Abstract
Background:
Despite studies demonstrating improved outcomes, pessimism persists regarding the effectiveness of surgery for pancreatic cancer. Our objective was to evaluate utilization of surgery in early stage disease and identify factors predicting failure to undergo surgery.
Methods:
Using the National Cancer Data Base (1995–2004), 9559 patients were identified with potentially resectable tumors (pretreatment clinical Stage I: T1N0M0 and T2N0M0). Multivariate models were employed to identify factors predicting failure to undergo surgery and assess the impact of pancreatectomy on survival.
Results:
Of clinical Stage I patients 71.4% (6823/9559) did not undergo surgery; 6.4% (616/9559) were excluded due to comorbidities; 4.2% (403/9559) refused surgery; 9.1% (869/9559) were excluded due to age; and 38.2% (3,644/9559) with potentially resectable cancers were classified as “not offered surgery.” Of the 28.6% (2736/9559) of patients who underwent surgery, 96.0% (2630/2736) underwent pancreatectomy, and 4.0% (458/2736) had unresectable tumors.
Patients were less likely to undergo surgery if they were older than 65 years, were black, were on Medicare or Medicaid, had pancreatic head lesions, earned lower annual incomes, or had less education (P < 0.0001). Patients were less likely to receive surgery at low-volume and community centers. Patients underwent surgery more frequently at National Cancer Institute/National Comprehensive Cancer Network-designated cancer centers (P < 0.0001). Patients who were not offered surgery had significantly better survival than those with Stage III or IV disease but worse survival than patients who underwent pancreatectomy for Stage I disease (P < 0.0001).
Conclusions:
This is the first study to characterize the striking underuse of pancreatectomy in the United States. Of early stage pancreatic cancer patients without any identifiable contraindications, 38.2% failed to undergo surgery.
Despite studies demonstrating improved outcomes, pessimism persists regarding the effectiveness of surgery for pancreatic cancer. Our objective was to evaluate the utilization of surgery in early stage disease and identify factors predicting failure to undergo surgery. This is the first study to characterize the striking underuse of pancreatectomy in the United States.
Pancreatic cancer is the fourth leading cause of cancer deaths in the United States. In 2007, the American Cancer Society estimates that over 37,000 patients will be diagnosed with pancreatic cancer, and more than 33,000 will die of the disease.1 Patients with pancreatic cancer have a particularly dismal prognosis due to multiple factors, including late presentation, aggressive tumor biology, complex surgical management, and the lack of effective systemic therapies.2,3 Overall survival rates have remained relatively unaffected with fewer than 5% of all patients surviving 5 years after diagnosis.4
Surgery remains the only potentially curative treatment of localized pancreatic cancer.3 During the last 20 years, significant advances in preoperative evaluation, surgical techniques, and postoperative care have reduced the perioperative morbidity and mortality associated with pancreatic surgery.5–8 Mortality after pancreaticoduodenectomy has dropped from ∼25% in the 1960s to less than 3% in some high-volume centers,7–11 and recent studies have suggested improvements in long-term survival rates after resection for localized disease that approach 30%.12
Despite numerous studies and guidelines establishing pancreatectomy as the primary treatment modality for localized pancreatic adenocarcinoma, pessimistic attitudes toward all patients with pancreatic cancer have perhaps led to skepticism regarding the efficacy of resection. Clinicians have long recognized that a diagnosis of pancreatic cancer encompasses little variability in long-term outcomes; however, these views are outdated in light of recent evidence. Our hypothesis was that these attitudes affect utilization of surgery for early stage pancreatic cancer after controlling for age, comorbidities, and patient refusal to undergo surgery. The objectives of this study were 1) to evaluate and characterize the utilization of surgery for pancreatic adenocarcinoma, 2) to identify factors predicting failure to undergo surgery for localized disease, and 3) to evaluate the effect of surgery on survival.
METHODS
Data Acquisition and Patient Selection
The National Cancer Data Base (NCDB) is a program of the American College of Surgeons (ACS) and the Commission on Cancer (CoC).13 The NCDB now contains data on over 19 million patients from over 1440 hospitals accounting for greater than 75% of all cancers in the United States annually. The NCDB collects data regarding patient demographics, socioeconomic status, tumor variables, clinical and pathologic staging, treatment details, recurrence, survival, and health systems/provider information. Based on national incidence estimates from the American Cancer Society, the NCDB captures greater than 76% of all pancreatic cancers in the United States.1 This study protocol was approved by the Northwestern University Institutional Review Board.
Patients from 1995 to 2004 with International Classification of Disease – Oncology, 2nd and 3rd Edition (ICD-O-2/3) codes specific for pancreas were selected (C25.0, C25.1, C25.2, C25.3, C25.7, C25.8, C25.9).14,15 Patients were limited by ICD-O-2/3 codes for histologies consistent with pancreatic adenocarcinoma yielding 192,565 patients. Due to variations in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual from 1995 to 2004 (editions 4 to 6), all patients were restaged based on the AJCC 6th Edition TNM classification.16
Unique to the NCDB is the requirement to report both clinical and pathologic staging information. A pretreatment clinical stage is recorded in the database as judged by the treating physicians based on clinical findings and radiographic imaging. If the patient undergoes a surgical resection, pathologic staging information is separately recorded. We limited our analysis to patients with complete staging information who had clinical Stage I disease (n = 9559), which includes T1N0M0 and T2N0M0. Based on clinical and radiographic evaluation, these patients have potentially resectable disease because the tumor is localized to the pancreas and there are no obviously involved lymph nodes or distant metastases.
Patients who underwent pancreatectomy were identified based on the CoC's Registry Operations and Data Standards (ROADS) and the Facility Oncology Registry Data Standards (FORDS) site-specific procedure coding.17,18 In addition, if a patient did not undergo pancreatectomy, a reason is recorded in the database as detailed in the FORDS manual. These potential explanations include “not offered surgery”; “excluded due to comorbidities”; “patient refused recommended surgery”; and “unknown”. Patients who undergo surgery but who do not undergo pancreatectomy (presumably unresectable at laparotomy) are also recorded separately. In addition, we categorized patients older than 85 years at the time of diagnosis as “advanced age”.
The NCDB began requiring reporting of 6 preexisting comorbidities based on International Classification of Disease, 9th Edition (ICD-9-CM) classification in 2003.19 The primary cancer diagnosis and postoperative complications are not included when these 6 codes are reported, and comorbidities are recorded regardless of whether the patient undergoes surgery. A modified Charlson Comorbidity score (the number of coexisting medical conditions weighted according to their relative effects on survival) was calculated to assess the severity of preexisting comorbidities.20,21
Hospital Classification
Hospitals in the NCDB are classified by the CoC into academic and community cancer centers based on case volume and services offered.22 Academic hospitals must be affiliated with teaching and research institutions, meet annual case-volume requirements, and fulfill criteria regarding the ability to provide a wide range of cancer-specific specialists and services. Academic institutions comprise 23.6% of CoC hospitals and account for 37.3% of cases in the NCDB. National Comprehensive Cancer Network (NCCN) hospitals and National Cancer Institute (NCI)-designated cancer centers contribute data to the NCDB. NCCN and NCI hospitals were combined for analysis. Hospital volume was based on the average annual volume of pancreatic cancer cases reported. Quartiles were calculated with equal numbers of patients in each quartile. Hospital volume was evaluated as a continuous and categorical variable, but because results were similar, volume is only shown as a categorical variable.
Statistical Analysis
Continuous variables were compared using independent-sample t tests. Categorical variables were analyzed with Pearson χ2 tests with Bonferroni correction. Graphs and tables were used as needed to examine the distribution of each variable; Pearson correlation coefficients were determined for each pair of variables to aid in model building. Multivariate analysis was performed with a binary logistic regression model to identify factors predicting failure to undergo surgery in clinical Stage I patients. The level of statistical significance was set to P < 0.05. All P values reported are 2-tailed. As surgical and nonsurgical patients were evaluated, 5-year survival rates were calculated as the time from the date of diagnosis to death or last follow-up. Five-year overall and relative survival was estimated by the Kaplan–Meier method and compared using the log-rank test.23 Overall and relative survival (ratio of observed to expected survival based on United States Census data matched for gender, age, race, and no history of cancer) were similar due to the short median survival for pancreatic adenocarcinoma patients, thus only overall survival is shown. Median follow-up was 13.1 months.
Cox proportional hazards modeling was used to evaluate the impact of surgery on survival while adjusting for potential confounders, including age (<55, 56–65, 66–75, 76–85, >85 years), race (white, black, Asian, Hispanic, Other), median income (quartiles), college education (quartiles by percent with a degree per zip code), insurance status (private, Medicaid, Medicare, governmental, uninsured), anatomic location within the pancreas (head, body, tail), hospital factors (case volume quartiles, academic versus community, and NCCN/NCI versus non-NCCN/NCI were inserted separately into the model due to a high degree of collinearity), year of diagnosis, and census region.24 Since patient-level socioeconomic data are not recorded, median household income and education (percent of patients with college degrees) were assessed at the zip code level based on 2000 United States Census Bureau data.25 Utilization of surgery was also examined by United States census region based on 2000 Census Bureau data. Statistical analyses were performed using SPSS, version 14 (Chicago, IL).
RESULTS
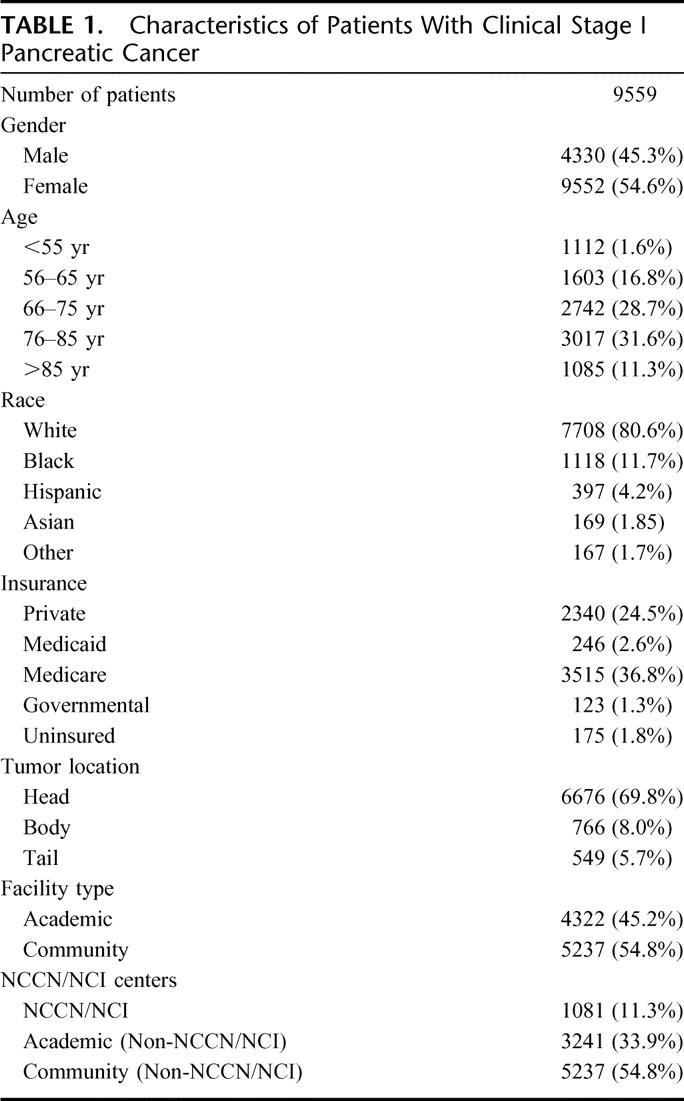
Of the 192,565 patients with pancreatic adenocarcinoma in the NCDB from 1995 to 2004, 9559 patients were selected who had pretreatment clinical Stage I disease and were thus thought to be potentially resectable (Table 1). The median age was 71.9 years (mean 72.4, range 18–107). More than 60% of the patients had Medicare or private insurance. The tumors were located in the head of the pancreas in 69.8%, body in 8.0%, and tail in 5.7%. The percentage of patients treated at academic hospitals was 45.2%, whereas 54.8% were treated at community hospitals. NCCN/NCI centers collectively cared for 11.3% of patients.
TABLE 1. Characteristics of Patients With Clinical Stage I Pancreatic Cancer
Utilization of Surgery
From 1995 to 2004, pancreatectomy utilization in Stage I patients increased from 21.8% to 35.7% (P < 0.0001); while the percentage of patients who did not receive treatment decreased (Fig. 1). Of the 9559 patients with clinical, pretreatment Stage I pancreatic cancer, only 28.6% underwent surgery (Fig. 2). Of those who underwent surgery, 96.0% were resectable and underwent pancreatectomy, while 4.0% were unresectable at laparotomy. Of clinical Stage I patients, 6.4% were excluded due to comorbidities, 4.2% refused surgery, and an additional 9.1% were excluded due to advanced age. Of patients with potentially resectable tumors 38.2% were not offered surgery. The reason for why the remaining 13.5% of patients did not undergo surgery was listed as unknown. Thus, 51.7% (38.2% not offered surgery + 13.5% unknown) of pancreatic cancer patients with clinical Stage I disease did not have a documented or identifiable reason for why they did not undergo surgery. Moreover, there was a minimal change in the reasons for not undergoing surgery from 1995 to 2004 (Fig. 3).
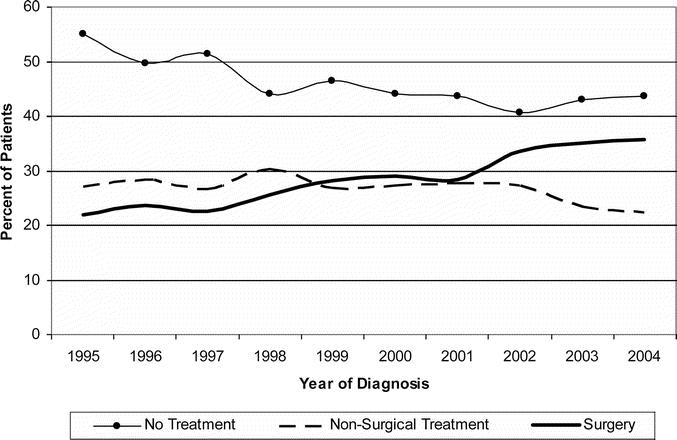
FIGURE 1. Treatment trends for Stage I pancreatic adenocarcinoma comparing utilization of pancreatectomy, nonsurgical treatment, and no treatment.
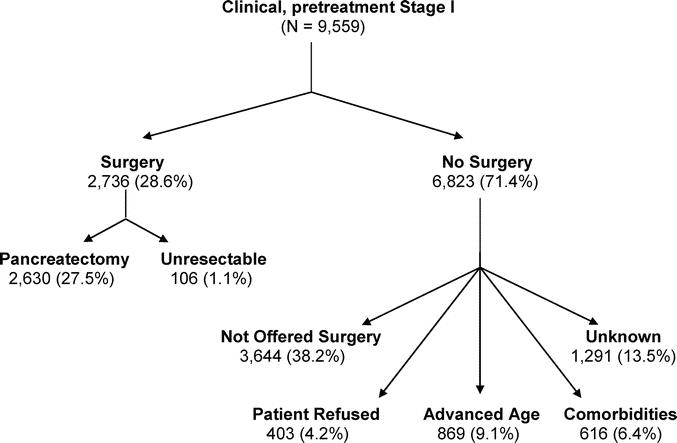
FIGURE 2. Management of 9559 patients with pretreatment, clinical Stage I pancreatic adenocarcinoma from 1995 to 2004.
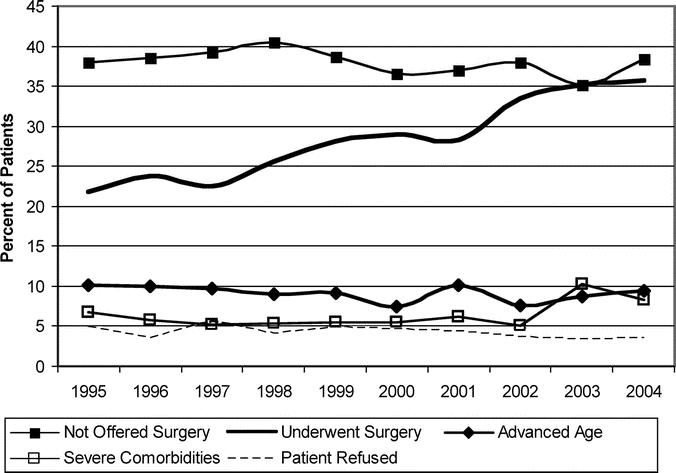
FIGURE 3. Reasons why patients did not undergo surgery for clinical Stage I pancreatic cancer over time compared with those undergoing surgery.
Factors Predicting Utilization of Surgery
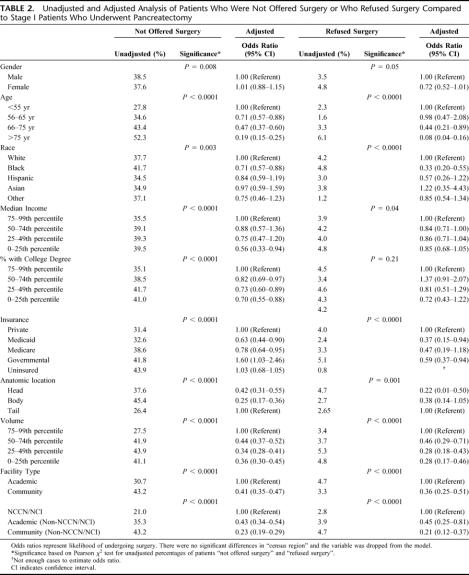
To identify factors predicting failure to undergo surgery, patients who underwent surgery were compared with those who were not offered surgery. The age of patients who were not offered surgery was higher than those who were selected to undergo surgery: 71.7 versus 65.1 years, P < 0.0001. The average age of patients not offered surgery and of those who underwent surgery remained similar over the course of the study (not offered surgery: 73.6 years in 1995 to 74.8 years in 2004, P = 0.34; underwent surgery: 63.3 years in 1995 to 64.7 years in 2004, P = 0.28). Charlson scores reflected more severe comorbidities in the group that underwent surgery compared with the group that was not offered surgery (Charlson >2: 32.3% versus 30.6%, P < 0.0001). Logistic regression was used to identify factors predicting failure to be offered surgery. Patients were significantly less likely to receive surgery if they were older, were black, had lower annual incomes, had less education, or were on Medicare or Medicaid (P < 0.0001) (Table 2). Patients were also nearly 3-fold less likely to undergo resection if the tumor was in the head/body of the pancreas compared with the tail (P < 0.0001). In addition, patients were less likely to receive surgery at low-volume centers (odds ratio [OR] 0.36, 95% confidence interval [CI] 0.30–0.45, P < 0.0001) and community hospitals (OR 0.41, 95% CI 0.35–0.47, P < 0.0001) than at high-volume and academic institutions. Moreover, patients treated at NCCN/NCI cancer centers were significantly more likely to undergo pancreatectomy than patients treated at other academic centers (OR 0.43, 95% CI 0.34–0.54, P < 0.0001) and community hospitals (OR 0.23, 95% CI 0.19–0.29, P < 0.0001).
TABLE 2. Unadjusted and Adjusted Analysis of Patients Who Were Not Offered Surgery or Who Refused Surgery Compared to Stage I Patients Who Underwent Pancreatectomy
Further analysis was done to compare patients who refused surgery to those who received surgery. Patients who refused surgery were older: 77.4 versus 65.1 year, P < 0.0001. Charlson scores reflected more severe comorbidities in the group who refused surgery compared with the group that underwent surgery (Charlson >2: 38.7% vs. 32.3%, P < 0.0001). On multivariate analysis, patients who refused surgery were significantly more likely to be older, black, on Medicaid, or to have lesions in the head/body of the pancreas (P < 0.0001) (Table 2). Patients treated at low-volume and community hospitals refused surgery more often than patients seen at high-volume and academic institutions (P < 0.0001). Patients treated at NCCN/NCI institutions were significantly less likely to refuse surgery (P < 0.0001). Annual income and level of education did not affect whether patients refused surgery.
Impact on Survival
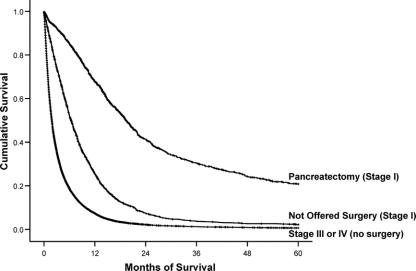
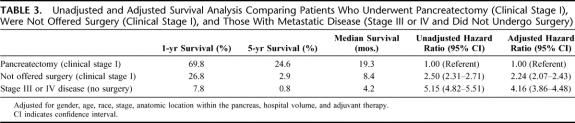
To evaluate the impact of surgery on survival, we compared 3 groups: clinical Stage I patients undergoing pancreatectomy, clinical Stage I patients who were not offered surgery, and patients with Stage III or IV disease who did not undergo any surgical treatment (n = 68,521). Five-year overall survival rates for clinical Stage I patients who underwent pancreatectomy were higher than for those patients with Stage III/IV disease, 19.3% versus 0.8%, P < 0.0001 (median survival 19.1 vs. 4.2 months) (Fig. 4). However, survival for clinical Stage I patients who were not offered surgery (median survival 8.4 months) was initially better than Stage III/IV patients but became more similar to patients with unresectable disease at ∼36 months from diagnosis. A Cox proportional hazards model was used to compare survival among these 3 groups while controlling for potential confounders (Table 3). Pancreatectomy was an independent predictor of a greater than 2-fold increase in the likelihood of survival when compared with patients who were not offered surgery and a greater than 4-fold increase compared with Stage III/IV patients (P < 0.0001). Clinical Stage I patients who were not offered surgery had better adjusted survival compared with Stage III/IV patients (P < 0.0001).
FIGURE 4. Five-year survival for pancreatic adenocarcinoma comparing patients who underwent pancreatectomy for clinical Stage I (n = 2736), were not offered surgery despite being clinical Stage I (n = 3644), and those with Stage III or IV who did not undergo surgery (n = 68,521).
TABLE 3. Unadjusted and Adjusted Survival Analysis Comparing Patients Who Underwent Pancreatectomy (Clinical Stage I), Were Not Offered Surgery (Clinical Stage I), and Those With Metastatic Disease (Stage III or IV and Did Not Undergo Surgery)
DISCUSSION
Pancreatectomy is the only curative therapy for early stage pancreatic cancer.4,26 However, nihilistic attitudes toward the disease may result in underuse of cancer-directed surgery. This study demonstrates that despite better survival after pancreatectomy, 51.7% of Stage I patients did not undergo surgery for potentially resectable pancreatic cancer even after accounting for patients who did not undergo surgery due to severe comorbidities, advanced age, or patient refusal. Patients were less likely to undergo surgery if they were older, were black, had lower annual incomes, had less education, or were on Medicare or Medicaid. Patients were more likely to receive surgery at academic institutions, high-volume hospitals, and NCCN/NCI centers. This is the first study to describe and characterize such striking underuse of pancreatectomy and identify factors predicting underutilization.
Our first objective was to assess and characterize utilization of pancreatic surgery. In 1996 Sener et al27 reported treatment and survival trends on 100,313 patients from the NCDB. They noted that 21.9% of Stage I patients from 1985 to 1995 underwent cancer-directed therapy. Similarly, our group's report on rising utilization of multimodality therapy found a similarly small percentage of patients receiving pancreatectomy for early stage disease.28 Two recent studies utilizing the NCI's Surveillance Epidemiology and End Results (SEER) database focused on the increasing utilization of surgery and reported that surgical management of resectable pancreatic cancer had increased to ∼35% in 2002.12,29 However, no study has attempted to define and characterize the underutilization of surgery. We found that after controlling for comorbidities, age, and patient refusal, 54.7% of patient did not have a documented or identifiable reason for why they did not undergo surgery for presumably resectable pancreatic malignancy. Of patients with potentially resectable pancreatic cancer 38.2% were simply not offered surgery.
Our second objective was to identify disparities in care associated with the failure to operate on clinical Stage I patients. Racial disparities in the care of pancreatic cancer have been well-described.30,31 In our analysis, patients were more likely to not be offered surgery if they were black. We also found that older age was associated with decreased utilization of surgery. Interestingly, we found that the mean age of patients undergoing surgery and not offered surgery remained unchanged over the past decade. While studies of pancreaticoduodenectomy in patients over 85 years of age have reported that surgery can be done safely in older patients, they do show an increase in morbidity and mortality with increasing age.26,32,33 Regardless of whether these racial and age discrepancies in the care of pancreatic cancer patients are due to access to care or hospital/physician factors, emphasis needs to be placed on offering resection to all appropriate patients.
In our analysis, patients were more likely to receive surgery for lesions in the tail compared with the head or body of the pancreas. This is an intriguing finding that may demonstrate historically nihilistic attitudes toward lesions in the head of the pancreas. Referring physicians, surgeons, and patients may be affected by historical data regarding the considerable perioperative morbidity and mortality of pancreaticoduodenectomy. Multiple studies in the last 20 years from academic and community institutions have demonstrated improved safety of surgery for lesions in the head of the pancreas.5,7,8,10,11,34,35 Our previous work on survival has demonstrated similar outcomes for patients undergoing surgery regardless of lesion location within the pancreas.36 In these Stage I patients with resectable disease, surgery is the only chance for cure and, if medically suitable, the location of the lesion within the pancreas should not preclude resection.
Numerous reports in recent years have demonstrated decreased perioperative complications and improved survival for patients undergoing pancreatectomy at academic, high-volume centers.11,26,37–43 We found that patients treated at high-volume hospitals, academic centers, and NCCN/NCI institutions were more likely to undergo surgery. This may be due to the increased willingness of surgeons at designated cancer centers to operate on pancreatic cancer. Similarly, it may be a function of the patient population served by NCCN/NCI institutions. Our results suggest that not only do specialty cancer centers have better outcomes, but they also offer stage-specific treatments more frequently.
A recent study focusing on pancreatic surgery in African American patients found that black patients were more likely to refuse surgery than white patients.30 Our results demonstrate similar findings in that black patients were 3 times more likely to refuse an operation. We also found that refusal of surgery for early stage pancreatic cancer was associated with advanced age, insurance status (Medicaid), and tumor location within the pancreas (head and body tumors). Moreover, patients treated at NCCN/NCI centers were significantly less likely to refuse surgery. This potentially reflects the type of patients who seek attention at NCCN/NCI centers but may also be associated with the increased willingness of surgeons at these hospitals to undertake high complexity operations.
Our third objective was to assess the impact of pancreatectomy on survival. Numerous studies have shown that surgery is the only potentially curative treatment of early stage pancreatic cancer.4 Similarly, we found that clinical Stage I patients selected to undergo pancreatectomy had significantly improved survival compared with Stage III/IV patients. More interesting was the pattern of survival in the clinical Stage I patients who were not offered surgery. For the first 2 years after diagnosis, these patients had survival that was clearly better than Stage III/IV patients who have unresectable disease; however, by the 3-year mark, that difference had nearly disappeared, and patients who were clinical Stage I at diagnosis and did not undergo surgery now had survival rates that were no different from patients presenting with advanced disease. Thus, although it may be tempting to theorize that the patients not offered surgery simply had their stage recorded improperly in the cancer registry or by the physician, these survival data demonstrate a clear difference between these potentially resectable patients who were not offered surgery and those that underwent pancreatectomy, implying that these patients initially had disease that was potentially resectable.
Our study has several potential limitations. First, we limited our analysis to patients with clinical, pretreatment Stage I (T1N0M0 and T2N0M0) disease to establish a population of pancreatic cancer patients who are potential candidates for resection.16 Certainly patients with T3 and/or node-positive disease are also candidates for surgical extirpation; however, we limited the ambiguity in the appropriateness of resection for these patients. Moreover, the AJCC pancreatic cancer staging system has changed during the last 10 years, particularly with respect to the definitions of Stage II and III disease. As such, we chose not to confound the analyses with the inclusion of Stage II patients. The finding of pancreatectomy underuse is likely to be strengthened by broadening our study to include Stage II patients because if patients with Stage I disease are not receiving surgery, then it is likely that a higher percentage of appropriate, resectable patients with Stage II disease are not undergoing surgery. A second potential limitation of this study is the under-reporting inherent to cancer registry data.44–47 It is possible that the number of patients receiving surgery or the reason for why they did not undergo pancreatectomy is under-reported. However, studies examining the accuracy of registry data have found that procedures are coded with high accuracy when compared with patient charts, with considerably more accuracy than diagnosis and comorbidity codes.48–50 Furthermore, the number of patients missing data on whether they had surgery or were excluded (age, comorbidities, patient refusal) is less than 5% in 2004. Thus, nearly all patients who do not undergo pancreatectomy have a documented reason explaining why they did not undergo cancer-directed surgery. Finally, the patients not offered surgery may have unreported comorbidities or performance status that cannot be evaluated in our dataset. However, our examination of available comorbidity data demonstrated that patients not offered surgery actually had lower Charlson score than those patients who underwent surgery.
CONCLUSIONS
The recognition of treatment underuse in other disease sites has led to numerous quality improvement measures through joint efforts of the American College of Surgeons, NCDB, CoC, and the National Quality Forum (NQF). For example, a standard of care is that Stage III colon cancer patients should receive adjuvant chemotherapy. Cases of Stage III colon cancer submitted to the NCDB are required to have a detailed explanation regarding why the patient did not receive chemotherapy, and “not offered” is not an acceptable justification. If a reason is not documented, the chart is sent back to the hospital and physician for an explanation. A deeper understanding of the underuse of pancreatectomy may be discovered by implementing a similar mechanism for pancreatic cancer where the hospital's cancer registrar or treating physician can provide a brief narrative explaining why the patient failed to undergo surgery, thus providing a better understanding of the not offered surgery category. Hospitals with outlying utilization rates can be identified and notified in the hope of improving cancer care.
Despite a demonstrated survival benefit for Stage I pancreatic adenocarcinoma patients who undergo curative resection, more than half of patients with resectable cancer fail to receive surgery. Patients treated at academic hospitals, NCCN/NCI institutions, and high-volume centers were more likely to undergo surgery. The only opportunity for cure in early stage patients is surgical resection, but nihilistic attitudes toward pancreatic cancer likely contribute to this striking underuse of curative resection for pancreatic adenocarcinoma. There is an opportunity to improve the care of pancreatic cancer patients in the United States by offering surgery to all appropriate patients with resectable disease.
ACKNOWLEDGMENTS
The authors thank the staff of the American College of Surgeons, National Cancer Data Base, for their assistance, particularly James Banasiak, MS, and E. Greer Gay, RN, MPH, PhD.
Footnotes
KYB is supported by the American College of Surgeons, Clinical Scholars in Residence program and a clinical research grant from the Northwestern University Department of Surgery.
Reprints: Mark S. Talamonti, MD, Department of Surgery, Feinberg School of Medicine, Northwestern University, 675 N. St. Clair Street, Galter 10-105, Chicago, IL 60611. E-mail: mtalamonti@nmff.org.
REFERENCES
- 1.American Cancer Society: Cancer Facts and Figures. Available at http://www.cancer.org/docroot/stt/stt_0.asp. Accessed December 15, 2006.
- 2.Brennan MF. Adjuvant therapy following resection for pancreatic adenocarcinoma. Surg Oncol Clin N Am. 2004;13:555–566, vii. [DOI] [PubMed] [Google Scholar]
- 3.Stojadinovic A, Hoos A, Brennan MF, Conlon KC. Randomized clinical trials in pancreatic cancer. Surg Oncol Clin N Am. 2002;11:207–229, x. [DOI] [PubMed] [Google Scholar]
- 4.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995;130:295–299, discussion 299–300. [DOI] [PubMed]
- 6.Richter A, Niedergethmann M, Sturm JW, et al. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt CM, Powell ES, Yiannoutsos CT, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718–725, discussion 725–727. [DOI] [PubMed]
- 8.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. [DOI] [PubMed] [Google Scholar]
- 9.Whipple AO. An evaluation of radical surgery for carcinoma of the pancreas and ampullary region. Ann Intern Med. 1949;31:624–627. [DOI] [PubMed] [Google Scholar]
- 10.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riall TS, Nealon WH, Goodwin JS, et al. Pancreatic cancer in the general population: improvements in survival over the last decade. J Gastrointest Surg. 2006;10:1212–1223, discussion 1223–1224. [DOI] [PubMed]
- 13.Winchester DP, Stewart AK, Bura C, Jones RS. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004;85:1–3. [DOI] [PubMed] [Google Scholar]
- 14.International Classification of Disease for Oncology. 2nd ed. Geneva: World Health Organization, 1990. [Google Scholar]
- 15.International Classification of Disease for Oncology. 3rd ed. Geneva: World Health Organization, 2000. [Google Scholar]
- 16.AJCC Cancer Staging Manual. 6th ed. Chicago, IL: Springer, 2002. [Google Scholar]
- 17.Standards of the Commission on Cancer Volume II: Registry Operations and Data Standards. Vol. II: Commission on Cancer, 1998.
- 18.Facility Oncology Registry Data Standards. Chicago: Commission on Cancer, 2004. [Google Scholar]
- 19.Department of Health and Human Services. The International Classification of Diseases. 9th revised. Clinical modification: ICD-9-CM. Washington, DC: Government Printing Office, 1998. [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 22.Commission on Cancer: Approvals Categories. Available at http://www.facs.org/cancer/coc/categories.html. Accessed December 17, 2006.
- 23.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Cox D. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 25.United States Census Bureau. Census 2000. Available at http://www.census.gov/main/www/cen2000.html. Accessed January 21, 2007.
- 26.Fong Y, Gonen M, Rubin D, et al. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242:540–544, discussion 544–547. [DOI] [PMC free article] [PubMed]
- 27.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. [DOI] [PubMed] [Google Scholar]
- 28.Bilimoria K, Bentrem D, Ko C, et al. Multimodality therapy for pancreatic surgery in the United States: utilization, outcomes, and the effect of hospital volume. 2006. Submitted for publication. [DOI] [PubMed]
- 29.Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14:1320–1326. [DOI] [PubMed] [Google Scholar]
- 30.Eloubeidi MA, Desmond RA, Wilcox CM, et al. Prognostic factors for survival in pancreatic cancer: a population-based study. Am J Surg. 2006;192:322–329. [DOI] [PubMed] [Google Scholar]
- 31.Lucas FL, Stukel TA, Morris AM, et al. Race and surgical mortality in the United States. Ann Surg. 2006;243:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohn TA, Yeo CJ, Cameron JL, et al. Should pancreaticoduodenectomy be performed in octogenarians? J Gastrointest Surg. 1998;2:207–216. [DOI] [PubMed] [Google Scholar]
- 33.Makary MA, Winter JM, Cameron JL, et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg. 2006;10:347–356. [DOI] [PubMed] [Google Scholar]
- 34.Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–435, discussion 435–438. [DOI] [PMC free article] [PubMed]
- 35.Afsari A, Zhandoug Z, Young S, et al. Outcome analysis of pancreaticoduodenectomy at a community hospital. Am Surg. 2002;68:281–284, discussion 284–285. [PubMed]
- 36.Bilimoria K, Bentrem D, Ko C, et al. Validation of the AJCC Staging System for Pancreatic Cancer. Cancer. 2007. In press.
- 37.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
- 38.Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–183. [PubMed] [Google Scholar]
- 39.Birkmeyer NJ, Goodney PP, Stukel TA, et al. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005;103:435–441. [DOI] [PubMed] [Google Scholar]
- 40.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725, discussion 726. [DOI] [PubMed]
- 41.Gordon TA, Bowman HM, Tielsch JM, et al. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998;228:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 44.Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21:1293–1300. [DOI] [PubMed] [Google Scholar]
- 45.Bickell NA, Chassin MR. Determining the quality of breast cancer care: do tumor registries measure up? Ann Intern Med. 2000;132:705–710. [DOI] [PubMed] [Google Scholar]
- 46.Cress RD, Zaslavsky AM, West DW, et al. Completeness of information on adjuvant therapies for colorectal cancer in population-based cancer registries. Med Care. 2003;41:1006–1112. [DOI] [PubMed] [Google Scholar]
- 47.Malin JL, Kahn KL, Adams J, et al. Validity of cancer registry data for measuring the quality of breast cancer care. J Natl Cancer Inst. 2002;94:835–844. [DOI] [PubMed] [Google Scholar]
- 48.Hawker GA, Coyte PC, Wright JG, et al. Accuracy of administrative data for assessing outcomes after knee replacement surgery. J Clin Epidemiol. 1997;50:265–273. [DOI] [PubMed] [Google Scholar]
- 49.Henderson T, Shepheard J, Sundararajan V. Quality of diagnosis and procedure coding in ICD-10 administrative data. Med Care. 2006;44:1011–1019. [DOI] [PubMed] [Google Scholar]
- 50.Kahn LH, Blustein J, Arons RR, et al. The validity of hospital administrative data in monitoring variations in breast cancer surgery. Am J Public Health. 1996;86:243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]