Abstract
There is much interest, derived from current neurochemical, genetic, and therapeutic research, in the role of brain neurotrophins in schizophrenia. Neurotrophins play key roles in neuronal development and differentiation (i.e., promoting dendritogenesis and synaptogenesis), and in orchestrating the neuronal response to stress/noxious stimuli. Additionally, neurotrophins are modulators across monominergic (dopamine and serotonin), gabaergic and cholinergic systems. These roles focus on important areas of the etiopathophysiology of schizophrenia. Clinical studies show reductions in brain-derived neurotrophic factor (BDNF) and nerve growth factor (NFG) in schizophrenic patients as compared to normal control subjects, as well as differences in patients receiving first-generation antipsychotics (FGAs) or second-generation antipsychotics (SGAs). We now report on BDNF levels in subjects with first-episode psychosis in comparison with normal healthy controls. Compared to normal controls (N = 14; 290.5 ± 38.81 pg/ml), first-episode psychotic patients showed significant reduction (N = 15; 135 ± 21.77 pg/ml; P = 0.001; f = 12.873) in plasma BDNF. Additionally, plasma BDNF levels showed a significant negative correlation (N=13' r=0.584, p=0.0362) only with positive symptom scores at base line and no significant correlations were found with any of the cognitive performance test battery or motor function test scores. Low BDNF levels at the onset of psychosis suggest that it may contribute to the pathogenesis of schizophrenia and perhaps could be a helpful neurobiological marker for possible early treatment intervention.
Keywords: Brain-derived neurotrophic factor, first-episode psychosis, schizophrenia, neurotrophins
1. Introduction
The study of First-episode psychosis (FEP) is particularly advantageous in understanding the neurobiology of schizophrenia, in part because of the opportunity to minimize the potential impact of confounds such as illness duration, medication effects, and the psychiatric and medical comorbidities that are associated with chronicity of illness (Buckley and Evans, 2006). Studies of brain structure in FEP reveal a widespread pattern of cortical tissue loss (Thompson et al, 2001; Vita et al, 2006; Narr et al, 2006; Hao et al, 2006). Although findings of whole brain tissue loss, venticular enlargement, and selective (fronto-temporal) loss may appear less pronounced in FEP samples than in more chronic patient groups, it is nevertheless salutary that such changes are evident even at first presentation of illness. Indeed, there is evidence, although less substantial in amount and less robust in content, to suggest that anatomical brain changes may well antedate the onset of florid psychosis. Recently, Lappin and colleagues (2006) have reported that the longer duration of untreated psychosis in a British FEP sample was associated with greater gray matter tissue loss, with a predeliction for the left temporal lobe. In an earlier account of a high risk cohort, Pantelis and colleagues (2003) reported a reduction in medical temporal lobe volume in high risk patients after they were scanned following the onset of frank psychosis. This was an important finding, suggesting brain changes associated with the evolution of psychosis itself. In a more recent and extended analysis (Velakoulis et al, 2006), high risk subjects were compared with first episode chronic schizophrenia and normal control groups according to hippocampal and amygdala volumes at baseline. The results show no difference in MRI volumes between the high risk subjects and normal controls, irrespective of whether the at-risk patients did or did not progress to overt psychosis. First episode schizophrenia (but not other psychoses) groups had reduced (left) hippocampal volume. The implication that the changes occur in concert with the transition is intriguing and accords well with the notion of psychosis as a biologically toxic event in itself. Additionally, it has been found that second-generation antipsychotics (SGAs) lead to less tissue loss than first-generation antipsychotics (FGAs) over the course of treatment, perhaps reflecting greater clinical stability with SGAs (Chakos et al, 2005; Lieberman et al, 2005).
While the factors(s) and mechanisms underlying such observations remain to be elucidated, one reasonable summation is that fundamental processes in the brain have already been affected to produce the extent of structural changes that are evident on MRI in FEP. Amidst a growing appreciation of the developmental neurobiology of schizophrenia – as well as the propensity for progressive brain changes among patients with an unfavorable illness course – there is emergent information on abnormalities in the expression of neurotrophins in schizophrenia. Our own group has also focused on brain neurotrophins as one potential mediator of the neurobiology of schizophrenia and we have previously produced evidence that nerve growth factor – a prominent neurotrophin – is reduced in FEP (Parikh et al, 2003).
Neurotrophins have been extensively studied and their role has been established in neuronal development, in promoting synaptogenesis, and in orchestrating the neuronal response to stress/anxious stimuli. Brain-Derived Neurotrophic Factor (BDNF) is the most ubiquitous and most comprehensively studied of these neurotrophins. BDNF is found all over the cortex but is located predominantly in the hippocampus. BDNF has been found to promote survival of a wide range of neuronal cells and is known to modulate dopamine, gabaergic, and serotonergic receptors (Shoval and Weizman, 2005).
As mentioned above, an earlier study from our group (Parikh et al, 2003) measured NGF levels in drug naïve and medicated patients. Compared with normal controls, plasma NGF levels were lower in both patient groups. Interestingly, however, NGF levels were higher overall in the medicated chronic schizophrenia sample than in the first episode schizophrenia sample. Furthermore, significantly higher levels of NGF were seen in patients on treatment with SGAs than in patients who were receiving FGAs. Thus, there is evidence both that neurotrophins (in the latter instance, NGF) may be reduced in FEP and that (subsequent) treatment with antipsychotics can influence the pattern of neurotrophins. The present study adds to this emergent profile of the neurobiology of FEP. Specifically, in this study we have examined plasma levels of BDNF in patients during their first episode of schizophrenia in comparison with BDNF from healthy control subjects.
2. Methods and Materials
2.1. Subjects
The patient and normal subjects were from our earlier studies and were described extensively before (Evans et al, 2003; Khan et al, 2002; Parikh et al, 2003). Briefly, never-medicated FEP patients (N = 15; male/female = 8/7) were enrolled at the Department of Psychiatry, Dwight David Eisenhower Army Medical Center (DDEAMC), Fort Gordon, GA and from the Medical College of Georgia (MCG), Augusta, GA. The patients were diagnosed as schizophrenia or schizophreniform disorder after follow-up during subsequent 6 months of hospitalization. The clinical state of the patients was evaluated independently by two of the authors at baseline and findings were recorded on an anchored brief psychiatry rating scale (BPRS) (Overall and Gorham, 1962), and PANSS-positive (PSS) and PANSS-negative (NSS) symptom scores (Kay et al., 1987). The patients were of mean age 21 ± 8.83SD years and had a mean duration of illness of 2.1 ± 2.75 years. Controls (N= 14; male/female = 9/5) with mean age of 25 ± 5.72SD years consisted of the healthy volunteers recruited via advertisements at the Medical College of Georgia, VAMC and DDEAMC. Control subjects were matched for gender and age with first-episode schizophrenia patients. Institutional review boards of both DDEAMC and MCG had approved the research protocol, and all the subjects signed the consent forms.
2.2. Preparation of plasma and storage
The fasting blood from each subject was drawn early in the morning between 8:00 AM and 10:00 AM in lavender vacutainer-containing EDTA. The blood was centrifuged at 2500 rpm for 10 min at 5°C. The plasma was carefully separated, supplemented with 20μg/ml aprotinin and stored at - 70°C until analyzed.
2.3. Plasma BDNF ELISA
All samples were available as plasma since our earlier published studies were primarily focused on RBC and plasma. Although, fresh serum samples may yield slightly higher levels of neurotrophins due to release from platelets, since our samples were frozen and thawed such neurotrophins should have released from the platelets. A double antibody sandwich ELISA was developed to quantitate human plasma BDNF and all the measurements were performed in duplicate. Ninety-six well immunoplates were coated overnight at 4°C with 100μl of 1μg/ml monoclonal antihuman β-BDNF antibody (R&D systems). After blocking with 1% BSA, wells were incubated with 100 μl of sample for 2 h at room temperature. Wells were then incubated with 100 μl of 100 ng/ml biotinylated human β-BDNF polyclonal antibody (R&D Systems) for 2 h followed by incubation with 100μl of streptavidin-HRP for 20 min. Reaction was initiated by incubating the wells with 100 μl of TMB substrate solution in dark for 20 min and stopped by adding 50 μl of 1 M sulfuric acid. The absorbance was measured at 450 nm with Multiskan microplate reader (Flow Labs, McLean, VA USA). Standard curve was obtained by fitting the absorbance to different concentrations of human β-BDNF (R&D systems). The sensitivity of the assay was 4 pg/ml. All the samples were analyzed in duplicated in one session by an investigator blind to experimental set up. All assays were carried out in duplicate, and values were presented as means ± S.E.M.
2.4. Statistical analysis
Statistical analysis was done using Sigma Stat software. Student's t-test with a two-tailed variance was used to determine significant differences in BDNF levels between the groups. Correlations between plasma BDNF contents and psychopathology scores were examined using Pearson's product moment test.
3. Results
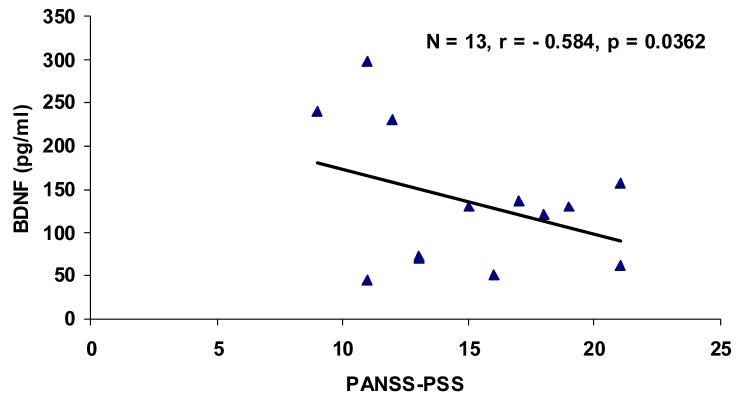
Patients and control groups were matched with respect to age and gender, although the first episode patients were younger. (This was not significant.) (Table 1). BDNF contents were markedly lower in plasma of never-medicated FEP (N = 14, 17.0 ± 3.0 pg/ml, P < 0.001) as compared to normal subjects (N = 15, 49.1 ± 6.7 pg/ml) (Fig. 1). Correlations were examined between plasma contents of BDNF and psychopathology scores. A highly significant negative correlation (N=13' r=0.584, p=0.0362) was found between plasma BDNF and scores of the baseline PANSS-Positive Symptom subscales (Figure 2). However, no correlations were found with other PANSS symptom or cognitive performance scores.
Table 1.
Demographic characteristics of first episode drug-naïve patients and normal controls
| Normal subjects | Schizophrenic patients | |
|---|---|---|
| N = 14 | N = 15 | |
| Mean age (years) | 25.28 ± 5.72SD | 21.8 ± 8.83SD |
| Sex (male/female) | 9/5 | 8/7 |
| Duration of illness | 0 | 2.1 + 2.75 |
p<0.001
Figure 1.
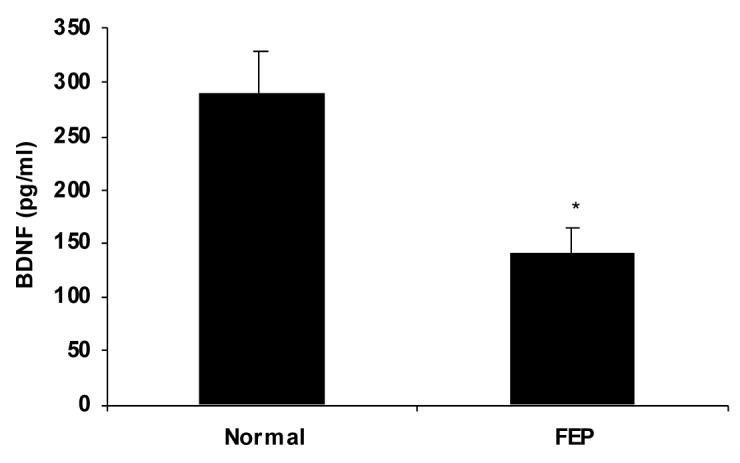
BDNF levels in plasma from first episode drug-naïve patients compared to normal controls
Figure 2.
Correlation between plasma BDNF contents and psychopathology scores.
4. Discussion
The present study reports markedly reduced BDNF levels in the plasma of FEP patients in comparison with healthy control subjects. The present study also extends our earlier comparative study of NGF in first episode patients, wherein lower NGF was observed in FEP patients (Parikh et al, 2003). These findings are consistent with an emergent literature that points to the abnormality in the physiology of BDNF in the brains of patients with schizophrenia (Shoval and Weizman, 2005; Buckley et al, submitted for publication). Reductions both with BDNF and in TrK B expression were found in the brains of patients with schizophrenia from the NIMH group (Weickert et al, 2003; 2005). In an analysis of MRI data from the Hillside first episode study, Szesko and colleagues (2005) found that the functional polymorphism of BDNF confers greater susceptibility to hippocampal tissue loss in schizophrenia. The effect of Val/Met heterozygosity on hippocampal volume was observed in both normal subjects and patients with schizophrenia but it was more pronounced in the patient group (Szesko et al, 2005). Wassink and colleagues (1999) also found a similar effect of BDNF genotype on brain imaging in schizophrenia. Other studies have focused on the impact of this Val/Met BDNF gene polymorphism on treatment response in schizophrenia. Anttilo and colleagues (2005) did not find any impact of BDNF polymorphisms on treatment response in a sample of 14 patients with schizophrenia who were treated with first generation antipsychotics. Liou and colleagues (2004) reported a relationship between Val/Met heterozygosity and severity of tardive dyskinesia in a sample of over 200 patients with schizophrenia. In another study (Ten et al, 2005) of BDNF levels among 80 patients with tardive dyskinesia (TD), patients with TD had lower BDNF levels than patients without TD or normal controls.
Toyooka and colleagues (2002) reported BDNF reductions in the serum of patients with schizophrenia. Shimizu and colleagues also reported a trend (not statistically significant) for lower BDNF levels in the serum in a sample of 15 drug naïve patients compared with medicated patients and normal subjects. Huang and Lee (2005) did not find overall patient – control differences, but rather reported higher BDNF levels in patients with paranoid and residual subtypes of schizophrenia. Earlier studies discussed and the present report support that neurotrophins may be associated with the expression of active psychotic symptoms. Particularly, the significant correlation between plasma BDNF and PANSS-PSS at baseline may indicate that BDNF may be a neurobiological predictor for change in psychopathology status, remission or exacerbation. Earlier, we reported correlation between NGF and the PANSS-NSS (Parikh et al, 2003). Earlier data that NGF levels were higher in patients medicated with SGAs vs FGAs (Parikh et al, 2003) and our extensive work on the brain NGF and BDNF in brain in rats treated with FGAs or SGAs, collectively suggest that plasma NGF and BDNF may be candidate biological markers for the differential actions of antipsychotics and the relationship between neurobiological events and clinical relapse.
Acknowledgements
This work was supported by a NIH/NCCAM Grant (AT000147).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anttilo S, Illia A, Kampman O, et al. Lack of association between two polymorphisms of brain-derived neurotrophic factor and response to typical neuroleptics. J Neural Transm. 2005;112:885–890. doi: 10.1007/s00702-004-0233-9. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Mahadik S, Pillai A, Terry A., Jr. Neurotrophins and schizophrenia. doi: 10.1016/j.schres.2007.01.025. review, revised submitted for publication in Schiz. Res. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Evans D. First episode schizophrenia – a of opportunigy for therapeutic intervention. J Postgrad Med. 2006;51:5–19. [PubMed] [Google Scholar]
- Chakos MH, Schobel SA, Gu H, et al. Duration of illness and treatment effects on hippocampal volume in male patients with schizophrenia. Br J Psychiatry. 2005;186:26–31. doi: 10.1192/bjp.186.1.26. [DOI] [PubMed] [Google Scholar]
- Hao Y, Liu Z, Jiang T, Gong G, Liu H, Tan L, Kuang F, Xu L, Yi Y, Zhang Z. White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuroreport. 2006;17(1):23–6. doi: 10.1097/01.wnr.0000195664.15090.46. [DOI] [PubMed] [Google Scholar]
- Huang TL, Lee CT. Associations between serum brain-derived neurotrophic factor levels and clinical phenotypes in schizophrenia patients. Journal of Psychiatric Research. 2005 doi: 10.1016/j.jpsychires.2005.11.004. in press. [DOI] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–275. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Lappin JM, Morgan K, Morgan C, Hutchison G, CChitnis X, Suckling J, et al. Gray matter abnormalities associated with duration of untreated psychosis. Schizophr Res. 2006;83(23):145–53. doi: 10.1016/j.schres.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollesfson GD, Charles C, Zipursky R, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62(4):361–70. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Liou YJ, Liao DL, Chen JY, et al. Association analysis of the dopamine D3 receptor gene ser9gly and brain-derived neurotrophic factor gene val66met polymorphisms with antipsychotic-induced persistent tardive dyskinesia and clinical expression in Chinese schizophrenic patients. Neuromolecular Med. 2004;5(3):243–51. doi: 10.1385/NMM:5:3:243. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Woods RP, Thompson PM, Szeszko P, et al. Regional specificity of cerebrospinal fluid abnormalities in first episode schizophrenia. Psychiatry Res. 2006;146(1):21–33. doi: 10.1016/j.pscychresns.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Overall JE, Graham DR. Brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Pantelis C, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005 Jul;31(3):672–96. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- Parikh V, Evans DR, Khan MM, Mahadik SP. Nerve growth factor in never-medicated first-episode psychotic and medicated chronic schizophrenic patients: possible implications for treatment outcome. Schizophr Res. 2003;60:117–123. doi: 10.1016/s0920-9964(02)00434-6. [DOI] [PubMed] [Google Scholar]
- Pillai A, Sharma S, Mahadik SP. Modulation of BDNF levels by antipsychotics regulate their temporal effects on neurogenesis in hippocampus. Biol Psychiatry. 2005a;57:201S. [Google Scholar]
- Shimizu E, Hashimoto K, Watanabe H, et al. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia are indistinguishable from controls. Neuroscience Letters. 2003;351(2):111–114. doi: 10.1016/j.neulet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Shoval G, Weizman A. The possible role of neurotrophins in the pathogenesis and therapy of schizophrenia. Eur Neuropsychopharmacol. 2005;15:319–329. doi: 10.1016/j.euroneuro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Molecular Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Tan YL, Zhou DF, Zhang XY. Decreased plasma brain-derived neurotrophic factor levels in schizophrenic patients with tardive dyskinesia: association with dyskinetic movements. Schizophrenia Research. 2005;74:263–270. doi: 10.1016/j.schres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T, Nawa H. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249–257. doi: 10.1016/s0165-1781(02)00127-0. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63(2):139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Rapoport JL, Hayashi KM, Geaga JA, et al. Dynamically spreading frontal and cingulated deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry. 2006;63(1):25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- Vita A, DePeri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitive magnetic resonance imaging studies. Schizophr Res. 2006;82(1):75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Nelson JJ, Crow RR, Andreasen NCA. Heritability of BDNF alleles and their effect on brain morphology in schizophrenia. Am J Med Genet. 1999;88:724–728. doi: 10.1002/(sici)1096-8628(19991215)88:6<724::aid-ajmg25>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, et al. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8(6):592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Ligons DL, Romancyzk T, et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10(7):637–650. doi: 10.1038/sj.mp.4001678. [DOI] [PubMed] [Google Scholar]