because of the poor quality. Thus, a selection of a subset of participants with tendency of slow drying was analyzed in drying times 7 and 8 with consequent relative improvement in scan quality.
A similar feature was noted for NFL measurements. A rapid deterioration was noted initially until drying time 3, when an increase in thickness appeared (Fig 4). After drying time 6, a second upswing was noted. In eyes with scans beyond 130 seconds, the first upswing was not apparent, thus demonstrating the same phenomenon as described in the quality parameters. The cause of the late upswing was unclear. Our hypothesis is that, despite the fact that the upper eyelid was taped, we could not prevent the eye movement that might allow for some tear film distribution. Many of the participants reported a blurring of vision toward the end of the drying sessions. It is possible that to minimize this blurring and to best fixate on the target, some participants moved their eye, resulting in a redistribution of the tear film.
Because of the upswing pattern of the graphs, the overall estimate of the deterioration rate as defined by using liner regression underestimates the true slope. Using a model to evaluate the changes at the early drying phase, it seemed that at drying time of 15 seconds the NFL thickness changes are beyond the 95% confidence interval of the reproducibility error of StratusOCT and thus may significantly affect the accuracy of the measurements. Moreover, this rate was calculated based on data obtained from a healthy and relatively young cohort of participants. It can be expected that in elderly patients and with the use of some of the commonly used topical medications, this rate may be accelerated further.
It should be noted that in the presence of dry cornea, OCT analysis tends to report thinner NFL values, which may be perceived as abnormal measurements in glaucomatous eyes even in eyes without actual damage.
When blinking was permitted, a gradual improvement appeared, although a significant difference was still observed in the first 2 scans while participants were allowed to blink freely. Therefore, participants who experience corneal dryness during scanning should be allowed appropriate time for blinking before an accurate scan can be acquired.
There were several limitations to this study. Topical anesthetics may affect the properties of the tear film layer. However, it would have been intolerable and unethical to conduct this experiment without their use. Because there were no significant differences in the outcome parameters between the 2 preanesthetic baseline scans and the postanesthetic scan, it seems that they did not impose a short‐term effect on the optical properties of the tear film layer. Moreover, in clinical practice, many patients are scanned after their intraocular pressure is measured, which includes the use of topical anesthetics. Another limitation of our study is that several eyes did not have measurements from the late drying scans because of the prominent deterioration in scan quality. However, if these censored eyes had been continued for the full drying time, it is likely that our findings would have been stronger.
The duration of the corneal exposure was purposely exaggerated by our experimental design to prove that OCT images could be affected in this manner. The substantial changes that appear after the very short corneal drying time emphasize the importance of this process in the routine clinical practice. Because blinking was able to restore both the image quality parameters and the thickness measurements to values that were close to baseline values, we suggest that interventions aimed to ensure a smooth optical surface, such as instructing patients to blink before scanning or instilling artificial tear drops, could lead to higher quality scans and more accurate clinical measurements. Additionally, because the drying effects were observed to be relatively long lasting, one may consider conducting imaging studies before conducting automated perimetry, during which patients are likely to be exposed to reduced blinking. Although this study was conducted using one specific imaging technology, we believe that these results and conclusions may be applicable to other imaging methods that depend on light passing through the cornea.
Acknowledgments
Supported in part by the National Institutes of Health, Bethesda, Maryland (grant nos.: R01‐EY013178‐6, P30‐EY008098); The Eye and Ear Foundation, Pittsburgh, Pennsylvania; and an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York.
Footnotes
Dr Schuman receives royalties for intellectual property licensed by the Massachusetts Institute of Technology to Carl Zeiss Meditec, Inc.
References
- 1.Fujimoto JG, Brezinski ME, Tearney GJ, et al. Optical biopsy and imaging using optical coherence tomography. Nat Med. 1995;1:970–972. doi: 10.1038/nm0995-970. [DOI] [PubMed] [Google Scholar]
- 2.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–332. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 4.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci. 2004;45:1716–1724. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guedes V, Schuman JS, Hertzmark E, et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology. 2003;110:177–189. doi: 10.1016/s0161-6420(02)01564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanner V, Chauhan DS, Jackson TL, Williamson TH. Optical coherence tomography of the vitreoretinal interface in macular hole formation. Br J Ophthalmol. 2001;85:1092–1097. doi: 10.1136/bjo.85.9.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995;102:748–756. doi: 10.1016/s0161-6420(95)30959-1. [DOI] [PubMed] [Google Scholar]
- 8.Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh‐resolution optical coherence tomography. Arch Ophthalmol. 2003;121:695–706. doi: 10.1001/archopht.121.5.695. [DOI] [PubMed] [Google Scholar]
- 9.Hee MR, Puliafito CA, Duker JS, et al. Topography of diabetic macular edema with optical coherence tomography. Ophthalmology. 1998;105:360–370. doi: 10.1016/s0161-6420(98)93601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuman JS, Hee MR, Arya AV, et al. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995;6:89–95. doi: 10.1097/00055735-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Dursun D, Monroy D, Knighton R, et al. The effects of experimental tear film removal on corneal surface regularity and barrier function. Ophthalmology. 2000;107:1754–1760. doi: 10.1016/s0161-6420(00)00273-6. [DOI] [PubMed] [Google Scholar]
- 12.Rolando M, Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol. 2001;45 suppl 2:S203–S210. doi: 10.1016/s0039-6257(00)00203-4. [DOI] [PubMed] [Google Scholar]
- 13.Schlote T, Kadner G, Freudenthaler N. Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefes Arch Clin Exp Ophthalmol. 2004;242:306–312. doi: 10.1007/s00417-003-0845-z. [DOI] [PubMed] [Google Scholar]
- 14.Tsubota K, Nakamori K. Dry eyes and video display terminals. N Engl J Med. 1993;328:584. doi: 10.1056/NEJM199302253280817. [DOI] [PubMed] [Google Scholar]