Abstract
Objective:
To determine the incidence and independent predictors of gastrointestinal complications (GICs) following cardiac surgery.
Summary Background Data:
Gastrointestinal ischemia and hemorrhage represent a rare but devastating complication following heart surgery. The profile of patients referred for cardiac surgery has changed during the last decade, questioning the validity of previously reported incidence and risk factors.
Methods:
We retrospectively analyzed prospectively collected data from 4819 patients undergoing cardiac surgery between 1998 and 2004. Patients with GICs were compared with the entire patient population. Study endpoints were mortality, postoperative morbidities, and long-term survival.
Results:
GICs occurred in 51 (1.1%) patients. Etiologies were intestinal ischemia (n = 30; 59%) and hemorrhage (n = 21; 41%). The incidence decreased during the study period (1998–2001: 1.3%, 2002–2004: 0.7%; P = 0.04). The incidence per type of procedure was as follows: coronary artery bypass grafting (CABG)/valve (2.4%), aortic surgery (1.7%), valve surgery (1.0%), and CABG (0.5%; P = 0.001). Multivariate analysis revealed age (odds ratio [OR] = 2.1), myocardial infarction (OR = 2.5), CHF (OR = 2.4), hemodynamic instability (OR = 2.8), cardiopulmonary bypass time >120 minutes (OR = 6.2), peripheral vascular disease (OR = 2.2), renal (OR = 3.2), and hepatic failure (OR = 10.8) as independent predictors of GICs. The overall hospital mortality among patients with GICs was 33%. Long-term survival was significantly decreased in patients with GICs compared with the control group.
Conclusions:
Gastrointestinal complications following cardiac surgery remain rare with an incidence <1% in a contemporary series. The key to a lower incidence of GICs lies in systematic application of preventive measures and new advances in intraoperative management. Identification of independent risk factors would facilitate the determination of patients who would benefit from additional perioperative monitoring. Future resources should therefore be redirected to mitigate GICs in high-risk patients.
We determined the incidence and independent predictors of gastrointestinal complications following cardiac surgery in a recent era and demonstrated a decrease of these complications during the study period. The key to a lower incidence lies in systematic application of preventive measures and advances in intraoperative management.
The incidence of gastrointestinal complications (GICs) after cardiac surgery varies between 0.3% and 3%.1–4 Although they occur infrequently, GI events are serious complications that carry high mortality and morbidity rates. Previous reports on the incidence and risk factors for this complication have focused on patients undergoing coronary artery bypass grafting (CABG) or large cohorts of cardiac surgery patients with predominantly CABG procedures.2,5 During the last few years, with the broader application of percutaneous transluminal coronary angioplasty (PTCA), the population of patients referred for cardiac surgery has significantly changed. Currently, a majority of patients in tertiary centers is referred for more complex procedures including combined valve/CABG, multiple valve and aortic procedures.
In addition, during the last decade, significant advances have also been made in the perioperative management of patients undergoing cardiac surgery, which could have impacted the incidence of GICs. These changes have raised the question of the validity of previously reported incidence and risk factors for the occurrence of this complication. In this study, we sought to determine the incidence, independent risk factors, and outcome following GICs in a heterogeneous cohort of cardiac surgery patients in a recent era.
PATIENTS AND METHODS
Study Population
We retrospectively analyzed a series of 4986 consecutive patients undergoing cardiac surgery at the Mount Sinai Medical Center between January 1998 and December 2004. Patients undergoing cardiac transplantation or assist device implantation (n = 167) were excluded from this study.
The protocol was approved by our local institutional review board and compliant to the Health Insurance Portability and Accountability Act regulations and the ethical guidelines of the 1975 declaration of Helsinki. The approval included a waiver of informed consent.
Data Collection and Outcome Analysis
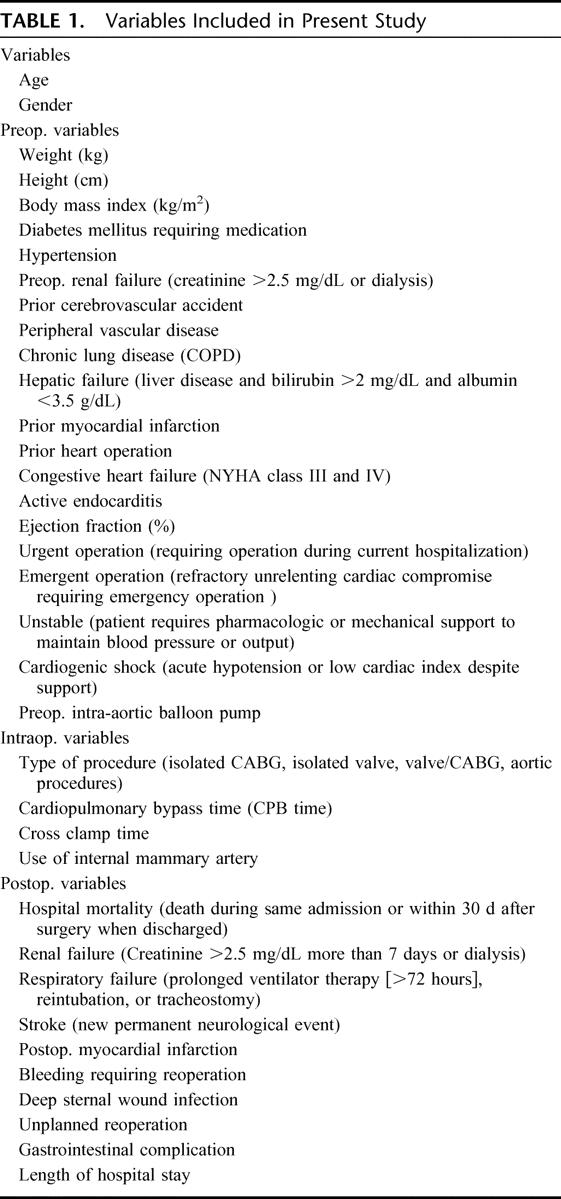
Clinical variables were prospectively entered into the New York State Department of Health (NYSDH, State Cardiac Advisory Committee) data registry (www.health.state.ny.us). The NYSDH data registry represents a mandatory verified peer-reviewed data collection system including all adult cardiac surgery procedures in the state of New York and records and analyzes data in a strictly supervised and widely reported fashion. Patient demographics and risk factors, operative information, and postoperative outcome data were retrospectively analyzed. Additional information was obtained from patient charts when necessary. Follow-up survival information was obtained by cross matching patient's social security number with the web-based social security death index (ssdi.rootsweb.com). When a patient was not registered as being dead, he was considered alive. Table 1 summarizes preoperative variables included in this study and their definition as indicated.
TABLE 1. Variables Included in Present Study
In addition, the logistic EuroSCORE was used for risk stratification.6 The EuroSCORE is a risk stratification system based on multiple preoperative risk factors to predict operative mortality. Patients were divided into 4 subgroups determined by their predicted mortality as follows: low risk (3%), moderate risk (3%–9%), high risk (9%–25%), and very high risk (>25%).
The main outcome parameter of this study is the occurrence of postoperative GICs. This complication was defined in accordance to the NYSDH data registry: any postoperative episode of vomiting blood, gross blood in the stool, perforation or necrosis of the stomach or intestine that required an invasive diagnostic or therapeutic intervention, such as gastroscopy, colonoscopy, or laparotomy. Other GI-related morbidities such as cholecystitis, pancreatitis, or paralytic ileus were not included in the analysis. The medical records, operative notes, radiographs, and autopsy reports of all patients with GICs were reviewed thoroughly when applicable. Patients without GICs served as the control group.
Further outcome measures for this study included hospital mortality, major postoperative complications (respiratory failure, renal failure, deep sternal wound infection, bleeding requiring reoperation, unplanned reoperation, stroke), length of hospital stay, and late survival. Hospital mortality was defined as death following the procedure before patients discharge regardless of the duration of hospitalization. Patients who died after discharge from hospital but within 30 days following the procedure were also considered as hospital deaths. Respiratory failure was defined as prolonged ventilator therapy (>72 hours) or need for reintubation or tracheostomy. Renal failure was defined as creatinine >2.5 mg/dL for more than 7 postoperative days or the need for dialysis. Stroke was defined as a new permanent neurologic event occurring perioperatively or postoperatively.
Intraoperative and Postoperative Management
All procedures were performed using standard anesthetic and surgical techniques adapted to the individual procedures. A small skin incision and a full or partial sternotomy were performed in all patients. Epi-aortic scanning of the ascending aorta was done to rule out ascending aortic atherosclerotic disease prior to cannulation (since January 2002). After systemic heparinization, cardiopulmonary bypass (CPB) was instituted between the ascending aorta and either the right atrium using a two-stage cannula or both venae cavae. Cardioplegia using high potassium cold blood was administered in an antegrade and/ or retrograde fashion for myocardial protection. In patients undergoing valve surgery, further myocardial protection was obtained with mild to moderate systemic cooling (28°C to 30°C). Procedures involving the aortic arch were performed in deep hypothermic circulatory arrest. After the completion of CPB, protamine was given based on the heparin level. Following surgery, all patients were transferred to the intensive care unit. Patients were weaned from ventilator when hemodynamic stability was achieved, no postoperative bleeding occurred, and adequate consciousness was obtained. Stable patients were transferred to the regular ward and discharged home or to a rehabilitation facility when appropriate. Intraoperative variables used in this study are reported in Table 1.
Ulcer Prophylaxis
Patients undergoing cardiac surgery at our institution routinely received H2-blockers as ulcer prophylaxis. Patients with a history of GI bleeding were treated with proton pump inhibitors instead.
Statistical Analyses
Normally distributed continuous variables are presented as mean ± SD and otherwise as median ± interquartile range (IQR). Categorical variables are shown as the percentage of the sample. A P value <0.05 was considered statistically significant for all used tests.
To identify preoperative and perioperative factors associated with the occurrence of GICs, data were explored by contingency table analyses to look for evidence that some values should be grouped and for evidence of linear trend in continuous variables. χ2 test, Fisher exact test, and Cochran-Armitage test for trend were used to identify factors that significantly influenced the risk of GICs when considered one at a time. These factors were then entered in a stepwise logistic regression analysis to identify a set of independent variables associated with postoperative GICs. The odds ratio (OR), corresponding 95% confidence interval (CI), and the P value are reported for each independent factor. Similar analyses were undertaken to identify independent factors predicting hospital mortality in patients suffering from postoperative GI complications. Long-term survival was analyzed using Kaplan-Meier survival curves. Differences in patient characteristics were controlled by Cox proportional hazard analysis. The statistical analysis was performed using SPSS for Windows, version 14.0 (SPSS Inc., Chicago, IL) and SAS version 9.1.3 (SAS Institute Inc., Cary, NC).
RESULTS
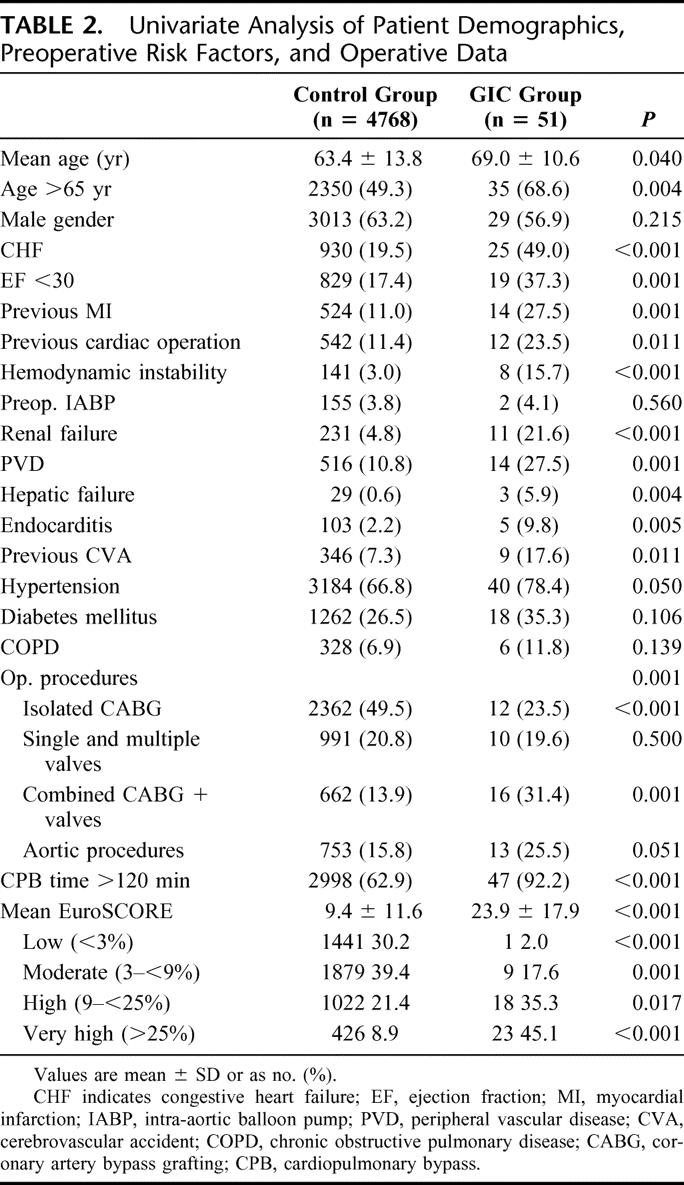
A total of 4819 adult patients were included in this study. The mean age was 63 ± 14 years, 52% (n = 2497) of patients were older than 65 years, and 64% (n = 3084) of patients were male. Preoperative risk factors included hypertension (n = 3226, 67%), diabetes mellitus (n = 1278, 26%), PVD (n = 534, 11%), COPD (n = 334, 7%), and renal failure (n = 240, 5%). Congestive heart failure was present in 980 patients (20%). The mean ejection fraction was 45.5% ± 15.2%. Eleven percent (n = 569) of patients had a history of at least one previous cardiac operation. Patient demographics and the distribution of preoperative risk factors are summarized in Table 2.
TABLE 2. Univariate Analysis of Patient Demographics, Preoperative Risk Factors, and Operative Data
Forty-nine percent (n = 2374) of patients underwent isolated CABG (conventional CABG, n = 2002, 84%; off-pump CABG, n = 372, 16%), 21% (n = 1001) had a single or multiple valve procedures, 14% (n = 678) of patients underwent combined valve and CABG procedures, and 16% (n = 766) underwent surgery involving the ascending aorta or the aortic arch. During the study period, a total of 2328 patient underwent isolated CABG procedures.
Fifty one patients (1.1%) suffered from postoperative GICs. The majority of these patients presented with intestinal ischemia (n = 30 of 51, 59%). The clinical manifestations included necrosis of the small intestine in 16 patients and necrosis of the colon/rectum in 14 patients. Clinical symptoms in patients with ischemic GICs were predominately abdominal distension (n = 20, 67%) and abdominal pain (n = 5, 17%). In addition, elevation of serum lactate was consistently observed in the majority of patients (n = 19, 63%). Abdominal CT scan was available in 12 patients and showed abnormalities suggestive of ischemic bowel disease in 8 (67%) patients (dilated small bowel loops, n = 8, wall thickening, n = 4, extraluminal air, n = 3, pneumatosis, n = 1). The remaining 21 patients (41%) presented with upper or lower gastrointestinal hemorrhage. The clinical manifestations in this subgroup were as follows: esophageal varicose bleeding (2 patients), erosive gastritis/gastric ulcer (12 patients), duodenal ulcer (2 patients), and colorectal bleeding (5 patients). In patients with hemorrhagic complications, hematemesis (n = 12, 57%) and decreased hematocrit level (n = 17, 81%) despite administration of red blood cells were the most common indicators. The diagnosis was confirmed in all patients with upper or lower gastrointestinal endoscopy. We observed a significant decrease of GICs during the study period: from 1.3% between 1998 and 2001 to 0.7% between 2002 and 2004 (P = 0.044).
The rate of GICs was different when the patient population was stratified by procedures: isolated CABG (n = 12, 0.5%), single or multiple valve surgery (n = 10, 1.0%), combined CABG and valve procedures (n = 16, 2.4%), and aortic surgery (n = 13, 1.7%) (P = 0.001). The incidence of gastrointestinal complications in the conventional and off-pump CABG groups were 10 (0.5%) and 2 (0.5%), respectively (P = not significant). When patients were stratified according to EuroSCORE, we observed an increasing rate of GICs with increased predicted mortality: low risk (n = 2, 0.1%), moderate risk (n = 8, 0.4%), high risk (n = 17, 2%), and very high (n = 24, 5%) (P < 0.001).
Predictors of Gastrointestinal Complications
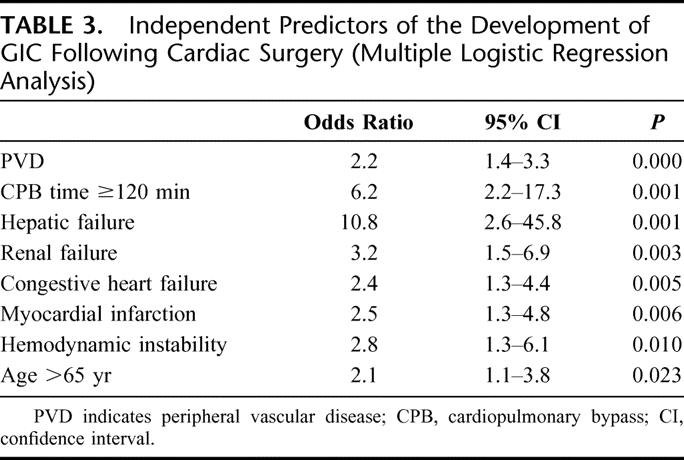
In univariate analysis, patients with GICs were more likely to present preoperative risk factors including age >65 years, severe impaired left ventricular function, history of myocardial infarction, CHF, hemodynamic instability on admission, need for intra-aortic balloon pump, previous cardiac procedure, CPB time >120 minutes, previous CVA, PVD, hepatic failure, preoperative renal failure, endocarditis, and EuroSCORE (Table 2). Stepwise multivariate logistic regression analysis revealed age over 65 years (odds ratio [OR] = 2.1, P = 0.023), previous myocardial infarction (OR = 2.5, P = 0.006), CHF (OR = 2.4, P = 0.005), hemodynamic instability (OR = 2.8, P = 0.010), CPB time >120 minutes (OR = 6.2, P = 0.001), PVD (OR = 2.2, P < 0.001), renal failure (OR = 3.2, P = 0.003), and hepatic failure (OR = 10.8, P = 0.001) as independent predictors for the occurrence of GICs after cardiac surgery (Table 3).
TABLE 3. Independent Predictors of the Development of GIC Following Cardiac Surgery (Multiple Logistic Regression Analysis)
Outcome of Patients With Gastrointestinal Complications
The treatment strategies varied according to the etiology of GICs. Sixteen of 30 patients with ischemic GICs underwent surgery. The procedures performed included small bowel resection (n = 6) and hemicolectomy/ colectomy (n = 10). Fourteen patients with ischemic GICs were not explored. The majority of these patients had a delayed diagnosis and were considered too critically ill to undergo an invasive procedure. All 21 patients with GI hemorrhage were treated with transfusion and proton pump inhibitors. In addition, 15 patients (71%) required additional endoscopic treatment, including clip placement, thermocoagulation, or epinephrine sclerotherapy. Finally, surgery was performed in 3 patients (14%). One of these 3 patients underwent gastrectomy for intractable upper GI bleeding, whereas 2 patients had a hemicolectomy for lower GI bleeding.
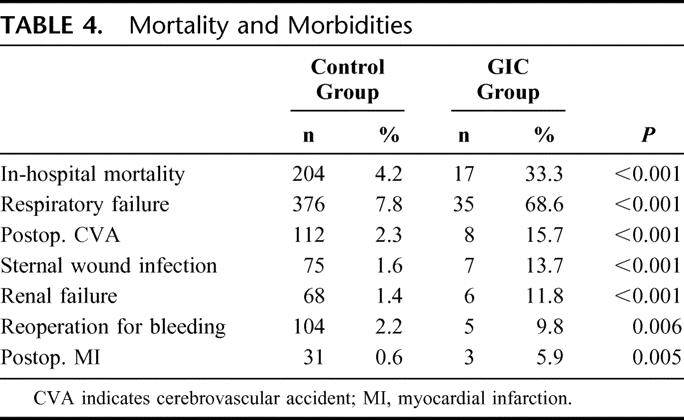
The overall hospital mortality among patients with GICs was 33% (n = 17) compared with a mortality rate of 4.3% (n = 204) in patients without postoperative GICs (P < 0.001). In the subgroup of patients presenting with bowel necrosis, the mortality rate was 47% (n = 14 of 30). The mortality rate among patients with GICs undergoing surgery was 25% (n = 4 of 16). In patients with ischemic event not undergoing surgery, the mortality rate was 71% (n = 10 of 14). Among patients with hemorrhagic GICs, the mortality rate was 14% (3 of 21).
Patients with GICs were more likely to present other major postoperative complications, including stroke, myocardial infarction, sternal wound infection, reoperation for bleeding, renal failure, and respiratory failure (Table 4). The median length of hospital stay was significantly increased in patients with GICs compared with the control group (7 days, IQR 5–11 days vs. 32 days, IQR 14–73 days, P < 0.001).
TABLE 4. Mortality and Morbidities
Follow-up Data
Follow-up was completed for 4591 patients (98%). The mean follow-up time was 4.2 ± 2.3 years. Long-term survival of discharged patients was significantly decreased in patients with GICs compared with patients without this complication. One-year and 5-year survival rates were 69% ± 8% and 59% ± 9% for patients with GICs and 94% ± 1% and 82% ± 1%, respectively, for the population without complication (P < 0.001). Figure 1 shows Kaplan-Meier survival curves.
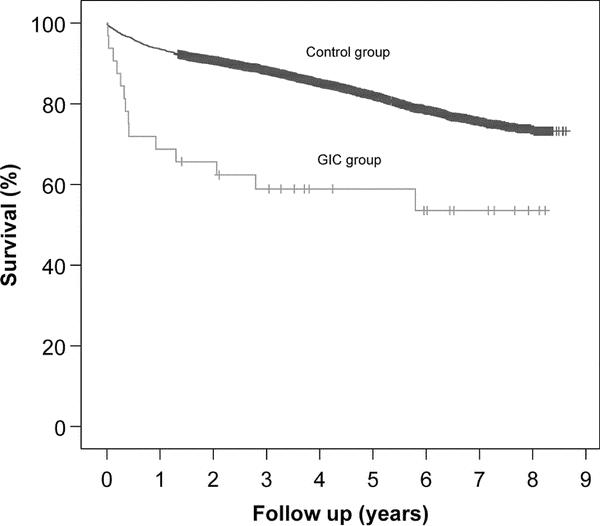
FIGURE 1. Kaplan-Meier survival curves showing long-term survival of discharged patients. GICs, gastrointestinal complications.
DISCUSSION
This study was conducted to analyze retrospectively the incidence, independent risk factors for the occurrence of GICs, and early and late survival following this condition in a large cohort of patients undergoing cardiac surgery within a recent period. In our study, using a precise definition of GICs based on the NYSDH data registry, the incidence of this complication was 1.1% (n = 51). The majority of patients (59%, n = 31) had ischemic bowel disease, while 41% (n = 21) had GI hemorrhage. The overall hospital mortality was 33% (47% in the ischemic group and 14% in patients with gastrointestinal hemorrhage). We were able to identify 8 independent predictors for the occurrence of GICs, including age over 65 years, previous myocardial infarction, CHF, hemodynamic instability, PVD, renal failure, hepatic failure, and CPB time >120 minutes. Long-term survival of patients with GICs was significantly reduced to patients without this complication.
GICs following open heart procedures represent a rare but serious event. The incidence of GI complications in the literature varies between 0.3% and 3%. This variation might be explained by an inconsistency in defining GICs in different studies. Mangi et al analyzed 8709 patients undergoing cardiac surgery and reported an incidence as low as 0.5%.2 These authors only reported patients with GICs, which required a general surgical consult. Using this definition, they only included the “sickest” patients presenting with advanced GICs, particularly ischemic bowel disease. Consequently, patients with GICs controlled by medical or endoscopic treatment without surgical consult were not reported in their series. In contrast, one of the highest rates of GICs was reported by Christenson et al.3 These authors used a broad definition of GICs, including acute cholecystitis, pancreatitis, and medically treated GI bleeding and reported an incidence of 2.9% in a series of 3493 patients. Despite differences in defining this postoperative complication, the 2 most common etiologies of GICs reported by most clinical series are ischemic bowel disease and GI hemorrhage.2,4,5 Based on these findings, the NYSDH Data Registry requires the report of these 2 major events in patients undergoing cardiac surgery. According to this precise definition, the incidence of GICs was 1.1% in our series, which is at the lower end of the reported range in the literature.
Another interesting finding of our study is that we were able to demonstrate a significant decrease in the incidence of this complication during the study period (from 1998 to 2001, 1.3%; and from 2002 to 2004, 0.7%, P = 0.04). This reduction was notified in the incidence of both, ischemic and more markedly of hemorrhagic GICs. Our study design does not allow us to determine the precise explanation for this finding; however, we think that the key to a lower incidence of GICs lies in systematic application of preventing measures and new advances in intraoperative management of patients undergoing cardiac surgery. The decrease in the incidence of hemorrhagic GICs during the recent era is probably related to a better patient selection preoperatively with the routine workup of patients with a history of GI hemorrhage and peptic ulcer. In addition, improved perioperative ulcer prophylaxis with the systematic use of H2-blockers or proton pump inhibitors might have contributed in decreasing the incidence of this condition. The decrease in the incidence of ischemic GICs might be explained by implementation of new intraoperative measurements, which were introduced in our practice since 2002. These measurements include routine epi-aortic ultrasonography to detect any atherosclerotic lesions2,7 prior to aortic manipulation and cannulation. The surgical strategy is then determined according to the presence or absence of calcification in the ascending aorta and its extent. The resulting reduced rate of plaque disruption and peripheral embolization due to aortic cannulation might have contributed in decreasing the incidence of GI ischemic events.7,8 Another important measurement is the use of axillary artery instead of femoral artery as an inflow for arterial cannulation in patients undergoing complex aortic surgery in the setting of ascending aortic/arch dissections or aneurysms.9 This technique preserves antegrade arterial perfusion during CPB and avoids retrograde perfusion, which carries the risk of complications such as retrograde atheroembolism or organ malperfusion.10 Maintenance of higher perfusion pressures (>70 mm Hg) in all patients, particularly those with atherosclerotic risk factors,11 and an adequate hematocrit on CPB12 may have also played a role in avoiding abdominal organ hypoperfusion.
Our data indicate that ischemic bowel disease remains the main etiology of GICs. Previous studies from the 1990s reported gastrointestinal hemorrhage as the most frequent etiology of GICs.1,4 In 1995, in a series of 1831 patients, Spotnitz et al4 reported an incidence of 2% GICs following cardiac surgery. In their series, 46% had upper or lower GI bleeding and only 5% suffered from bowel necrosis.4 In contrast, in a more recent study, Mangi et al reported mesenteric ischemia as the dominant etiology of GICs. In their study, 67% patients with GICs had an intestinal ischemia.2 In our series, ischemic GICs occurred in 59% (n = 30 of 51) of patients confirming the finding of the Mangi et al study. The changes in the profile of patients undergoing cardiac surgery may be a potential explanation for the increasing incidence of ischemic GI events. Today cardiac surgical patients are older, present with significant preoperative comorbidities including atherosclerotic diseases and require more complex and prolonged cardiac procedures. The presence of these factors potentially increases the risk of abdominal organ hypoperfusion and thromboembolic events, which represent the 2 main pathophysiologic mechanisms of ischemic GICs. This explanation is further corroborated by a clear identification of independent risk factors for the occurrence of this complication using multivariate analysis. Only a few studies have performed this type of statistical analysis and were able to find the influence of age,1,5,13 NYHA class,1,5 postoperative vascular complications,5 postoperative low cardiac output syndrome,13 need for IABP support,2 and inconsistently CPB time1,5 as predictors for the occurrence of GICs following cardiac surgery. Our study revealed 8 independent factors associated with this complication, demonstrating the different pathophysiologic mechanisms involved, namely, hypoperfusion and thromboembolic events. Age over 65 years, previous myocardial infarction, PVD, and renal failure reflect a high incidence of atherosclerotic burden increasing the risk of thromboembolic events following cardiac surgery. Other risk factors, such as CHF, hemodynamic instability, and CPB time >120 minutes reflect the risk for perioperative and postoperative hypoperfusion due to low cardiac output or decreased systemic blood pressure.
Most of our patients with GICs presented with more than one complication. This is in accordance with other published data.5,14 Recht et al reported a significantly increased rate of neurologic, pulmonary, renal, and infectious complications in patients with GICs, but were not able to identify a cause-relationship between different organ dysfunctions and GI events.14 Similarly, our study was not designed to clarify the association between the occurrence of this complication and other major morbidities following cardiac surgery.
Despite the fact that we were able to show the reduction in the incidence of this complication during the recent years, the associated mortality, particularly of intestinal ischemia remains high.2,5 Most patients with bleeding complications are diagnosed early and can be treated medically or endoscopically with a relatively low mortality. In contrast, in patients with intestinal ischemia diagnosis is often delayed due to masked symptoms and the surgical therapy is performed often as a salvage operation resulting in a high mortality. Therefore, in patients with high risk for this complication careful postoperative examination is recommended to establish early diagnosis and surgical therapy when this complication occurs.
Reports on long-term survival in patients with GICs remain scarce. In our study, 1-year and 5-year survival in discharged patients with GICs were 68% ± 8% and 59% ± 9%, respectively, compared with 94% ± 1% and 82% ± 1% in patients without this complication. This is similar to the results reported by Andersson et al, which have shown a 1-year survival rate of 50% in patients with GICs.5 Our observation of a decreased survival in these patients even after hospital discharge might be based on the possibility that GICs may be a marker of predisposition to death rather than a direct cause. This assumption is supported by our finding that the incidence of GI events following cardiac surgery is correlated to the predicted mortality by EuroSCORE. This implies that at least some patients who die after a GI event were independently disposed to perioperative and postoperative death regardless of the GICs. However, we were not able to determine the exact causes of death in patients who died during follow-up.
Limitations
This study retrospectively analyzed prospectively collected data and has therefore certain limitations. Databases may underreport events and risk factors. However, the NYSDH periodically visits participating center for validation and there has not been any question of the quality of our data, or underreporting of adverse events in our center. Another problem all databases face is that of incomplete data. Risk models can only adjust for data that has been collected. Our study did not examine some previously reported risk factors for the occurrence of GICs such as heparin-induced thrombocytopenia, atrial fibrillation, and transfusion requirements.2,15
CONCLUSION
Gastrointestinal complications, particularly ischemic GICs, remain a rare but life-threatening event following cardiac surgery. The systematic application of measurements such as epi-aortic scanning, avoidance of retrograde perfusion by the use of axillary artery instead of femoral artery cannulation, and the preservation of a high perfusion pressure during CPB have probably contributed in the decrease of the incidence of this complication in recent years. Identification of independent risk factors would facilitate the determination of patients who would benefit from additional workup prior to undergoing cardiac surgery. In addition, these patients would benefit from close surveillance for early diagnosis, which should lead to an aggressive treatment approach. Future resources should therefore be redirected to mitigate GICs in high-risk patients.
ACKNOWLEDGMENTS
The authors thank Carol A. Bodian, DrPH, Department of Biomathematical Sciences, Mount Sinai School of Medicine, New York, NY, for expert statistical support.
Footnotes
Reprints: Farzan Filsoufi, MD, Department of Cardiothoracic Surgery, Mount Sinai School of Medicine, 1190 Fifth Avenue, Box 1028, New York, NY 10029. E-mail: Farzan.filsoufi@mountsinai.org.
REFERENCES
- 1.Zacharias A, Schwann TA, Parenteau GL, et al. Predictors of gastrointestinal complications in cardiac surgery. Tex Heart Inst J. 2000;27:93–99. [PMC free article] [PubMed] [Google Scholar]
- 2.Mangi AA, Christison-Lagay ER, Torchiana DF, et al. Gastrointestinal complications in patients undergoing heart operation: an analysis of 8709 consecutive cardiac surgical patients. Ann Surg. 2005;241:895–901; discussion 901–904. [DOI] [PMC free article] [PubMed]
- 3.Christenson JT, Schmuziger M, Maurice J, et al. Postoperative visceral hypotension the common cause for gastrointestinal complications after cardiac surgery. Thorac Cardiovasc Surg. 1994;42:152–157. [DOI] [PubMed] [Google Scholar]
- 4.Spotnitz WD, Sanders RP, Hanks JB, et al. General surgical complications can be predicted after cardiopulmonary bypass. Ann Surg. 1995;221:489–496; discussion 496–497. [DOI] [PMC free article] [PubMed]
- 5.Andersson B, Nilsson J, Brandt J, et al. Gastrointestinal complications after cardiac surgery. Br J Surg. 2005;92:326–333. [DOI] [PubMed] [Google Scholar]
- 6.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- 7.Wareing TH, Davila-Roman VG, Daily BB, et al. Strategy for the reduction of stroke incidence in cardiac surgical patients. Ann Thorac Surg. 1993;55:1400–1407; discussion 1407–1408. [DOI] [PubMed]
- 8.Sharony R, Grossi EA, Saunders PC, et al. Propensity case-matched analysis of off-pump coronary artery bypass grafting in patients with atheromatous aortic disease. J Thorac Cardiovasc Surg. 2004;127:406–413. [DOI] [PubMed] [Google Scholar]
- 9.Hedayati N, Sherwood JT, Schomisch SJ, et al. Axillary artery cannulation for cardiopulmonary bypass reduces cerebral microemboli. J Thorac Cardiovasc Surg. 2004;128:386–390. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Fukuda I, Osaka M, et al. Axillary artery and transapical aortic cannulation as an alternative to femoral artery cannulation. Eur J Cardiothorac Surg. 2003;23:842–843. [DOI] [PubMed] [Google Scholar]
- 11.Gold JP, Charlson ME, Williams-Russo P, et al. Improvement of outcomes after coronary artery bypass: a randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110:1302–1311; discussion 1311–1314. [DOI] [PubMed]
- 12.DeFoe GR, Ross CS, Olmstead EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting: Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 2001;71:769–776. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K, Matsumoto M, Sugita T, et al. Gastrointestinal complications in patients undergoing coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2005;11:25–28. [PubMed] [Google Scholar]
- 14.Recht MH, Smith JM, Woods SE, et al. Predictors and outcomes of gastrointestinal complications in patients undergoing coronary artery bypass graft surgery: a prospective, nested case-control study. J Am Coll Surg. 2004;198:742–747. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, Roberts N, Firmin RK, et al. Risk factors for intestinal ischaemia in cardiac surgical patients. Eur J Cardiothorac Surg. 2002;21:411–416. [DOI] [PubMed] [Google Scholar]


