Abstract
Background:
To analyze clinical courses and outcome of postpancreatectomy hemorrhage (PPH) after major pancreatic surgery.
Summary Background Data:
Although PPH is the most life-threatening complication following pancreatic surgery, standardized rules for its management do not exist.
Methods:
Between 1992 and 2006, 1524 patients operated on for pancreatic diseases were included in a prospective database. A risk stratification of PPH according to the following parameters was performed: severity of PPH classified as mild (drop of hemoglobin concentration <3 g/dL) or severe (>3 g/dL), time of PPH occurrence (early, first to fifth postoperative day; late, after sixth day), coincident pancreatic fistula, intraluminal or extraluminal bleeding manifestation, and presence of “complex” vascular pathologies (erosions, pseudoaneurysms). Success rates of interventional endoscopy and angiography in preventing relaparotomy were analyzed as well as PPH-related overall outcome.
Results:
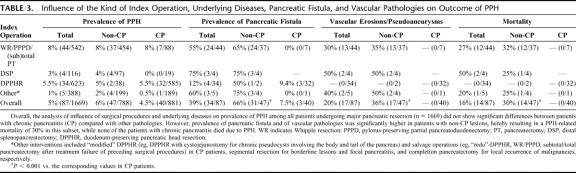
Prevalence of PPH was 5.7% (n = 87) distributed almost equally among patients suffering from malignancies, borderline tumors, and focal pancreatitis (n = 47) and from chronic pancreatitis (n = 40). PPH-related overall mortality of 16% (n = 14) was closely associated with 1) the occurrence of pancreatic fistula (13 of 14); 2) vascular pathologies, ie, erosions and pseudoaneurysms (12 of 14); 3) delayed PPH occurrence (14 of 14); and 4) underlying disease with lethal PPH found only in patients with soft texture of the pancreatic remnant, while no patient with chronic pancreatitis died. Conversely, primary severity of PPH (mild vs. severe) and the kind of index operation (Whipple resection, pylorus-preserving partial pancreaticoduodenectomy, organ-preserving procedures) had no influence on outcome of PPH. Endoscopy was successful in 3 from 15 patients (20%), who had intraluminal PPH within the first or second postoperative day. “True,” early extraluminal PPH had uniformly to be treated by relaparotomy. Seventeen patients had “false,” early extraluminal PPH due to primarily intraluminal bleeding site from the pancreaticoenteric anastomosis with secondary disruption of the anastomosis. From 43 patients subjected to angiography, 25 underwent interventional coiling with a success rate of 80% (n = 20). Overall, relaparotomy was performed in 60 patients among whom 33 underwent surgery as first-line treatment, while 27 were relaparotomied as rescue treatment after failure of interventional endoscopy or radiology.
Conclusion:
Prognosis of PPH depends mainly on the presence of preceding pancreatic fistula. Decision making as to the indication for nonsurgical interventions should consider time of onset, presence of pancreatic fistula, vascular pathologies, and the underlying disease.
The armory of interventional options for treatment of postpancreatectomy hemorrhage encompasses endoscopy, angiography, or relaparotomy. A standardized therapeutic algorithm has to consider an individual risk profile. The risk of lethal course is increased when hemorrhage occurs after the sixth postoperative day, especially when it is associated with pancreatic fistula.
Although mortality after pancreatic surgery in most high-volume centers has decreased to less than 3%,1–3 morbidity still remains considerably high, ranging from 18% to 52%.4–9 The most frequent causes for morbidity are anastomotic insufficiencies (pancreatic, biliary, gastric/duodenal, and enteral), pancreatic fistulas, and delayed gastric emptying.
Postpancreatectomy hemorrhage (PPH) is a less frequent, however, in some patients, devastating complication. Since both its pathophysiologic and clinical features may differ considerably, it is difficult to establish diagnostic and therapeutic algorithms for adequate management of PPH: 1) time of onset (early PPH occurring within 24 to 48 hours postoperatively versus delayed PPH after several days to weeks); 2) severity [(a) mild, (b) moderate, (c) severest, ie, life-threatening]; 3) intraluminal or extraluminal manifestation; 4) underlying disease (pancreatic carcinoma vs. chronic pancreatitis); 5) kind of index operation; and 6) a possible association to erosive vascular pathologies due to pancreatic fistula are factors that are important for estimating the prognosis of PPH. A customized risk analysis should precede individual decision-making. The armory of diagnostic and therapeutic means ranges from observant monitoring and fluid replacement, interventional procedures, ie, endoscopy and radiology, to surgical relaparotomy.
Early bleeding within the immediate postoperative period is unlikely due to vascular erosions but rather a result of simple technical failures. In case of PPH in the abdominal cavity, no doubt exists that immediate relaparotomy is indicated. However, in case of early bleeding in the gastrointestinal tract, endoscopy may also be an option for interventionally treating bleeding sites located at the gastrojejunostomy or, if accessible, at the enteroenteric anastomosis.
Because of the life-threatening potential of PPH, standardized rules with respect to its management are urgently needed. So far, in clinical routine, the decision about how to handle PPH is often arbitrary and is usually based on institutional or even individual experiences.
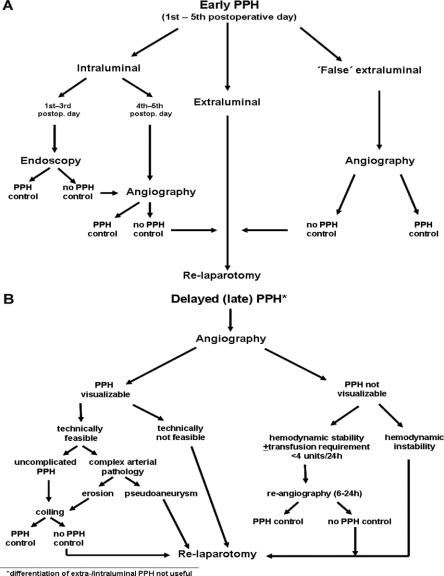
The aim of the presented evaluation was therefore to “dissect” the heterogeneous causes of PPH. Onset of manifestations, clinical features, courses, and success rates of nonsurgical options, ie, interventional radiology and endoscopy, and surgical procedures for treatment of PPH were analyzed. Based on a 15-year institutional experience, we sought, in particular, to classify different bleeding types and suggest a diagnostic and therapeutic algorithm that may help to allocate patients to a customized therapy (Fig. 1).
FIGURE 1. Suggested algorithm for treatment of early (A) and delayed (B) postpancreatectomy hemorrhage after major pancreatic surgery.
METHODS
Between 1992 and 2006, 1524 patients who underwent a total of 1669 resectional pancreatic operations were included in a prospective, pancreatic database. Classic resectional procedures were performed in 1281 patients. This included duodenum preserving pancreas head resection (DPPHR, n = 623), Whipple resection (n = 358), pylorus preserving pancreatoduodenectomy (n = 160), distal pancreatectomy (n = 116), and total pancreatectomy (n = 24). In the remaining 388 patients, other interventions, eg, DPPHR combined with cystojejunostomy, redo pancreas head resection, segmental resection, and resectional salvage procedures after primary treatment failure in both carcinoma and chronic pancreatitis patients were performed. Patients with limited draining procedures, eg, simple cystojejunostomy without any resectional aspect of the operation, were not included in this analysis.
The following parameters were evaluated:
Hemorrhage
The severity of bleeding was classified with slight modifications of the recommendation of the International Study Group of Pancreatic Surgery.10 Mild bleeding was defined as a decrease of hemoglobin concentration less than 3 g/dL without or with discrete clinical impairment (tachycardia, decrease of mean arterial blood pressure), not obligatorily requiring surgical or nonsurgical intervention. Severe PPH was defined as a decrease of hemoglobin concentration ≥3 g/dL, with clinical impairment and requiring either surgical or nonsurgical treatment. “Sentinel” bleeding was defined as 1) discrete but evident blood loss via abdominal drains or nasogastric tubes, hematemesis, or melena; 2) decrease of hemoglobin concentration ≤1.5 g/dL; 3) spontaneous cessation of hemorrhage without need for transfusion with red blood cells; and 4) rehemorrhage after a symptom-free time frame of at least 12 hours. According to the time of onset, PPH occurring within the first to fifth postoperative day was termed “early,” while after the sixth postoperative day the term “delayed” or “late” PPH was used.
Pancreatic Fistula
Until 2005, we defined fistula as drain fluid >20 mL/24 hours after 3 days postoperatively with amylase activity more than 3 times the serum activity. In August 2005, we decided to adopt our definition according to the International Study Group for Pancreatic Fistula (ISGPF) classification.11 After implementation of the ISGPF definition, a total of 92 patients were operated on for pancreatic diseases. Since only marginal differences between both definitions existed, an analysis of the subset of patients operated on before 2005 showed that ISGPF classification would also have defined all of these patients to have pancreatic fistula.
Nonsurgical, Interventional Bleeding Management
Angiographic evaluation or upper gastrointestinal endoscopy was only performed in case of successful maintenance of hemodynamic stability by fluids, transfusion with packed red blood cells, and fresh frozen plasma.
Angiography sought specifically to exclude bleeding sites originating from the following main vessels and their branches: hepatic artery, gastroduodenal artery, splenic artery, and superior mesenteric artery, respectively. In case of a transsection of the gastroduodenal artery, special attention was addressed to rule out stump insufficiency. Vascular access for interventional embolization was achieved by puncturing the common femoral artery. Afterward, the catheter was advanced in the visceral aortic branches, ie, the hepatic artery, gastroduodenal artery, splenic artery, and the superior mesenteric artery. Embolization was performed using a coaxial technique and microcoils, hereby embolizing the proven or assumed site of hemorrhage. In patients in whom index angiography failed to localize the bleeding source, who were hemodynamically stable at the time of investigation and had blood requirement ≤4 units/24 hours, the introducer sheath was left in the common femoral artery for a maximum of 24 hours to maintain vascular access. In case of recurrent bleeding within this period, patients underwent immediate reangiography. After 24 hours, the introducer was removed.
In case of successful bleeding control by interventional angiography, CT scans and/or abdominal sonographies were performed checking for fluid collections close to the pancreatic anastomosis. When such pathologies suggestive for inadequately drained pancreatic fistula, eg, due to dislocation of target drains, drain clotting, or both, were evidenced, indication for CT-guided placement of further drains within the collection, if interventionally accessible, or relaparotomy was established, depending on the patient's clinical condition and on whether patients had pancreatic fistula.
Surgical Reexploration
Indication for surgery was based on the following findings: 1) acute life-threatening hemodynamic deterioration with decrease of hemoglobin ≥3 g/dL or evident bleeding drained percutaneously or via the nasogastric tube; and 2) critical hemodynamic instability with continuing requirement of packed red blood cells exceeding 6 units per 12 hours without evidence for the bleeding source by angiography/endoscopy. In case of life-threatening situations, bedside decisions without measurement of hemoglobin were made.
In patients that had early extraintestinal bleeding without vascular erosions or pseudoaneurysms, appropriate hemostasis was usually achieved by suture ligating the bleeding site. When hemorrhage originated from ominous vascular erosions or pseudoaneurysms, operative decision making was based on the patients individual risk profile. Surgical procedures ranged from simple suture ligation or angioplastic reconstructions to completion pancreatectomy in patients with severe pancreatic fistula. Only in the absence of severe upper abdominal pancreatitis due to inadequately drained pancreatic fistula, vascular reconstruction was combined with completion pancreatectomy. When significant peri-anastomotic pancreatitis had occurred, severe vascular damages, ie, erosions and pseudoaneurysms, were treated by oversewing or ligating the bleeding source. In these cases, intraoperative decision whether 1) to drain pancreatic fistula externally, 2) to perform completion pancreatectomy, or 3) to subject patients to repeated, planned peritoneal lavage was based on several aspects, especially on the presence or absence of: concomitant pancreatitis/peritonitis, severe coagulopathy, and severe adhesions.
Our standard method of reconstruction after Whipple resection/PPPD consists of a separate end-to-side anastomosis of the pancreatic remnant with the proximal jejunal stump after removal of the specimen. Afterward, 30 to 40 cm distal to the pancreaticojejunostomy, the jejunal continuity is interrupted. A choledochojejunal anastomosis in end-to-side technique and an antecolic end-to side gastrojejunostomy are performed. Reconstruction is completed by reinsertion of the isolated, jejunal loop anastomosed with the pancreatic remnant in side-to side technique into the alimentary continuity 30 to 40 cm distal to the gastrojejunostomy. Intraluminal and “false” extraluminal PPH occurring until the fifth postoperative day were consistently located at the pancreaticojejunostomy. We treated such pathologies usually by opening the stapled, blind ends of the jejunal loop, which had been separated out of the gastrointestinal continuity during the index operation. This enabled us to maintain the integrity of the pancreaticojejunostomy as long as the bleeding site had not been clearly proven. When bleeding from the pancreaticojejunostomy was excluded, the further anastomoses were explored depending on the intraoperative situs.
Statistical Analysis
SPSS 11.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. Data were analyzed using the χ2 test, Student t test, and Fisher exact test as appropriate. A P value <0.05 was considered statistically significant. Since this analysis was intended to be explorative, no adjustment for multiple testing was carried out.
RESULTS
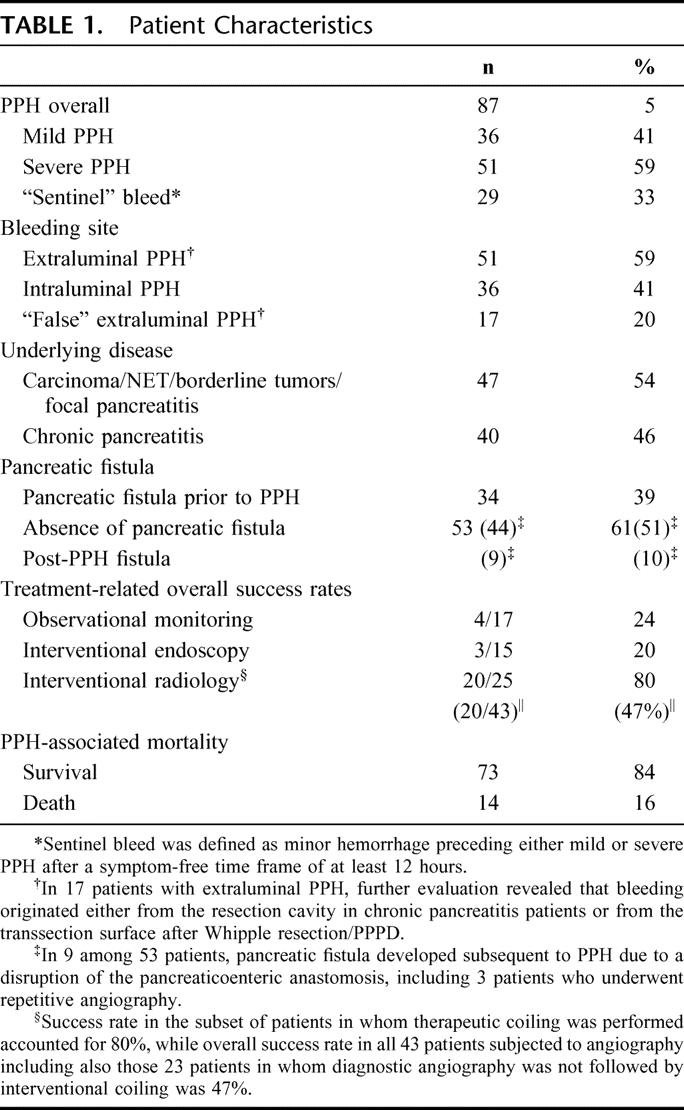
Patient Characteristics
The overall prevalence of PPH was 5.7% (n = 87). The underlying diseases were in 47 patients, pancreatic carcinoma, neuroendocrine tumors, tumors of borderline pathology, and focal pancreatitis, whereas 40 patients suffered from chronic pancreatitis involving the entire gland (Table 1). Intraductal papillary mucinous tumors of the pancreas (IPMT), mucinous-type cystadenoma, and nonmetastatic neuroendocrine tumors (NET) of the pancreas <3 cm without invasion in adjacent organs, respectively, were defined as borderline pathologies. Mild PPH (decrease of Hb <3 g/dL) was found in 36 patients, while 51 patients had primarily severe PPH (decrease of Hb ≥3 g/dL). Seventeen patients were primarily objected to observational monitoring without interventional procedures because they were hemodynamically symptom-free and had a decrease of Hb concentration ≤1.5 g/dL. However, in 13 of these 17 patients (76%), primarily discrete bleeding turned to be “sentinel PPH” with severe rehemorrhage (range, 14–85 hours) in their further clinical course requiring subsequent intervention, ie, angiography, endoscopy, or surgery, respectively. This resulted in a total of 83 patients who had endoscopic, radiologic, or surgical interventions, whereas in only 4 patients mild PPH came spontaneously to rest.
TABLE 1. Patient Characteristics
Interventions Performed
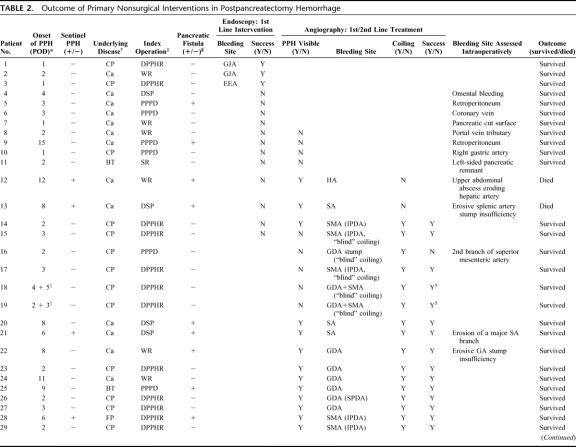
Including the 13 patients undergoing conservative therapy first but who underwent interventional procedures in their further course, the following first-line interventions were performed: 43 of 83 patients (52%) were subjected to angiography, 15 (18%) patients to endoscopy with an overlap of 8 patients subjected first to endoscopy and afterward to angiography (Table 2). Thirty-three patients (40%) underwent primary surgical relaparotomy either due to extraluminal blood loss evidenced by abdominal drains or to severe hemodynamic instability. In 27 patients, relaparotomy as rescue treatment after failure of interventional endoscopy, interventional angiography, or both, was performed. This resulted, overall, in 27 patients (31%) who could successfully treated by nonsurgical conservative monitoring and interventional procedures, while 60 patients (69%) required relaparotomy (Tables 1, 2).
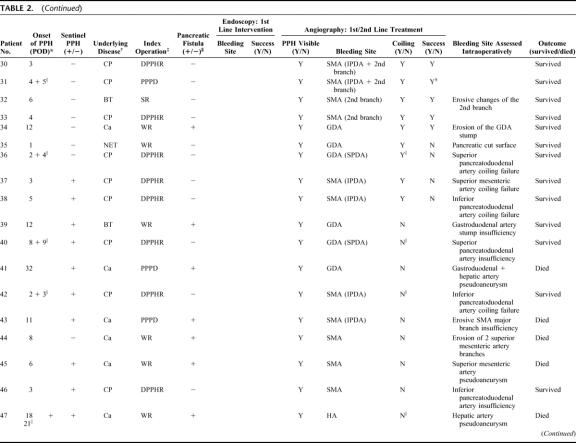
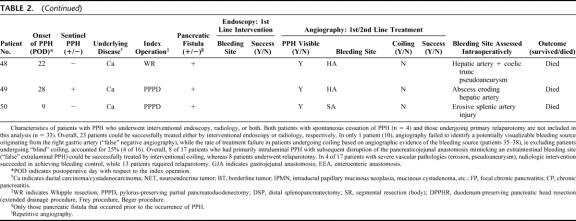
TABLE 2. Outcome of Primary Nonsurgical Interventions in Postpancreatectomy Hemorrhage
TABLE 2. (Continued)
TABLE 2. (Continued)
Site and Onset of PPH
Fifty-one patients had extraluminal PPH, whereas 36 patients had intraluminal PPH. Fifty-three patients had early PPH, while in the remainder of 30 patients, PPH occurred delayed. Seventeen of 34 patients who had extraluminal PPH until the fifth postoperative day had “true” extraintestinal bleeding sites due to insufficient hemostasis (eg, retroperitoneal and omental bleeding sites). These patients consistently underwent relaparotomy. In 17 patients, however, PPH was due to primary intraluminal bleeding originating from the pancreaticoenteric anastomosis with secondary disruption and subsequent bleeding into the abdominal cavity (“false” extraluminal PPH). Index operations in the latter subset were duodenum-preserving pancreatic head resection (n = 11) or Whipple resection/PPPD (n = 6). In 16 patients, the pancreaticoenteric anastomosis withstood elevated anastomotic pressure, hereby resulting in early intraluminal PPH originating from the pancreatic transsection surface. In only 3 patients who were treated by interventional endoscopy, early PPH at the first or second postoperative day was located at the gastroenteric or proximal enteroenteric anastomosis (Table 2). Patients with “false” extraluminal PPH until the fifth postoperative day, especially those who had undergone DPPHR for chronic pancreatitis, showed a characteristic clinical feature differing considerably from PPH due to other reasons: 1) in all but one patient, the leading primary symptom was an attack of acute, severe upper abdominal pain; 2) no association to pancreatic fistula prior to PPH was detected in any case; 3) an alteration of abdominal drainage fluids being serous first and becoming sanguineous in the further course and/or hematemesis or melena occurred after a characteristic delay of at least 5 hours after the occurrence of pain; and 4) in none of these patients were complex vascular irregularities found angiographically or during relaparotomy.
The likelihood of devastating outcome due to severe vascular abnormalities increased in patients with late extraluminal as well as intraluminal PPH after the fifth postoperative day (n = 17). Endoscopic intervention failed to achieve bleeding control in any of these patients (Table 2).
Pancreatic Fistula
In the subset of the 87 patients with PPH, 34 (39%,) had proven fistula prior to PPH, while in the entire cohort of 1669 cases the overall pancreatic fistula rate was 9% (n = 185, P < 0.001). In 53 patients (61%), fistula did not precede PPH. In the latter subset, 9 patients developed pancreatic fistula subsequent to PPH due to a disruption of the pancreaticoenteric anastomosis. The detailed analysis of patient distribution revealed a significantly lower prevalence of fistula-associated PPH in patients with chronic pancreatitis (3 of 40; 7.5%) compared with those who had other pathologies (31 of 47; 66%; P < 0.001). PPH associated with preceding pancreatic fistula (n = 34) was lethal in 13 patients (38%). In contrast, in the subgroup of 44 patients without pancreatic fistula, only one died due to PPH (P < 0.001).
Sentinel Bleed
The overall prevalence of “sentinel” bleed was 33% (n = 29). Relaparotomy rate associated with “sentinel” bleed was 83% (24 of 29). Risk stratification according of whether patients with “sentinel” PPH additionally had pancreatic fistula (n = 20) or not showed that mortality in patients with sentinel bleed and concomitant fistula accounted for 57% (8 among 14 patients with lethal course).
Interventional Angiography
In 34 of 43 patients who underwent angiography, the bleeding site could be localized. However, in 14 of these patients, interventional coiling was not feasible due to the direct vicinity of the bleeding source to the hepatic artery or the superior mesenteric artery. Further analysis showed that none of those 9 instances with negative angiographic findings had bleeding episodes in their previous course, rendering a temporary cessation of a minor primary sentinel bleed in these patients rather unlikely. Overall, interventional coiling was performed in 25 patients (Table 2). Among these, in 20 patients, the bleeding site had been angiographically visualized. In another 5 patients with chronic pancreatitis, “blind” coiling was performed despite the lack of definitive angiographic proof of the bleeding site because PPH was clinically suspected to originate from either the gastroduodenal artery or the first 2 branches of the SMA. In 17 patients with extraluminal PPH evidenced via abdominal drains, the bleeding source was found to originate either from the resection cavity after DPPHR or from the transsection surface after Whipple resection and PPPD, respectively. Overall, in 8 of these patients, “false” extraluminal PPH due to a disruption of the pancreaticojejunal anastomosis could be successfully treated by interventional coiling, whereas 8 patients underwent relaparotomy (Tables 1, 2). Repetitive angiography was performed in 7 patients with hemodynamic stability at the time of investigation and transfusion requirement ≤4 units/24 hours. Overall, the success rate of radiologic coiling in terms of definitive hemostasis was 80% (n = 20), including 3 patients in whom reangiography was successful (Fig. 2). Among 17 patients with erosive (“complex”) arterial pathologies, 4 could be successfully treated by angiography (Table 2). Interestingly, in patients without definitive angiographic visualization in which, based on the surgeons suspicion, “blind” coiling of either superior or inferior pancreaticoduodenal branches or the gastroduodenal artery itself was performed, interventional angiography was successful in 4 among 5 patients, whereas only 1 patient required relaparotomy (Table 2).
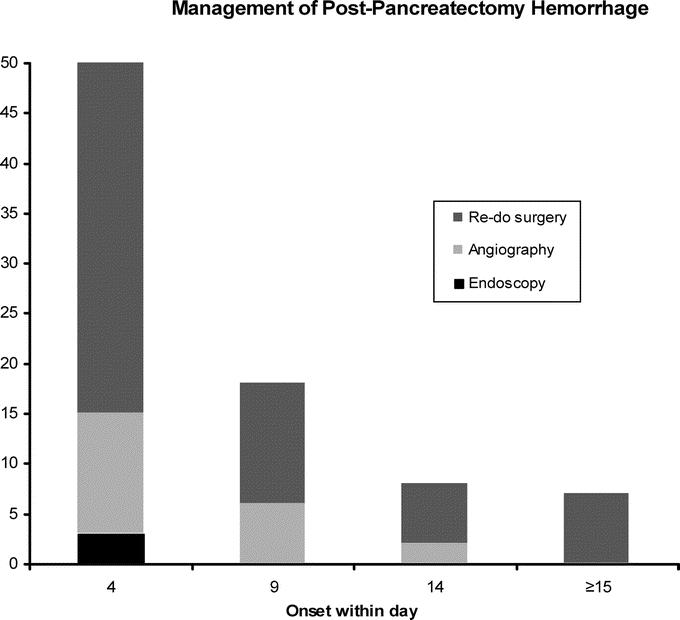
FIGURE 2. Definitive treatment of postpancreatectomy hemorrhage. Endoscopy successfully achieved bleeding control in only 3 patients with intraluminal PPH within the first and second postoperative day after the index operation. Afterward, even in case of intraluminal bleeding site, patients had either to undergo interventional angiography or relaparotomy.
The following bleeding sites were identified angiographically: hepatic artery (n = 4), stump or branches of the gastroduodenal artery or superior pancreaticoduodenal artery (n = 12), proximal branches of the SMA, ie, inferior pancreaticoduodenal artery (n = 14), and branches of the splenic artery (n = 4).
After successful bleeding control by interventional coiling, 3 patients were subjected to CT-guided interventional placement of additional drains close to the pancreaticojejunostomy due to considerable fluid collections not reached by abdominal drains. Analysis of fluid secretion in these 3 patients confirmed significantly elevated pancreatic enzymes with amylase activity ranging from 4 to 8 times the serum activity.
Uncomplicated/Complex Arterial Pathologies
In case of arterial bleeding, PPH was classified according to radiologic or intraoperative features either as “uncomplicated” (n = 63) or “complex” (n = 17) depending on the presence or absence of arterial erosions or pseudoaneurysms. PPH due to complex vascular pathologies differed considerably from uncomplicated bleedings. First, they occurred only in patients who had pancreatic fistula first. Second, they were observed only in patients who did not suffer from chronic pancreatitis, whereas no patient with chronic pancreatitis experienced complex arterial erosions or pseudoaneurysms irrespective from the kind of the index operation (duodenum-preserving procedures versus pylorus-preserving or classic Whipple resection). Last, fistula-associated complex arterial bleedings consistently occurred in a delayed fashion (late onset PPH) with a peak at the 9th postoperative day (range, sixth to 32nd postoperative day). Conversely, in patients with pancreatic carcinoma without pancreatic fistula and in those with chronic pancreatitis, uncomplicated arterial bleedings peaked at the fourth postoperative day (range, first to 26th postoperative day).
Interventional Endoscopy
Because of intraluminal PPH evidenced by apparent blood loss through the nasogastric tube, 15 patients underwent endoscopy between the first to 15th postoperative day. In 3 patients (20%), all of which experienced PPH within the first 48 postoperative hours, endoscopy succeeded in localizing and interventionally treating bleeding at the gastroenteric or first enteroenteric anastomosis (Table 2). In the remainder of 12 patients with PPH after 48 hours, intraluminal bleeding was located beyond endoscopic accessibility, eg, the pancreaticojejunostomy or the second (distal) enteroenteric anastomosis (Fig. 2). These patients underwent either interventional angiography (n = 8, successful in 2 patients) or relaparotomy (n = 10, Table 2).
Relaparotomy
Ten of 33 patients undergoing relaparotomy as first-line treatment of PPH not only had pancreatic fistula but also an episode of “sentinel” PPH in their previous postoperative course. Among the latter, 8 patients died due to disastrous vascular pathologies.
Relaparotomy, which was performed, overall, in 60 patients either as first-line procedure or as rescue treatment after failure of interventional endoscopy or radiology (Fig. 2), revealed the following bleeding sites: hepatic and gastroduodenal arteries (n = 15), splenic artery (n = 3), branches of the superior mesenteric artery (n = 16), tributaries of the portal venous axis (n = 2), pancreatic resection surface or suture line of the pancreaticoenteric anastomosis (n = 8), other anastomosis (n = 3), and other bleeding sites, eg, the retroperitoneal space (n = 13). Relaparotomy due to technical infeasibility of interventional coiling consistently confirmed bleeding sources identified in preceding diagnostic angiography (Table 2). Only 1 patient underwent relaparotomy, although bleeding control had been definitively achieved by interventional angiography. This patient who had a huge fluid collection in CT scans due to an insufficiency of the pancreaticojejunostomy underwent oversewing of the anastomosis and replacement of dislocated drains.
Thirteen of 17 patients with complex vascular pathologies due to pancreatic fistula underwent relaparotomy encompassing the following procedures: 1) completion pancreatectomy with vascular reconstruction of the hepatic artery (n = 3) and the superior mesenteric artery (n = 1), 2) completion pancreatectomy with suture ligating the bleeding source (n = 2), and 3) suture ligating the bleeding source with external drainage of pancreatic fistulas with (n = 3) or without (n = 4), repeated, planned peritoneal lavage.3
All patients with late PPH after the fifth postoperative day (n = 17) had either pancreatic fistula or even an insufficiency of the pancreaticoenteric anastomosis.
Overall Outcome
Overall mortality based on the analysis of 1669 pancreatic resections, including the 14 patients who died due to PPH accounted for 3% (n = 50). Stratifying patients for presence or absence of PPH resulted in adjusted mortality of 2.3% for patients without PPH (36 of 1582). PPH was associated with a mortality of 16% (14 of 87; P < 0.0001, χ2 test), while it could be successfully treated in 73 patients (84%). Ten of 14 patients with lethal outcome suffered from pancreatic carcinoma, while the remainder had borderline pathologies (eg, IPMN, cystadenoma) and focal pancreatitis. No patient with typical chronic pancreatitis involving the entire gland died due to PPH (Table 3). Twelve of 14 patients with lethal course had complex vascular irregularities subsequent to pancreatic fistula, ie, pseudoaneurysms and arterial erosions. Thirteen of 14 patients had pancreatic fistula. Initial bleeding severity was severe only in 6 patients, while 8 had mild PPH. All patients who eventually died had delayed PPH occurring earliest at the sixth postoperative day (Table 2), hereby resulting in a mortality in this subset of patients (n = 30) of even 46.7%. Reasons of lethal outcome were as follows: diffuse peritonitis (n = 7); hepatic failure (n = 3) in patients with complex vascular erosions or pseudoaneurysms of the hepatic artery and the celiac trunk; recurrent, surgically intractable PPH episodes (n = 2); pulmonary embolism (n = 1); and fungal sepsis (n = 1).
TABLE 3. Influence of the Kind of Index Operation, Underlying Diseases, Pancreatic Fistula, and Vascular Pathologies on Outcome of PPH
DISCUSSION
Despite a reported prevalence of 5% to 12%,12–14 PPH remains a diagnostic and therapeutic black box. Standardized rules as to its management do not exist. Because of the diversity of bleeding types, PPH-associated outcome and mortality are unknown.6,9,15–17 Substantial differences include the onset and intensity of PPH, underlying diseases, kind of index operations, concomitant pancreatic fistula, extraluminal or intraluminal manifestation of bleeding, presence or absence of a “sentinel” bleed, and vascular irregularities, ie, devastating arterial erosions and pseudoaneurysms. With the aim to produce diagnostic and therapeutical algorithms for management of PPH, we retrospectively analyzed a prospective data base in which more than 1500 patients operated on for pancreatic pathologies have been included from 1992 on. In particular, we sought to identify risk “profiles” according to the following criteria: 1) early versus late onset of PPH; 2) presence or absence of concomitant pancreatic fistula; 3) underlying disease (pancreatic malignancy and borderline pathologies with “weak” pancreatic remnant texture versus chronic pancreatitis with “fibrotic” texture; and 4) kind of index operation. Taking these variables into account, success rates of interventional endoscopic and radiologic procedures were evaluated.
The distinction of “early” and “late” PPH has an important, if not even crucial impact on therapeutical management. Regardless of its intraluminal or extraluminal manifestation, early PPH is in most series reported to have much better prognosis than does ominous late PPH.12,18 Based on institutional experiences, Choi et al18 and Tien et al7 suggested setting the cutoff for differentiating early and late PPH at the fifth and seventh postoperative day, respectively.
Early PPH in the presented series was due to 3 reasons: 1) technical failures in terms of inadequate hemostasis in the operative field always associated with extraluminal PPH; 2) suture line of gastroenteric or one of the enteroenteric anastomosis leading uniformly to intraluminal PPH at the first or second postoperative day; and 3) resection cavity (chronic pancreatitis) or transsection surface (Whipple resection) of the pancreas resulting in PPH originating from the pancreatico-enteral anastomosis. The latter bleeding origin was of particular interest because in only half (n = 17) of these patients it led, as would be expected, to intraluminal PPH with hematemesis or melena. In the other half (n = 17), the intraluminal bleeding site became clinically apparent by extraluminal PPH, which resulted from a bursting of the pancreaticojejunostomy. Therefore, because it only mimicked extraluminal PPH, we entitled this subset of bleedings “false” extraluminal PPH. Growing experience with these patients has meanwhile prompted us to change our institutional policy for management of early bleeding designating patients in whom “false” extraluminal PPH is assumed to interventional radiology first, whereas emergency relaparotomy is restricted as a rescue procedure to patients in whom angiography fails or is technically not feasible.
A key finding of the presented analysis was that the likelihood of devastating and in 14 patients lethal outcome increased the later PPH occurred. Since lethal courses were only observed in delayed PPH, “overall” mortality of 16% in the entire cohort (n = 87) of patients with PPH is in some respects misleading. When only those 30 patients with delayed PPH were considered, its mortality was even 47%.
The core difference between early and delayed PPH after the fifth postoperative day was the high coincidence of delayed PPH with preceding pancreatic fistula. This finding is consistent with the surgical literature reporting significantly elevated risk of delayed PPH in patients with pancreatic fistula18 as well as a near 100% prevalence of preliminary pancreatic fistula in patients who exhibit delayed arterial bleeding.12 Some surgical series suggest a sequel of events at the beginning of which pancreatic fistula causes erosions, pseudoaneurysms, and other vascular irregularities, which eventually result in disastrous bleeding.12,18,19
However, why, overall, only a paucity of patients with pancreatic fistula develops PPH remains enigmatic. Reasons for the discrepant prevalence of pancreatic fistula, which is still the most frequent specific postoperative comorbidity and fistula-related PPH occurring with much lower likelihood, have not been clearly identified so far. Extended lymphadenectomy in the course of oncologic resections, exocrine competency indicated by soft texture of the pancreatic remnant producing highly aggressive pancreatic juice, or insufficient drainage of pancreatic fistula, respectively, may be cofactors increasing the risk of fistula-induced vascular damage with consecutive bleeding. Regarding the prediction of postoperative pancreatic juice volume, assessment of preoperative exocrine function may be helpful in identifying such “at-risk” patients, as suggested in one study.12
Also, the data reported here seem to confirm that the likelihood of pancreatic fistula depends on the underlying disease. It is likely that pancreatic carcinoma patients with “competent” pancreatic remnant as to its excretory function produce more pancreatic juice with higher erosive potential than do chronic pancreatitis patients with excretory insufficiency. Indeed, clinical experience seems to support the assumption that patients with oncological indications are more “at risk” to develop fistula-associated PPH compared with chronic pancreatitis patients. In the Johns Hopkins experience based on a retrospective analysis of 1891 patients undergoing pancreaticoduodenectomy, a soft texture of the pancreatic remnant was associated with a 20-fold increase in fistula risk over patients with a medium or firm gland.20 Several other studies confirmed the crucial impact of parenchymal texture on fistula formation.12,21–23 In the present series, the prevalence of fistula-related PPH in patients with pancreatic pathologies usually associated with a “physiological,” soft texture of the pancreatic remnant (carcinoma, NET, borderline tumors, etc.) was significantly higher (66%) than in patients with a firm glandular texture due to chronic pancreatitis (7.5%). That these few (n = 3) chronic pancreatitis patients had uniformly undergone duodenum-preserving pancreatic resections, while none of chronic pancreatitis patients subjected to “oncologic” resections, ie, Whipple operation or PPPD, had fistula-related delayed PPH suggested that its occurrence was associated with the underlying disease rather than with the kind of index operation. Further evidence that a soft texture of the pancreatic remnant represents an important risk factor for delayed PPH is provided by our observation that devastating (“complex”) arterial erosions and pseudoaneurysms, which were the predominant cause for lethal outcome were never found in chronic pancreatitis patients but only occurred in patients operated on for other reasons.
Last, fistula-related delayed PPH was closely related to “sentinel” bleed defined as insignificant amounts of blood loss. Minor “sentinel” bleed may herald consecutive devastating arterial bleeding originating from pseudoaneurysms or vascular erosions.12 Its reported prevalence ranges from 30% to 100%.7,12,15,24 It has been emphasized that its timely diagnosis may be essential to prevent fatal outcome,11 which is confirmed by the presented data. Not only that the great majority of patients (83%) with sentinel bleed eventually required relaparotomy, but its presence considerably worsened prognosis of delayed PPH with an increase of mortality from 38% associated with fistula-related PPH to 57% in patients who additionally had sentinel bleed.
CONCLUSION
We suggest a therapeutic algorithm, which is depicted in the figure, and encompasses endoscopy, angiography, and surgery for interventionally therapy for PPH. In particular, a customized decision-making should consider the following aspects:
Prognosis of PPH is closely associated with preceding pancreatic fistula. Therefore, devastating in some patients, lethal courses occur predominantly in late-onset PPH after the sixth postoperative day, while early PPH until the fifth postoperative day carries a good prognosis.
Management of early PPH within the first 5 days following the index operation depends on whether bleeding is located intraluminally or extraluminally. “True” extraluminal PPH is mostly due to insufficient hemostasis, occurs within 24 to 48 hours postoperatively, and requires immediate relaparotomy without diagnostic delay. “False” extraluminal PPH resulting from disruption of the pancreaticoenteric anastomosis with subsequent evidence of bleeding via abdominal drains has a reasonable chance to be treated by interventional angiography.
Interventional endoscopy is only indicated within 2 to 3 days after primary surgery when intraluminal PPH is suspected to originate from the gastroenteric or enteroenteric anastomosis. Intraluminal PPH occurring in the later postoperative course is unlikely to originate from anastomotic lesions, except for the pancreaticoenteric anastomosis and hence require either interventional angiography or relaparotomy.
Interventional angiography is indicated for “false” extraluminal PPH within the first 5 days following the index operation after exclusion of gastroenteric and enteroenteric anastomotic bleeding sites by endoscopy. In case of late PPH usually associated with pancreatic fistula formation, angiography as first-line intervention should be performed irrespective of the intraluminal or extraluminal site of bleeding.
In case of nonvisualizable PPH during index angiography, reangiography may be performed within 6 to 24 hours when patients remain hemodynamically stable with blood requirement ≤4 units. “Blind” coiling of branches of the gastroduodenal and superior mesenteric arteries may provide bleeding control after duodenum-preserving pancreatic head resections for chronic pancreatitis.
The coincidence of sentinel bleed prior to PPH and pancreatic fistula is associated with a mortality of >50%. Angiographic evaluation in these patients should be performed with the awareness of this specific risk. Therefore, when interventional angiography fails to visualize the bleeding source or when coiling is technically not feasible, relaparotomy is usually mandatory, even in case of temporary cessation of PPH.
Footnotes
Reprints: Emre F. Yekebas, MD, Department of Surgery, University Hospital Eppendorf, University of Hamburg, Martinistrasse 52, 20251 Hamburg, Germany. E-mail: yekebas@uke.uni-hamburg.de.
REFERENCES
- 1.Izbicki JR, Bloechle C, Knoefel WT, et al. Surgical treatment of chronic pancreatitis and quality of life after operation. Surg Clin North Am. 1999;79:913–944. [DOI] [PubMed] [Google Scholar]
- 2.Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–435; discussion 435–438. [DOI] [PMC free article] [PubMed]
- 3.Buchler MW, Wagner M, Schmied BM, et al. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310–1314; discussion 1315. [DOI] [PubMed]
- 4.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257; discussion 257–260.4 [DOI] [PMC free article] [PubMed]
- 5.van Berge Henegouwen MI, Allema JH, van Gulik TM, et al. Delayed massive haemorrhage after pancreatic and biliary surgery. Br J Surg. 1995;82:1527–1531. [DOI] [PubMed] [Google Scholar]
- 6.Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tien YW, Lee PH, Yang CY, et al. Risk factors of massive bleeding related to pancreatic leak after pancreaticoduodenectomy. J Am Coll Surg. 2005;201:554–559. [DOI] [PubMed] [Google Scholar]
- 8.Miedema BW, Sarr MG, van Heerden JA, et al. Complications following pancreaticoduodenectomy: current management. Arch Surg. 1992;127:945–949; discussion 949–950. [DOI] [PubMed]
- 9.de Castro SM, Kuhlmann KF, Busch OR, et al. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg. 2005;241:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veit J, Wente MN, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery definition (ISGPS). Surgery. In press. [DOI] [PubMed]
- 11.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 12.Sato N, Yamaguchi K, Shimizu S, et al. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: the importance of early angiography. Arch Surg. 1998;133:1099–1102. [DOI] [PubMed] [Google Scholar]
- 13.Balladur P, Christophe M, Tiret E, et al. Bleeding of the pancreatic stump following pancreatoduodenectomy for cancer. Hepatogastroenterology. 1996;43:268–270. [PubMed] [Google Scholar]
- 14.Halloran CM, Ghaneh P, Bosonnet L, et al. Complications of pancreatic cancer resection. Dig Surg. 2002;19:138–146. [DOI] [PubMed] [Google Scholar]
- 15.Shankar S, Russell RC. Haemorrhage in pancreatic disease. Br J Surg. 1989;76:863–866. [DOI] [PubMed] [Google Scholar]
- 16.Rumstadt B, Schwab M, Korth P, et al. Hemorrhage after pancreatoduodenectomy. Ann Surg. 1998;227:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reber PU, Baer HU, Patel AG, et al. Life-threatening upper gastrointestinal tract bleeding caused by ruptured extrahepatic pseudoaneurysm after pancreatoduodenectomy. Surgery. 1998;124:114–115. [PubMed] [Google Scholar]
- 18.Choi SH, Moon HJ, Heo JS, et al. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg. 2004;199:186–191. [DOI] [PubMed] [Google Scholar]
- 19.Munoz-Bongrand N, Sauvanet A, Denys A, et al. Conservative management of pancreatic fistula after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg. 2004;199:198–203. [DOI] [PubMed] [Google Scholar]
- 20.Lin JW, Cameron JL, Yeo CJ, et al. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951–959. [DOI] [PubMed] [Google Scholar]
- 21.Popiela T, Kedra B, Sierzega M, et al. Risk factors of pancreatic fistula following pancreaticoduodenectomy for periampullary cancer. Hepatogastroenterology. 2004;51:1484–1488. [PubMed] [Google Scholar]
- 22.Muscari F, Suc B, Kirzin S, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery. 2006;139:591–598. [DOI] [PubMed] [Google Scholar]
- 23.Yang YM, Tian XD, Zhuang Y, et al. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodsky JT, Turnbull AD. Arterial hemorrhage after pancreatoduodenectomy: the ‘sentinel bleed. ’ Arch Surg. 1991;126:1037–1040. [DOI] [PubMed] [Google Scholar]