Abstract
Background:
The correlation of the amylase value in drains (AVD) with the development of pancreatic fistula (PF) is still unclear.
Aim:
The purpose of this study was to identify within the first postoperative day (POD1) the predictive role of different risks factors, including AVD, in the development of PF.
Patients and Methods:
We prospectively investigated 137 patients who underwent major pancreatic resections. PF was defined and graded in accordance with the International Study Group on PF.
Results:
We considered 101 pancreaticoduodenectomies and 36 distal resections. The overall incidence of PF (A, B, and C grades) was 19.7% and it was 14.8% after pancreaticoduodenectomy and 33.3% after distal resection. All PF occurred in “soft” remnant pancreas. The PF developed in patients with a POD1 median AVD of 10,000 U/L, whereas patients without PF had a median AVD of 1222 U/L (P < 0.001). We established a cut-off of 5000 U/L POD1 AVD for univariate and multivariate analysis. The area under the receiver operating characteristic (ROC) curve was 0.922 (P < 0.001). The predicting risk factors selected in the univariate setting were “soft” pancreas (P = 0.005; odds ratio [OR]: 1.54; 95% CI: 1.32–1.79) and AVD (P < 0.001; OR: 5.66; 95% CI: 3.6–8.7; positive predictive value 59%; negative predictive value 98%), whereas in multivariate analysis the predicting risk factor was the POD1 AVD (P < 0.001; OR: 68.4; 95% CI: 14.8–315). Only 2 PFs were detected with AVD <5000 U/L and both were in pancreatogastric anastomosis (P = 0.053).
Conclusions:
AVD in POD1 ≥5000 U/L is the only significant predictive factor of PF development.
The range of postoperative fistula after pancreatic resections is reported from 2% to 30% of cases and in the literature there are few studies that investigate the predictive role of different risks factors in the development of pancreatic fistula, including the amylase value in drains. The authors show that the drain's amylase value on postoperative day 1 is the only significant predictive factor of pancreatic fistula development.
Pancreaticoduodenectomy (PD) and distal pancreatic resection (DP) are now standardized interventions for benign and malignant pancreatic lesions. Because of recent improvements in surgical techniques and perioperative management, the incidence of mortality and morbidity have decreased, even if the latter is still around 20% to 50% in high volume centers.1–22 The most frequent postoperative complication is pancreatic fistula (PF), which is often associated with abdominal collections, abscesses, sepsis, and hemorrhage, with the necessity of reintervention, extended hospitalization, and postoperative mortality in 40% of cases with complications.1–8
Despite its importance, PF has still not been uniformly defined.12,13 Recently, the International Study Group on Pancreatic Fistula (ISGPF)13 established that the most appropriate PF definition and grading should be based on the clinical impact of PF-related complications. In addition, there are still no reliable correlations between the output of drains and their concentration of amylase contained within, which is useful for the diagnosis of chemical PF; moreover only a few studies have attempted to correlate the concentration of amylase in drain fluid with the risk of developing complications.23–26
In the present study we examined, within the first postoperative day (POD1), the predictive role of different risks factors, including the value of amylase in drains in the development of PF.
PATIENTS AND METHODS
At the Department of Surgical and Gastroenterological Sciences at the University of Verona, 137 consecutive patients who underwent pancreatic resection (101 PD and 36 DP) between April 2005 and February 2006 were admitted to the study. In all patients daily drainage output and drain fluid quality, and the levels of serum and drain fluid amylase were determined starting from POD1 to POD5. If the drains had not been removed, then measurements were also made on POD7 and POD9. For measurement of amylase, 10 mL of liquid was used. The upper limit of normal serum amylase in our laboratory is 100 U/L.
Patient demographics, histology, type of intervention, surgical operator, type of pancreatic anastomosis (ie, pancreatojejunal anastomosis [PJ], pancreatogastric anastomosis [PG]), risk factors for PF (diameter of main pancreatic duct ≤3 mm or >3 mm, soft or hard pancreatic texture, and duration of operation),14–17 and days of postoperative length of stay were recorded. In the postoperative period the development of complications (PF, abdominal hemorrhage, delayed gastric emptying, acute pancreatitis of the pancreatic stump, enteric drainage from bedsore fistula), percutaneous drainage of abdominal collections, reintervention, readmission to the hospital, and mortality were collected. The definitions of the complications are provided in Table 1. The definition of PF used was that proposed by the ISGPF. A grading system was also used (A, B, and C)13 and, when indicated, a fistulography was performed.
TABLE 1. Definitions of Abdominal Complications After Pancreatic Resection
All patients were administered preoperative antibiotic prophylaxis with amoxicillin plus clavulanic acid (2.4 g); antithrombic prophylaxis was given for 30 days (low molecular weight heparin, 0.4 mL subcutaneously once a day). In patients undergoing PD octreotide prophylaxis was also given (0.1 mg subcutaneously for 3 times/24 h for 5 days after intervention).27
Surgical Technique
For PD a pylorus-preserving resection (PPPD) was performed in 91 cases (90%) whereas a Whipple resection was done in 10 cases (10%). The surgical operator was free to choose the type of anastomosis. During PG the anastomosis was performed on the posterior wall of the stomach in a single layer with nonresorbable button sutures with an anterior gastrostomy.28 PJ was performed in a single layer with nonresorbable button sutures. In both types of reconstructions 2 “easy-flow” drains (12 mm; Chimed R Livorno, Italy) were positioned, one behind the pancreatic anastomosis and the other at the level of the biliary anastomosis. DP was performed by using a TIA mechanical stapler to suture the distal shear. An “easy flow” drain (12 mm; Chimed R Livorno, Italy) was positioned near the pancreatic stump.
Statistical Analysis
Nonparametric tests (Kruskal-Wallis, Mann-Whitney U) were used to evaluate ordinal data, while a χ2 test was used for nominal data. Yate's continuous correction in a 2-way contingency table or Fisher exact test was used in the case of a small expected frequency. Study of potential prognostic factors for PF was carried out by employing logistic analysis; only variables available within 24 hours after intervention were considered. The diagnosis or lack thereof of a PF within the postsurgical observation was considered as a dependent dichotomy variable. The process that led to the identification of the most parsimonious model was performed using a backward technique by elimination of a variable that did not reach a P < 0.05 according to Wald's test. The odds ratios are presented with the respective confidence intervals to 95%. For logistic regression analysis, the amylase drain value in POD1 was considered as a continuous value; then for easier reading of the data an arbitrary cut-off in POD1 was inserted (70th percentiles corresponding to 5000 U/L). SPSS (rel. 13) programs were used for statistical analysis (SPSS Inc., Chicago, IL).
RESULTS
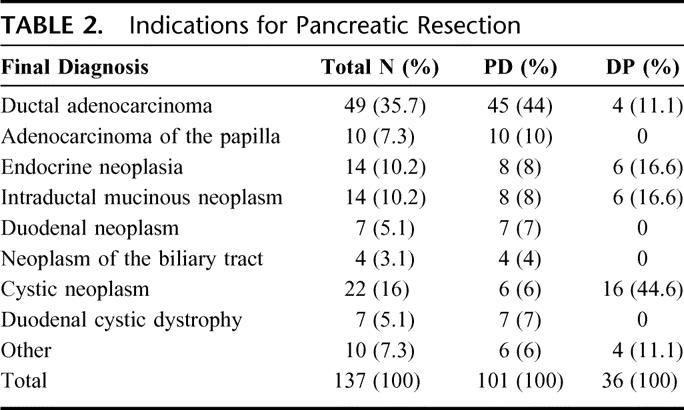
Of the 137 consecutive patients enrolled in the study, 101 underwent PD (73.6%) (PJ 78, PG 23) and 36 DP. There were 71 males (51.8%; mean age 58.5 ± 12.7 years) and 66 females (mean age 56 ± 12.5 years). For DP the mean time of intervention was 194 ± 19 minutes, while for PD the mean interventional time was 346 ± 36.8 minutes. The indications for surgical resection are described in Table 2. In PD the pancreatic texture of the stump was “soft” in 71 cases (52 PJ and 19 PG), whereas the diameter of the main pancreatic duct was divided into 2 groups according to the risk of developing PF (≤3 mm; >3 mm).15 There were 39 cases (38.6%) with a diameter ≤3 mm and 62 (61.4%) with a diameter >3 mm. Abdominal complications were recorded in 47 cases (34%), 31.6% in PD, and 41% in DP.
TABLE 2. Indications for Pancreatic Resection
The most frequent abdominal complication was clinically suspected PF, observed in 27 patients (19.7%): 15 patients (14.8%) who underwent PD (10 PJ, 5 PG) and 12 (33.3%) who underwent DP. Considering the ISGPF grading, PF were classified as follows: 12 grade A (5 PD, 7 DP); 11 grade B (6 PD, 5 DP); and 4 grade C (4 PD, 0 DP). The incidence of PF with clinically significant impact (grade B + C) was therefore present in 15 patients (10.9%): 10 (9.9%) after PD and 5 (13.8%) after DP.
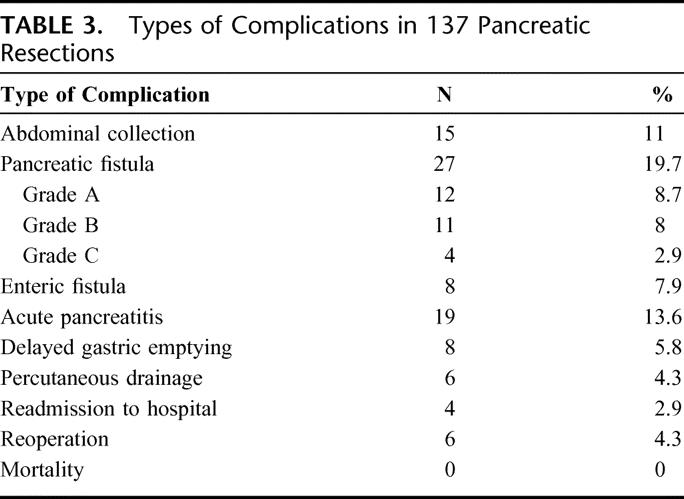
Abdominal collections were present in 15 patients (11%) requiring interventional radiologic procedures in 6 cases. An enteric drainage bedsore fistula was present in 8 cases (7.9% of patients undergoing PD), and in all cases the pancreatic texture was “soft.” A reoperation was performed in 6 cases (4%): 5 for hemorrhage (4 PD and 1 DP) and 1 for sepsis following a complete dehiscence of the pancreatic anastomosis. Acute pancreatitis of the pancreatic stump was present in 19 cases undergoing PD (13.6%; 10 PJ and 9 PG), which in 5 cases was associated with PF (3 grade B and 2 grade C). Overall complications are reported in Table 3. There was no mortality.
TABLE 3. Types of Complications in 137 Pancreatic Resections
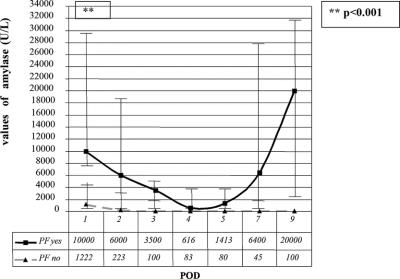
The median of the level of amylase in pancreatic drains tended to decrease from POD1 to POD5 (Fig. 1) in patients with normal postoperative recovery as well as in those with a complicated postoperative course. Although the amylase levels were higher in the latter group of patients, this difference was not statistically significant.
FIGURE 1. Median values of amylase in drains during postoperative recovery. Vertical bars show 25th and 75th percentiles.
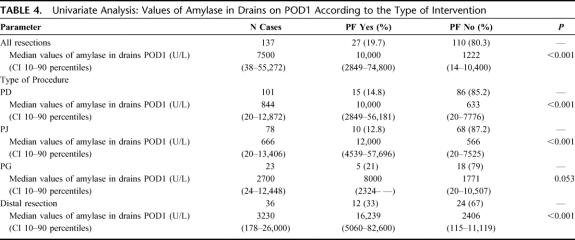
In univariate analysis, the median value of amylase in drains on POD1 was 1222 U/L (10th and 90th percentiles: 14–10,400 U/L) in patients who did not develop PF and 10,000 U/L (10–90 percentiles: 2849–74,800 U/L) in patients who developed PF (P < 0.001) (Table 4). This difference was independent of the type of intervention, the type of pancreas in PD (soft or hard, P < 0.001), and diameter of main pancreatic duct (≤3 mm and >3 mm, P < 0.001). The median difference of the level of amylase on POD1 was not statistically significant in the PG group (P = 0.053). Moreover, in univariate analysis, the presence of PF was significantly dependent on the “soft” texture of pancreas (P = 0.005; OR: 1.54; 95% CI: 1.32–1.79) and value of amylase in drains on POD1 (P < 0.001). For all analyses only those parameters available within the first postoperative day were considered.
TABLE 4. Univariate Analysis: Values of Amylase in Drains on POD1 According to the Type of Intervention
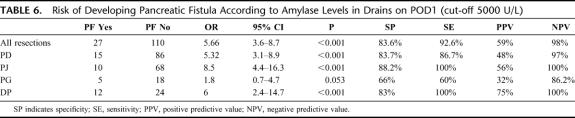
The univariate analysis was repeated by inserting a value of amylase at 5000 U/L in drains on POD1 as an arbitrary cut-off value (70th percentile). Even in this case the concentration of amylase was the variable selected (P < 0.001; OR: 5.66; 95% CI: 3.6–8.7).
For logistic regression analysis the dependent dichotomic variable considered was the development of PF, whereas the independent variables with a potential prognostic significance were gender, age, concentration of amylase in drains in POD1, drainage output, serum amylase, type of intervention, type of pancreatic texture, diameter of main pancreatic duct, surgical operator, and acute postoperative pancreatitis. The only significant variable was the quantity of amylase in the left drain (that placed near the pancreatic stump) on POD1 (P < 0.001; OR: 68.4; 95% CI: 14.8–315) without relationship with high or low amylase serum level.
Considering the sensitivity and specificity of amylase in drains on POD1 an area under the ROC curve of 0.922 was obtained (P < 0.001; 95% CI: 0.878–0.966) (Fig. 2).
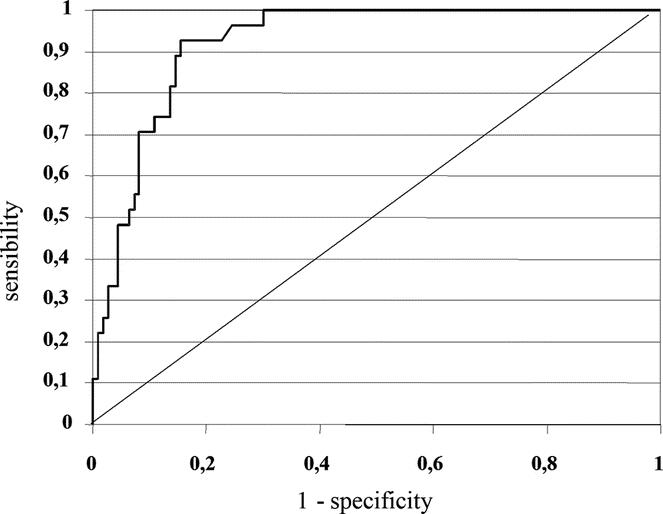
FIGURE 2. ROC curve based on amylase levels in drains on POD1 (area under the curve 0.922; P < 0.001; 95% CI: 0.878–0.966).
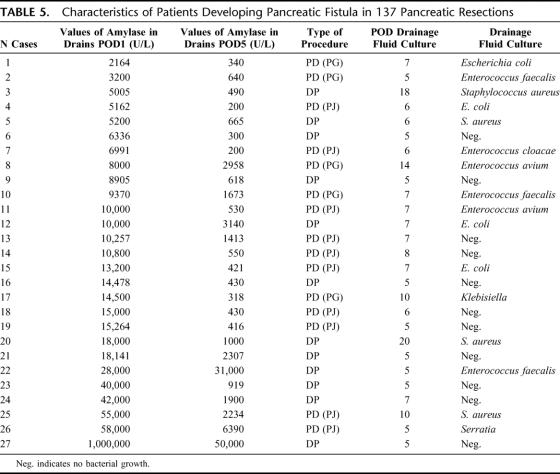
After analysis of each of the cases involving complications individually (Table 5), it was noted that there were only 2 of 95 (2%) cases presenting an amylase level ≤5000 U/L in drains on POD1 (both with PG), with values of 2164 and 3200 (P < 0.001; OR: 5.66; 95% CI: 3.6–8.7) with a specificity of 83.6% and a sensitivity of 92.6% (positive predictive value [PPV]: 59%; negative predictive value [NPV]): 98%. No patients subjected to PD with PJ reconstruction or to DP with amylase levels ≤5000 U/L in drains on POD1 developed PF (P < 0.001; OR: 7.7; 95% CI: 4.52-12.99) with a sensitivity of 100% and a specificity of 87% (PPV: 72%; NPV: 100%). The risk of developing PF on the basis of amylase in drains on POD1 is shown in Table 6. At our institute there was no significant difference in the incidence of PF between surgical operators of supervisors and likewise, there was no difference based on the different reconstruction technique used, PJ or PG.21
TABLE 5. Characteristics of Patients Developing Pancreatic Fistula in 137 Pancreatic Resections
TABLE 6. Risk of Developing Pancreatic Fistula According to Amylase Levels in Drains on POD1 (cut-off 5000 U/L)
In patients who developed PF the concentration of amylase in drains increased on POD5 with a median of 1413 U/L (10-90 percentiles: 222 U/L–38,600 U/L) with a minimum value of 200 U/L (Table 5). Alterations in the quality of output on POD1 were not a prognostic indicator for PF and were indicative of PF only on POD5. In fact, in patients developing PF, alterations in the quality of drainage were apparent only from POD5. Microbiological analysis of drainage fluid was positive in 16 patients (60%) who developed PF. In 81% of these cases a positive result was observed after POD5 (Table 5).
In 3 cases subjected to percutaneous drainage of abdominal collections under ultrasound guidance, drains had been previously removed without awareness of the complication. In fact, the amylase levels in drains on POD1 in these patients were all >5000 U/L (5005 U/L, 10,000 U/L, and 10,000 U/L), whereas they were ≥200 U/L on POD5 (520 U/L, 460 U/L, and 550 U/L, respectively). Lastly, there was no significant difference in the quantity of liquid on the different postoperative days (Fig. 3).
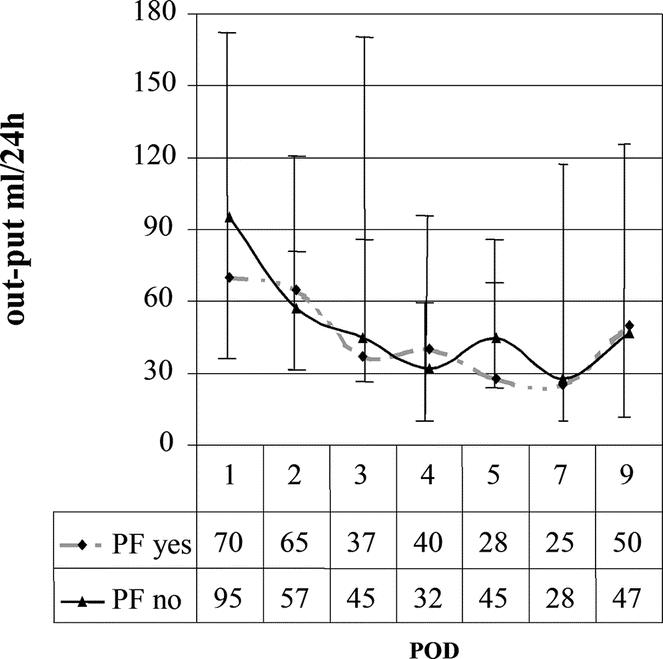
FIGURE 3. Median postoperative liquid output in drains. Vertical bars show 25th and 75th percentiles.
DISCUSSION
Pancreatic resection is still associated with an elevated incidence of complications. In clinical practice postoperative management is primarily governed on whether complications are present and, in particular, the development of PF determines the underlying management strategy. For example, the early removal of drains and the timing of recovery of eating influence both the period of hospitalization and further appearance of morbidity.
Recently, Kawai et al29 demonstrated that early removal of drains on POD4 (whenever possible) is an independent factor for reducing the incidence of abdominal infections; patients with drains still in place after POD8 had a significantly higher incidence of abdominal complications in general (P = 0.00003) and PF (P = 0.003), suggesting that infection of drains occurs around POD7 (positive cultures in 94% in patients in which the drain was not removed; OR: 6.7; 95% CI: 1.9–22.7). These data were also confirmed in the present study, since 81% of infected drains were revealed after POD5 in cases that developed PF.
In the literature there are few studies that have attempted to evaluate the predictive value of amylase in drains with the risk of developing PF, despite the fact that amylase values significantly influence postoperative management.23,26 The retrospective study by Shinchi et al26 on 207 PD defined pancreatic leak as an output >30 mL/24 h with amylase values on POD5 that were more than 5 times the serum value. Using this definition, a positive biochemical predictive value (59%) was not sufficient to justify the incidence of PF reported (29 cases, 14%). Likewise, the study by Shyr et al23 on 37 PD and with the development of a single case of PF with amylase in drains on POD7 of 74 U/L provides little insight into the physiological mechanisms of complications. Only a single study25 with a small patient cohort (n = 26) and a high incidence of PF (46%) reported a mean level of amylase in drains on POD1, 10,878 ± 14,800 U/L in cases with complications compared with 1482 ± 1615 U/L (maximal level 4838 U/L) in cases without complications (P < 0.01).
The present study demonstrates that, among all of the different risk factors within the POD1 in the development of PF, only the level of amylase in drains on POD1 has a predictive value for the appearance of PF, and in particular when the level of amylase is greater than 5000 U/L (P < 0.001; OR: 5.66; 95% CI 3.6–8.7) with a sensitivity of 92.6% and a specificity of 83.6% (PPV: 59%; NPV: 98%). Considering patients undergoing PD, no PF occurred in those with PJ and amylase in drains on POD1 <5000 U/L (P < 0.001; OR: 8.5; 95% CI: 4.4–16.3) with a sensitivity of 100% and a specificity of 88.2% (PPV: 56%; NPV: 100%); however, 2 patients who were reconstructed with PG developed PF. Therefore, this stresses the utility of amylase levels on POD1, especially in cases with PJ anastomosis, whereas the amylase levels did not reach statistical significance for PG (P = 0.053), perhaps because of the small number of patients (n = 23). In the group of patients who underwent DP, the incidence of PF was 33% among those presenting amylase levels ≥5000 U/L on POD1 (P < 0.001; OR: 6; 95% CI: 2.45–14.7) with a sensitivity of 100% and a specificity of 83% (PPV: 75%; NPV: 100%). Independently on the normal serum amylase level range, in our experience, the value of amylase drain level on POD1 >5000 U/L has to be considered in absolute terms. Moreover, the value we found is close to that (4838 U/L) reported by Hashimoto and Ohyanagi in a smaller series.25
In agreement with the results of Wada and Traverso, this would suggest that amylase levels on POD1 are increased in patients who will develop complications due to an early and imperceptible leak of the anastomosis.30 During the successive postoperative recovery period, the pancreas seems to decrease in terms of functionality, thereafter increasing on POD5 (Fig. 1). As hypothesized by Shinchi et al, the increase in amylase in drains during this period may be due to the stimulus provided by realimentation.26 We therefore compared the amylase levels in drains before and after realimentation. In the 27 cases of PF observed, in 9 patients (33%) there was an increase in amylase levels in drains after realimentation; thus only in a subgroup of cases amylase levels might be correlated with realimentation.
Lastly, if we consider that in the group of patients that presented amylase levels ≥5000 U/L (25 of 42 cases) only those with amylase levels ≥200 U/L on POD5 developed complications (Table 5). In those patients reconstructed with PJ after PD and treated with DP (114 cases in total), PF developed when amylase levels on POD1 were ≥5000 U/L (P < 0.001; OR: 7.7; 95% CI: 4.52-13) with a sensitivity of 100% and a specificity of 87% (PPV: 72%; NPV: 100%).
Considering the results of this study, the physiopathologic mechanism of the development of PF appears in the first instance because of a small intraoperative leak that would explain the presence of amylase in drains on POD1 ≥5000 U/L, with a small ischemic necrosis of the anastomosis on POD5 (amylase ≥200 U/L). Moreover, from this study it can be inferred that the quantity of liquid drained is not an essential parameter in defining PF (Fig. 3).
Other studies23–26 do not confirm the results reported herein, which may be due to a number of factors including surgical technique and management of the pancreatic stump (duct-to-mucosa pancreaticojejunostomy, anastomosis performed in double or triple layers, and use of a stent in the Wirsung duct) and by the different types of abdominal drains used (closed suction drains or soft easy flow). Surprisingly, no authors have cited the problem of enteric fistula from bedsore drainage,2,21,22 which was observed in our experience between POD5 and POD7 in patients with amylase levels on POD1 ≤5000 U/L, and in 8 cases (7.9%) of those undergoing PD. It should be stressed that only fistulography reveals drainage inside the intestinal loop of the anastomosed Roux with the pancreas, and for this reason fistulography is always performed at our institution when there is the clinical suspicion of fistula; in cases with fistula from bedsores, once the drain is mobilized under endoscopic guidance, the complication resolves within 48 hours in all cases. Lastly, this is the first prospective study to value amylase content in drains using the ISGPF definition and grading system. It should thus be evident how all of these different factors can influence the optimal time for the removal of drains.
The mortality rate we report in this cohort of 137 major resections is nil. Currently, the overall mortality rate for PD in our unit is less than 1% despite the still high morbidity rate.2,21–22,28 This seems to be a common experience, even in other “high volume” centers around the world. In general, we all are approaching a 0 rate of mortality because of our capability to manage the still high complications rate.1,3–6,10,14–19,27,29,30
As a consequence, we must be careful in changing our actual drain management, basing each single variation in the protocols only on the evidence. The data reported in the present article have been the background of a prospective randomized clinical trial on drain management that is running in our unit. Such a trial, on the base of amylase drain POD1 value, compares our standard drain management versus an earlier drain withdrawal.
In conclusion, the present study indicates how it is possible to identify the risk of PF formation on POD1 by analysis of amylase levels in drains. This possibility opens new frontiers in postoperative management. In fact, it is possible to identify a subgroup of patients at high risk for developing complications (POD1 amylase levels ≥5000 U/L) in which the patient may benefit from lengthening the time of intensive postoperative therapy including prolonged fasting and “in situ” drainage (Table 6). On the other hand, those patients not at risk (NPV 98% on all the pancreatic resection procedures) may be candidates for earlier removal of drains, thus avoiding infections, bedsore lesions, and favoring faster realimentation and discharge from the hospital.29
Footnotes
Supported by Fondazione Cariverona, Fondazione Zanotto Verona, MIUR Cofin 2005060715_004.
Reprints: Claudio Bassi, MD, Department of Surgery and Gastroenterology, Hospital “GB Rossi” University of Verona, Piazzale LA Scuro, 10, 37134 Verona, Italy. E-mail: claudio.bassi@univr.it.
REFERENCES
- 1.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassi C, Falconi M, Salvia R, et al. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg. 2001;18:453–457. [DOI] [PubMed] [Google Scholar]
- 3.Balcom JH 4th, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. [DOI] [PubMed] [Google Scholar]
- 4.Bottger TC, Junginger T. Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World J Surg. 1999;23:164–171. [DOI] [PubMed] [Google Scholar]
- 5.Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balzano G, Zerbi A, Cristallo M, et al. The unsolved problem of fistula after left pancreatectomy: the benefit of cautious drain management. J Gastrointest Surg. 2005;9:837–842. [DOI] [PubMed] [Google Scholar]
- 9.Sheehan MK, Beck K, Creech S, et al. Distal pancreatectomy: does the method of closure influence fistula formation? Am Surg. 2002;68:264–267. [PubMed] [Google Scholar]
- 10.Buchler MW, Friess H, Wagner M, et al. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87:883–889. [DOI] [PubMed] [Google Scholar]
- 11.Knaebel HP, Diener MK, Wente MN, et al. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92:539–546. [DOI] [PubMed] [Google Scholar]
- 12.Bassi C, Butturini G, Molinari E, et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Dig Surg. 2004;21:54–59. [DOI] [PubMed] [Google Scholar]
- 13.Bassi C, Dervenis C, Butturini G, et al. International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 14.Sato N, Yamaguchi K, Chijiiwa K, et al. Risk analysis of pancreatic fistula after pancreatic head resection. Arch Surg. 1998;133:1094–1098. [DOI] [PubMed] [Google Scholar]
- 15.Yang YM, Tian XD, Zhuang Y, et al. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popiela T, Kedra B, Sierzega M, et al. Risk factors of pancreatic fistula following pancreaticoduodenectomy for periampullary cancer. Hepatogastroenterology. 2004;51:1484–1488. [PubMed] [Google Scholar]
- 17.Adam U, Makowiec F, Riediger H, et al. Risk factors for complications after pancreatic head resection. Am J Surg. 2004;187:201–208. [DOI] [PubMed] [Google Scholar]
- 18.De Castro SM, Busch OR, van Gulik TM, et al. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg. 2005;92:1117–1123. [DOI] [PubMed] [Google Scholar]
- 19.Marcus SG, Cohen H, Ranson JH. Optimal management of the pancreatic remnant after pancreaticoduodenectomy. Ann Surg. 1995;221:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berberat PO, Friess H, Kleeff J, et al. Prevention and treatment of complications in pancreatic cancer surgery. Dig Surg. 1999;16:327–336. [DOI] [PubMed] [Google Scholar]
- 21.Bassi C, Falconi M, Molinari E, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005;242:767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassi C, Falconi M, Molinari E, et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134:766–771. [DOI] [PubMed] [Google Scholar]
- 23.Shyr YM, Su CH, Wu CW, et al. Does drainage fluid amylase reflect pancreatic leakage after pancreaticoduodenectomy? World J Surg. 2003;27:606–610. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Nakano H, Midorikawa T, et al. Prediction of pancreatic fistula by amylase levels of drainage fluid on the first day after pancreatectomy. Hepatogastroenterology. 2003;50:1155–1158. [PubMed] [Google Scholar]
- 25.Hashimoto N, Ohyanagi H. Pancreatic juice output and amylase level in the drainage fluid after pancreatoduodenectomy in relation to leakage. Hepatogastroenterology. 2002;49:553–555. [PubMed] [Google Scholar]
- 26.Shinchi H, Wada K, Traverso LW. The usefulness of drain data to identify a clinically relevant pancreatic anastomotic leak after pancreaticoduodenectomy? J Gastrointest Surg. 2006;10:490–498. [DOI] [PubMed] [Google Scholar]
- 27.Connor S, Alexakis N, Garden OJ, et al. Meta-analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. Br J Surg. 2005;92:1059–1067. [DOI] [PubMed] [Google Scholar]
- 28.Bassi C, Butturini G, Salvia R, et al. Open pancreaticogastrostomy after pancreaticoduodenectomy: a pilot study. J Gastrointest Surg. 2006;10:1072–1080. [DOI] [PubMed] [Google Scholar]
- 29.Kawai M, Tani M, Terasawa H, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wada K, Traverso LW. Pancreatic anastomotic leak after the Whipple procedure is reduced using the surgical microscope. Surgery. 2006;139:735–742. [DOI] [PubMed] [Google Scholar]