Abstract
Natural enzymes were selected to function inside the cell, not in the test tube; therefore, their performance is optimized for the crowded conditions encountered in vivo. Most man-made matrices for enzyme confinement lead to suboptimal catalytic activity. Ackerman and colleagues showed that an entrapping environment consisting of functionalized mesoporous silica actually enhances enzyme activity beyond the test-tube levels of free enzymes in solution. These findings provide an approach for dissecting the effect of various contributors to enzyme activity and thereby provide a means for finetuning the entrapping matrices to optimize enzyme performance in a rational way.
Introduction
Natural enzymes have evolved to perform functions in the crowded environments of living cells, where biological macromolecules can take up as much as 30% of the intracellular space [1,2]. This level of crowding substantially limits the space available for molecular events such as two-body encounters between enzyme and substrate or intramolecular rearrangements. Thus, by excluding volume, macromolecules reduce the configurational entropy of unfolded or misfolded proteins and increase the probability of effective encounters, leading to protein compaction and associations. These effects are likely to affect enzymatic activity considerably because they bear on the structural and/or functional integrity of the enzyme and on the catalytic turnover.
Although protein enzymes have optimized their catalytic performance to function in crowded cells, biotechnological and industrial advances in enzyme confinement or immobilization have not been fine-tuned to reach the standard of performance achievable under in vivo conditions [3]. This situation is likely to change, in light of recent research reported by the Ackerman group [4]. These researchers showed that an appropriate confinement matrix, such as functionalized mesoporous silica (FMS) with open pores, can lead to a dramatic improvement in enzymatic activity well above the levels achieved by free enzymes in solution. This model of enzyme entrapment is illustrated in Figure 1. Although precursors to this level of ‘pore engineering’ have been reported [5,6], including previous work by the same group [7], the improvement in catalytic activity beyond test-tube solution levels in the present work [4] is highly novel and promising.
Figure 1.
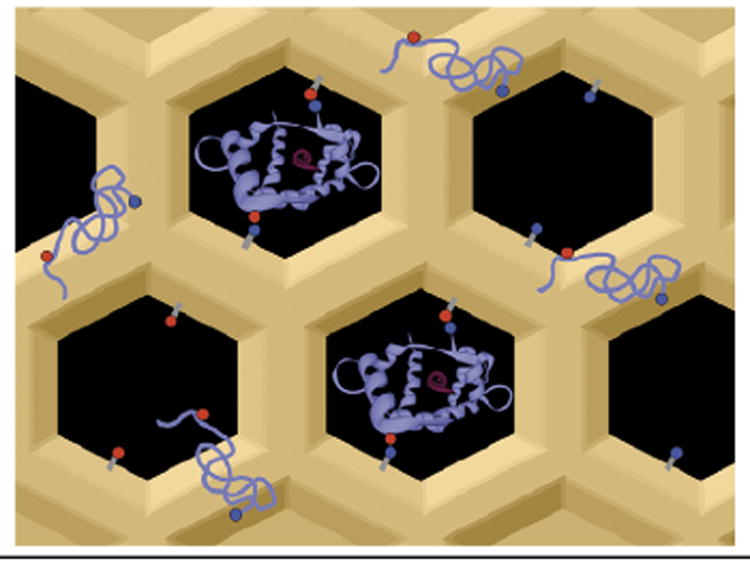
Proposed model for protein entrapment. The protein chain, in its native enzymatically competent fold, is indicated in ribbon representation, displaying matching polar groups on its surface. Protein entrapment is probable inside pores with matching patterns of functionalizing groups (red and blue balls) [4]. The pore entrapment is conducive to improved enzyme activity because the protein maintains its native structural integrity. By contrast, the free enzyme is more prone to misfolding (string representation).
The finding of improved activity by using FMS opens up an avenue for further theoretical and experimental work, ultimately aimed at optimizing confining matrices for enzymatic activity. A major task lies ahead, as we first dissect the impact of crowding on all molecular aspects of enzymatic activity [1]: the catalytic turnover [1,4]; the structural integrity of the enzyme [1,2]; framing refolding routes to enzyme recovery [2]; preventing aggregation and misfolding [8]; and even tempering the solvent around the catalytic site [9]. These studies should ultimately guide the engineering of entrapping matrices that are rationally optimized to enhance enzymatic activity.
A protein-confining matrix that enhances enzymatic activity
The Ackerman group developed a singular protein-confining matrix consisting of hexagonally ordered, functionalized mesoporous silica (SBA-15) with 300 Å pore size [4,7]. The pores have enough exposed surface area to prevent protein denaturation and are open, to facilitate mass transport and substrate access to the entrapped enzyme. Entrapment of the enzyme molecules in the pores is encouraged through electrostatic interactions between the protein and amino or carboxyl groups appended to the silica matrix with short aliphatic intercalation. The pore diameter seemed adequate to reduce, locally, the polarization of water or enhance electrostatic fields to the extent required for effective recognition of opposite charges [9]. Three commercially available enzymes were confined in the matrix: glucose oxidase, glucose isomerase and organophosphorous hydrolase. In all cases, the specific activity of the confined enzyme was enhanced beyond the levels in solution.
The Ackerman group conducted FTIR (Fourier-transform infrared spectroscopy) measurements of the immobilized protein to confirm that the confinement did not alter the native secondary structure of the protein. The bands associated with the two amide vibrational modes matched those of the protein in solution. In addition, the kinetic parameters for the enzymatic activity, namely the Michaelis-Menten constant and the turnover number, were determined for the confined protein and revealed only a small departure (same order of magnitude) from the values associated with the free enzyme in solution.
Arguably, FTIR measurements might be insufficient to discern local structural changes and any concurrent modulation of the microenvironment that can affect catalytic activity. However, by ruling out other factors, the spectroscopic and kinetic evidence points to the large-scale structural recovery of denatured molecules, with the confinement as the dominant factor enhancing enzymatic activity.
Tailoring matrices for enzyme recovery
Modeling studies pioneered by Minton [1,10] have delineated the effect of crowding on enzymology in terms of local and large-scale effects. Although crowding has been reported to promote protein denaturation or maintain structural disorder in certain instances [11,12], it is generally assumed that confinement introduces excluded volume effects that promote chain compaction and protein associations [1,2,10] (Figure 1). With regards to the latter, the probabilities of effective protein–substrate encounters or successful enzyme–product dissociations do not seem to be substantially altered by the FMS confinement developed by the Ackerman group. This observation is supported by the moderate variation in the enzyme kinetic parameters when compared with the solution values [4]. Furthermore, the results of the kinetic analysis prompted these researchers to postulate large-scale effects of excluded volume as the reason for increased enzymatic activity.
Excluded volume increases the frequency of intramolecular collisions of the protein chain with itself and reduces the conformational entropy of the unfolded or misfolded state [1,2] by reducing the 3D space available for exploration. The combination of these effects funnels the protein chain into compaction. However, chain compaction does not necessarily equate with protein renaturation, unless natural proteins evolved to misfold only into solvent-exposed extended conformations – an unverified hypothesis. Thus, plenty of experimental and theoretical effort will be required to prove that the particular pore size adopted (hexagons with a 300 Å diameter) optimizes, or at least promotes, refolding through volume exclusion. The results might, of course, be dependent on the enzymes used for confinement or, more specifically, on the relationships between pore size, radius of gyration of the native fold and contour length of the chain. Although protein confinement leads to chain compaction, it does not necessarily guarantee renaturation: compact misfolded states might occur and often result from non-native patterns of disulfide linkages [2]. Furthermore, there is evidence for crowding-induced denaturation, albeit on proteins with a marked misfolding propensity (e.g. amyloidogenic proteins) or those with a propensity for intrinsic disorder (e.g. oncogenic transcription factors) [11,12]. In such cases, confinement appears to induce aberrant aggregation [11] or oligomerization [12], respectively.
Besides promoting chain compaction, other alternatives to enzyme recovery promoted by the matrix are, in principle, plausible; these include a chaperone-like role for annealing and correcting misfolded states, the prevention of aggregation, or the preservation of structural integrity by preventing partial unfolding [2,8,10]. These possibilities will need to be evaluated as rational pore design develops.
The promising results obtained by the Ackerman group suggest that future rational optimization of the enzyme-confining matrix will ultimately depend on a clear delineation of excluded-volume effects on large-scale conformational rearrangements of the protein. Because the enzymes evolved to function under in vivo crowding conditions, and not under in vitro man-made confining matrices, lessons from the living cell might need to be drawn to optimize pore design.
Acknowledgments
The work described herein by the Ackerman group was funded by the Office of Biological and Environmental Research within the Department of Energy. AF and AKD are funded by the National Institutes of Health.
References
- 1.Minton AP. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem. 2001;276:10577–10580. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berg B, et al. Macromolecular crowding perturbs protein refolding kinetics: implications for folding inside the cell. EMBO J. 2000;19:3870–3875. doi: 10.1093/emboj/19.15.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yiu HH, et al. Enzyme immobilization using SBA-15 mesoporous molecular sieves with functionalized surfaces. J Mol Catal B: Enzym. 2001;15:81–92. [Google Scholar]
- 4.Lei C, et al. Characterization of functionalized nanoporous supports for protein confinement. Nanotechnology. 2006;17:5531–5538. doi: 10.1088/0957-4484/17/22/001. [DOI] [PubMed] [Google Scholar]
- 5.Miyahara M, et al. Immobilization of lysozyme onto preengineered mesoporous AISBA-15. J Nanosci Nanotechnol. 2006;6:1765–1771. doi: 10.1166/jnn.2006.221. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H, et al. Catalytic activity in organic solvents and stability of immobilized enzymes depend on the pore size and surface characteristics of mesoporous silica. Chem Mater. 2000;12:3301–3305. [Google Scholar]
- 7.Lei C, et al. Entrapping enzyme in a functionalized nanoporous support. J Am Chem Soc. 2002;124:11242–11243. doi: 10.1021/ja026855o. [DOI] [PubMed] [Google Scholar]
- 8.van den Berg B, et al. Effects of macromolecular crowding on protein folding and aggregation. EMBO J. 1999;18:6927–6933. doi: 10.1093/emboj/18.24.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández A. What caliber pore is like a pipe? Nanotubes as modulators of ion gradients. J Chem Phys. 2003;119:5315–5319. [Google Scholar]
- 10.Minton A. Excluded volume as a determinant of macromolecular structure and reactivity. Biopolymers. 1981;20:2093–2120. [Google Scholar]
- 11.McNulty BC, et al. Macromolecular crowding in the Escherichia coli periplasm maintains alpha-synuclein disorder. J Mol Biol. 2006;355:893–897. doi: 10.1016/j.jmb.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Flaugh SL, Lumb KJ. Effects of macromolecular crowding on intrinsically disordered proteins c-Fos and p27kip1. Biomacromolecules. 2001;2:538–540. doi: 10.1021/bm015502z. [DOI] [PubMed] [Google Scholar]


