Abstract
Objective:
To examine the outcome of technical variant liver transplant techniques relative to whole organ liver transplantation in pediatric liver transplant recipients.
Background:
Technical variant liver transplant techniques comprising split, reduced, and live-donor liver transplantation evolved to address the need for timely and size appropriate grafts for pediatric recipients.
Methods:
Analysis of data from the Studies of Pediatric Liver Transplantation (SPLIT) registry, a multicenter database of 44 North American pediatric liver transplant programs. The outcome (morbidity and mortality) of each of the technical variants were compared with that of whole organ recipients.
Results:
Data were available on 2192 transplant recipients (1183 whole, 261 split, 388 reduced, and 360 live donor). Recipients of all technical variant graft type were significantly younger than whole organ recipients, but on average spent 2.3 months less on the waiting list. Thirty-day post-transplant morbidity was increased for each type of technical variant relative to whole organ (45.1% whole, 66.7% split, 65.5% reduced, 51.9% live-donor). Biliary complications (30 day: 7.5% whole, 18.8% split, 16% reduced, 17.5% live-donor) and portal vein thrombosis (30 day: 3.6% whole, 8% split, 8% reduced, 7.5% live-donor) were more common in all technical variant types. Graft type was an independent predictor of graft loss (death or retransplantation) in a multivariate analysis. Split and reduced (relative risk = 1.74 and 1.77, respectively) grafts had a worse outcome when compared with whole organ recipients.
Conclusions:
Technical variant techniques expand the pediatric donor pool and reduce time from listing to transplant, but they are associated with increased morbidity and mortality.
Technical variant liver transplantation comprising reduced, split, and live-donor grafts evolved to address the need for size appropriate organs for pediatric liver recipients. This study will examine the outcome and morbidity of the technical variant techniques relative to whole organ transplants in the Studies of Pediatric Liver Transplantation (SPLIT) database.
Liver transplantation is considered to be an accepted treatment method for children and adults with end-stage liver disease, with survival rates approaching 90%.1 However, the demand for livers is far greater than the supply, and technical variant transplant techniques have been developed to increase the availability of organs for transplantation. These techniques are based on the principle that a partial liver graft with appropriate arterial and portal inflow, the corresponding venous and biliary drainage, and sufficient hepatocyte mass is able to fulfill the role of a whole organ.2 There are 3 technical variant techniques, namely, reduced,3 split,4 and live-donor.5,6 Reduced grafts arise when a graft is “cut-down” based on segmental anatomy, and the segments not transplanted are discarded. Since there is only 1 recipient, there is no need to share portal structures, thus maximizing the length of conduit for biliary and vascular anastomosis. Although the reduced technique expands the pediatric donor pool, this is to the detriment of adult recipients.7 Consequently, split techniques evolved, whereby a liver is divided such that it can be transplanted into 2 recipients. Live-donor techniques were a direct extension of split liver transplantation, whereby a partial liver graft from a live-donor is transplanted.
Studies of Pediatric Liver Transplantation (SPLIT) was established in 1995, with the primary objective to characterize and follow trends in patient and graft survival, rejection, growth, and immunosuppression to identify the potential morbidity and mortality factors and patterns of graft failure. Herein, we present the surgical outcomes from the SPLIT database with particular attention to variations in outcome by type of graft.
METHODS
We analyzed prospectively collected data by SPLIT since 1995. At the time of data closure for this report, June 1, 2006, 44 centers in the United States and Canada had registered 3161 patients, with 2445 of them receiving at least 1 transplant (0 to 212 patients per center). Participation in the SPLIT registry was approved by each center's institutional review board, and all parents or legal guardians provided written informed consent; subject assent was also obtained when appropriate.
Enrolled patients are followed every 6 months for 2 years and then yearly from the time of listing to transplant. After transplant, enrollees are followed every 6-months for 2 years and then yearly thereafter until the 18th birthday. Data captured include demographics, primary diagnosis, pretransplant conditions and morbidity, surgical data, perioperative and long-term morbidity such as lymphoproliferative disease, rejection, retransplantation, and death.
Data Analysis
We analyzed data for all children who received their first liver transplant between the inception of SPLIT in 1995 and June 1, 2006. Children were excluded if they received any grafts in addition to the liver. Univariate statistical analyses included χ2 or Fisher exact test to make comparisons between categorical variables, Wilcoxon rank sum test for comparison of means, and log-rank test for comparison of time to event outcomes. A P value of 0.05 was considered to be statistically significant and values between 0.05 and 0.1 a trend. All statistical analyses were performed using the SAS System for Windows, version 8.02 (SAS Institute, Cary, NC.).
Statistical Analyses
Impact of Graft Type
Subjects were grouped according to graft type (whole, split, reduced, live-donor) for the primary analyses regarding morbidity. In all cases, each group of patients who received a technical variant graft (split, reduced, live-donor) was individually compared with those who received a whole organ. Morbidity at 30 days was assessed for all patients who underwent transplantation. However, to obtain estimates of long-term morbidity (at 24 months), data were only analyzed for those who survived to that point and had undergone a 24-month follow-up visit. Causes of graft loss (death or retransplantation) were also tabulated. Kaplan-Meier curves were generated to examine the impact of graft type on graft and patient survival, as well as time to first episode of rejection.
Predictors of Graft Loss
To assess the impact of graft type on outcome when adjusting for other significant predictors, univariate analyses using a Cox proportional hazards model were performed to examine factors predictive of a composite outcome of graft loss, which was defined as either death or retransplantation. Both donor and recipient factors were considered for inclusion in the model, as was the type of immunosuppressant used and use of polyclonal antibodies. To account for changes in the field of liver transplantation over time, year of transplant was also considered in the analyses.
For the development of the multivariate model to predict post-transplant survival, factors significant at P ≤ 0.20 in the univariate analyses were initially included. Model reduction was performed using the backward elimination variable selection method. Factors remaining significant at P ≤ 0.05 were maintained in the final model. To control for center-specific effects, we performed 2 analyses. In the frailty method, each center was assumed to have a random effect that indicates the possibility of different baseline risks for patients at different centers. The robust variance method was also used to adjust for the possible correlations for the transplants at the same center using a robust “sandwich” variance approach.
RESULTS
During the index time period, 2291 children received their first liver transplant. Data on graft type was unavailable for 99 (4.3%) children and these were excluded; thus, the study population comprised 2192 children. One thousand eight hundred thirty-two liver (1832) transplants were performed using deceased donors (1183 whole organ, 261 split, and 388 reduced). Three hundred and sixty (360) children received grafts from live-donors.
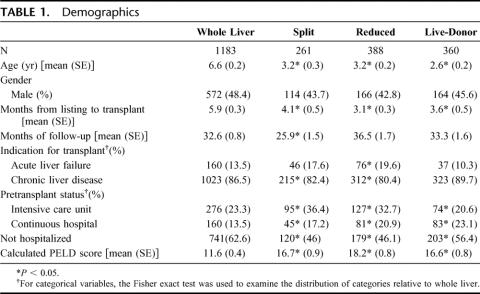
Demographic data are presented in Table 1. Those who received a split or reduced graft were more likely to be transplanted for acute liver failure. Acuity of disease as evidenced by the distribution of the intensity of care received, or the mean calculated pediatric end-stage liver disease score per group was greater in all of the technical variant groups relative to whole organ. Also, younger children were more likely to receive a technical variant graft. Only 33.9% (260 of 766) of patients 1-year-old or younger received a whole organ compared with 49.1% (358/729) of patients aged 1–5 years, 65.3% (309 of 473) aged 5–12, and 79.4% (255 of 321) of those 12 years or older. All technical variant graft types spent less time on the waiting list relative to whole organ recipients [5.9 months whole vs. 4.1 month split (P = 0.003), 3.1 month reduced (P < 0.001), 3.6 months live-donor (P = 0.0002)].
TABLE 1. Demographics
Operative Details
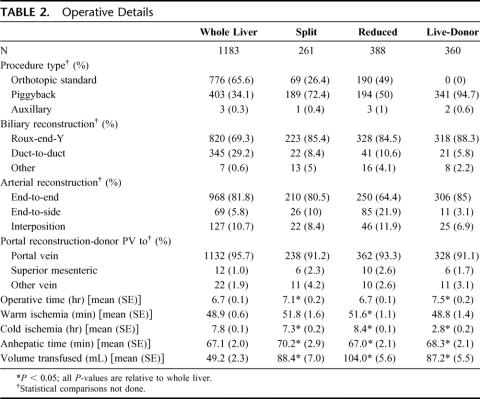
Details of the operative techniques employed are presented in Table 2. Roux-en-Y choledochojejunostomy was the predominant type of biliary reconstruction in all patients, although it was more frequently used in those undergoing a technical variant transplant. Twenty nine percent of patients who underwent whole organ transplantation had a duct-to-duct anastomosis, whereas the range for the technical variant types was between 5.8% and 10.6%. Although the operating time for split and live-donor and transplants were statistically longer than for whole organ (7.1 and 7.5 hours vs. 6.7 hours, respectively), the difference was small and not of clinical significance. Similarly, there were small but statistically significant differences in ischemic and anhepatic times between the technical variants and whole organ transplants. As would be expected, those who underwent a live-donor transplant had a significantly shorter cold-ischemia time (2.8 hours vs. 7.8 hours for whole organ, P < 0.001). The average amount of blood per kilogram body weight required for transplant recipients of whole donor organs was significantly lower than required for each of the technical variant types.
TABLE 2. Operative Details
Morbidity Within 30 Days of Transplant
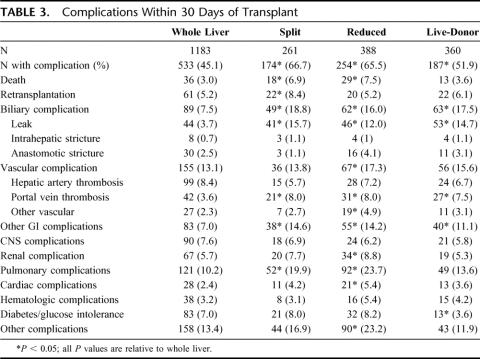
Data on morbidity within the first 30 days are presented in Table 3. Those receiving one of the technical variant grafts were more likely to develop complications within 30 days of transplant [66.7% (P < 0.001) split, 65.5% (P < 0.001) reduced, 51.9% (P = 0.01) live-donor] than those who received a whole organ graft (45.1%). Those undergoing a technical variant procedure also were more likely to require reoperation within 30 days of transplant. 29.5% of those who received a whole-organ underwent reoperation, whereas the proportion in the technical variant groups were 47.1% (P < 0.001) split, 51.0% (P < 0.001) reduced, and 41.9% (P < 0.001) of live-donor grafts. Furthermore, 3 or more reoperations were conducted in 5.0% of those with a split organ (P = 0.2), 7.7% of the recipients receiving a reduced organ (P = 0.001), and in 8.1% of live-donor recipients (P < 0.001). Only 3.1% of the whole organ recipients were thus affected.
TABLE 3. Complications Within 30 Days of Transplant
Patients who received any of the technical variant grafts were more likely to experience biliary tract leaks. The overall incidence of vascular complications was only increased for those who received a reduced graft, whereas all technical variant graft types were associated with an increased incidence of portal vein thrombosis. The incidence of portal vein thrombosis was at least double that of whole organ for all groups. Mortality within 30 days was increased for recipients of split and reduced grafts, although only recipients of split grafts were more likely to be retransplanted during this time period. The incidence of other gastrointestinal complications was increased for the technical variant grafts, as was the incidence of pulmonary complications for all technical variant types and cardiac complications for split and reduced recipients.
Patients who received a graft from a live-donor were significantly less likely than those who received a whole organ graft (3.6% vs. 7.0%, P = 0.007) to develop diabetes or glucose intolerance. As well, patients who received a live-donor graft were less likely, at any point following the transplant, (RR 0.82; 95% CI: 0.68–0.99; P = 0.034) to experience rejection than those who received a whole organ. Recipients of split (P = 0.217) and reduced (P = 0.326) grafts had similar incidences of rejection relative to whole organ. By 1-year, 46.6% of those who received a whole organ, 49.6% split, 44.4% reduced, and 40.5% live-donor had experienced at least 1 rejection episode.
Morbidity at 2 Years
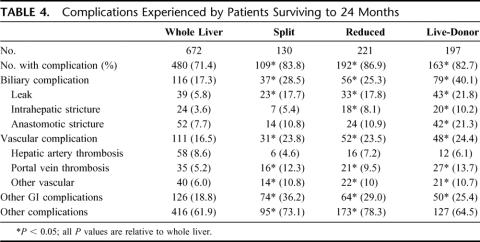
Morbidity experienced by those who survived to 24 month post-transplant is presented in Table 4. This cohort comprised 1245 patients (672 whole, 130 split, 221 reduced, and 197 live-donor). Before 24 months, 240 children died (88 whole, 35 split, 67 reduced, and 41 live-donor). The remaining 807 children had not yet reached their 24-month follow-up, or did not have data for that visit. The overall incidence of complications in the first 24-months was greater for each of the technical variant types relative to the whole organ group. The overall incidence of vascular complications was increased in recipients of technical variant grafts, primarily due to an increased risk of portal vein thrombosis in all graft types. There was also a significantly greater incidence of biliary tract complications among recipients of technical variants. All types were associated with an increased incidence of leaks; recipients of reduced and live-donor grafts had an increased incidence of intrahepatic biliary strictures [8.1% reduced (P = 0.005), 10.2 live (P < 0.001) vs. 3.6% whole organ]. Recipients of live-donor grafts also had a 2-fold increase in the incidence of anastomotic strictures. The incidence of other gastrointestinal and other nongastrointestinal complications was also increased in recipients of the technical variant types.
TABLE 4. Complications Experienced by Patients Surviving to 24 Months
Graft and Patient Survival
In univariate analyses graft and patient survival were superior in children who received a whole organ relative to those who received a technical variant graft. Kaplan-Meier curves for graft and patient survival are presented in Figures 1 and 2, respectively. All 3 technical variant techniques were associated with decreased graft survival relative to whole organ (split RR: 1.496, 95% CI: 1.107–2.022; reduced RR: 1.841, 95% CI: 1.454–2.322; live donor RR: 1.321, 95% CI: 1.007–1.732). In terms of patient survival, those who received a split (RR: 1.603; 95%CI: 1.094–2.349) or reduced graft (RR: 2.11; 95% CI: 1.571–2.835) and were less likely to survive than recipients of a whole organ. Those who underwent a live-donor transplant had similar survival relative to those who received a whole-organ (RR: 1.366; 95% CI: 0.962–1.941; P = 0.082). Indications for retransplantation and causes of death are listed in Table 5.
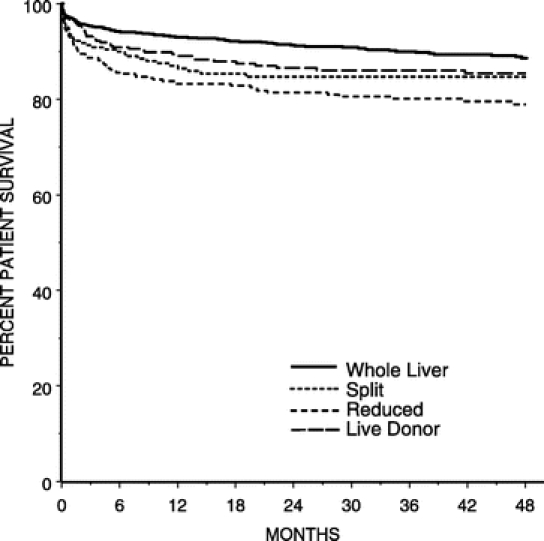
FIGURE 1. Kaplan-Meier probability of patient survival.
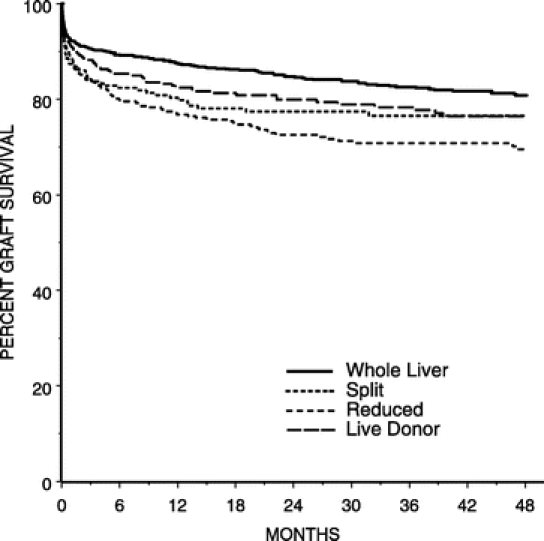
FIGURE 2. Kaplan-Meier probability of graft survival.
TABLE 5. Death and Retransplantation
Multivariate Predictors of Survival
To control for other confounders and to better understand the impact of graft type on survival, we performed a multivariate Cox proportional hazards regression. The outcome for this analysis was graft loss as defined by either death or retransplantation. The factors that were initially included the multivariate model (P < 0.2 in univariate analyses) were: primary diagnosis (P < 0.001), graft type (P < 0.001), donor age (P < 0.001), patient status at transplant (P < 0.001), year of transplant (P = 0.0005), warm ischemia time (P = 0.001), donor–recipient blood type match (P = 0.001), International Normalized Ratio (P = 0.001), bilirubin (P = 0.009), intravenous immune globulin use in the first 7 days post-transplantation (P = 0.15), initial immunosuppressant (P = 0.002), and monoclonal antibody use (P = 0.013). Although the pediatric end-stage liver disease score met criteria for inclusion in the model (P = 0.001), due to concerns of collinearity with its components of international normalized ratio and bilirubin, it was excluded from the model. Recipient age did not meet statistical criteria for inclusion in the model (P = 0.204), although a secondary analysis was performed, to ensure that its absence did not significantly alter the final multivariate model.
The final multivariate model is presented in Table 6. Graft type, primary diagnosis, donor–recipient blood type match, donor age, year of transplant, and warm ischemia time remained significant predictors of graft loss. Children who received a split (RR = 1.74) or reduced (RR = 1.54) graft were more likely suffer graft loss than those who received a whole organ. Recipients of grafts from live-donors did not have a worse outcome. In terms of diagnosis, children with fulminant liver failure as well as those with disease other than other cholestatic diseases or cirrhosis were more likely to experience graft loss than those with biliary atresia. Children undergoing transplant from a donor with a nonidentical blood type had a worse outcome, as did those transplanted before 2002. Young donors, less than 5 months of age, also had an increased risk for graft loss. Warm ischemia time, included as a continuous variable, was a predictor of graft loss, with each additional minute of ischemia resulting in a decrement in survival.
TABLE 6. Multiple Variable Predictors of Graft Loss
The analyses for center-specific effects failed to demonstrate that center was a significant confounder in explaining the differential survival of the various graft types. The differences between graft types observed using the standard analysis remained significant in the robust-variance approach to account for center variations. The variance of the center random effects in the frailty model was 0.084 (P = 0.06), which does not provide evidence for a center-specific effect in explaining our results.
DISCUSSION
Liver transplantation is considered to be the standard of care for children and adults with end stage liver disease.1 However, the promise of transplantation is limited by the availability of donor organs for transplant. Historically, this shortage was most acute for pediatric recipients because of the need for size matching of donors and recipients. To address this disparity, surgeons developed technical variant transplant techniques (split, reduced, and live-donor) for the pediatric population.2 The literature suggests no difference in transplant survival for technical variants relative to whole organ transplantation in a number of pediatric series describing the experience of high-volume centers.8–10 By contrast, reports of major cumulative series indicated improved outcome for whole organs versus the technical variant types, particularly reduced and split transplants.11 Although live-donor grafts in some series have demonstrated either equivalent or even a survival advantage over whole organ transplantation,12,13 with the possibility that this advantage may be most apparent in those less than 2-years of age.14
The present study was an analysis of the outcome of 2192 children undergoing liver transplantation at 1 of the 44 participating centers of the SPLIT research group over an 11-year period. Our univariate results demonstrated worse overall graft survival for all of the technical variant techniques and worse patient survival for those undergoing split and reduced liver transplantation with a trend to worse survival for those undergoing transplantation from a live-donor. However, on a multivariate analysis, with the composite outcome of graft loss (death or retransplantation), both split and reduced graft types had a worse outcome relative to whole organ. Recipients of organs from live-donors did not demonstrate this disadvantage. The other variables that predicted graft loss were primary diagnosis, donor–recipient blood type match, donor age, year of transplant, and warm ischemia. All of the technical variant transplant types were also associated with an increased risk of complications both at 30 days post-transplantation and in those who survived to 24 months.
It is important to put the relative poorer outcome of technical variant techniques into perspective. At 1-year, whole organs had a 93% patient survival rate, whereas the rate for reduced was 83.2%, split 87%, and live-donor 89%. Four-year survival was 89% whole, 79% reduced, 85% split, and 85% live-donor. Thus, although relative to a whole organ, reduced and split grafts have a slightly decreased probability of survival, the overall results for each of the transplant types are excellent. The slight decrement in overall survival must be balanced with the fact that almost all of these children would have died without transplantation, particularly when one considers the generally higher acuity of patients receiving technical variant grafts.
It must also be noted that using technical variant techniques significantly decrease the waiting list mortality.2,9 In this study, patients receiving a technical variant waited on average 2.3 months less than those who received a whole liver. This highlights the fact that the relative decrease in post-transplantation survival needs to be balanced with the gains in survival to transplant. Redding et al using Whitington's method to calculate an overall survival based on pretransplant and post-transplant survival,15 demonstrated a significant advantage to live-donor over deceased-donor (87% vs. 70%) transplantation primarily due to enhanced survival prior to transplant. Thus, although associated with slight decrements in post-transplantation survival, because of the excellent overall outcome and significant improvements in graft availability and survival to transplant as a result of the technical variant techniques, the net effect of such techniques is of significant benefit to the pediatric patient with liver failure.
Despite the excellent overall results, it is still important to note and understand the increased morbidity of technical variant procedures with the ultimate goal of developing strategies to overcome these challenges. The perioperative morbidity after pediatric liver transplantation typically includes biliary, portal, and arterial complications, followed by intestinal perforation and bleeding.16 Biliary complications in our series included bile leaks and anastomotic strictures. All 3 technical variant types had an increased incidence of bile leak at 30-days ranging from 12.0% for recipients of reduced grafts to 15.7% for split recipients. The corresponding incidence in recipients of whole organ grafts was 3.7%. Recipients of both reduced and live-donor grafts had an increased incidence of stricture at 24-months post-transplantation. Multiple hepatic ducts and damage to the delicate peribiliary vasculature are said to increase the complication rate. Technical maneuvers such as stentless microsurgical biliary reconstruction and enteric biliary drainage are known to significantly lower the incidence of anastomotic strictures and bile leaks.17,18 Given the increased incidence of biliary complications among recipients of technical variant grafts, we believe that potential improvement in the biliary morbidity can be expected by future microsurgical advances. The importance of these advances is highlighted by the outcome at 24-months post-transplantation. At this time point, over 20% of recipients of reduced grafts and 30% of live-donor recipients had biliary tract strictures (intrahepatic or anastomotic). These numbers must be interpreted in the context that all of the patients who were included in these analyses had survived to 24 months, and thus they are successes that we strive for, and a significant long-term biliary complication in more than 1 in 5 is concerning. Clearly, there is yet much that needs to be done to improve the outcome for these patients.
The spectrum of vascular complications after pediatric liver transplant is related to both the venous and arterial supply of the graft. Reconstructions of the portal venous supply in the pediatric population varies from slight portal vein mismatches in size-matched donor to significant discrepancies in diameter and decreased length in live-donor grafts.19 More reconstructions can be successfully performed using interposition grafts; however, these are associated with a significant increase in complications.20,21 Such reconstructions are more frequently employed in technical variant liver transplantation. We considered type of biliary and vascular reconstruction in our univariate analyses of graft loss; however, these did not achieve statistical significance for consideration in the multivariate model.
The incidence of hepatic artery thrombosis has been reported to be as high as 26% and is among the major causes for graft loss.22,23 Very young age and small body mass are probably the most significant risk factors, although these factors can be overcome with microvascular techniques.24,25 In this series, none of the technical variant techniques were associated with an increased risk of hepatic artery thrombosis, and at 30 days, the overall incidence of this complication was 7.5%. Although the nature of our data does not permit one to address the reasons for the low incidence of hepatic artery thrombosis, one can speculate that this may be due to an increase in the use of microvascular techniques for arterial reconstruction.
Portal venous thrombosis was a more common complication among recipients of all technical variant grafts both at 30 days and 24 months post-transplantation. The increased risk of portal venous thrombosis among live-donor recipients was previously identified by Millis et al.20 However, we believe that in the same way that microvascular techniques have resulted in a reduction in hepatic arterial complications, such techniques may play a role in future advances in portal reconstruction.
It is unclear why children who received technical variant grafts had an increased risk of cardiac, pulmonary, and respiratory complications, but it is important to note that patients tended to be younger and sicker at the time of transplant. Although the differences in pretransplant clinical status may explain these discrepancies in outcome, graft type was an independent predictor of graft loss on multivariate analysis, which controlled for baseline acuity. Therefore, one cannot fully account for the increased risk of morbidity and mortality following a technical variant procedure on the basis of differences in pretransplant acuity. A recent study found that the perioperative complications were independent risk factors for survival.26 Thus, the detrimental impact of graft type on survival may be related to the increased incidence of immediate perioperative complications experienced by recipients of technical variant grafts. Thus, surgical innovations, such as increased use of microsurgical reconstructive techniques, not only have the potential to improve immediate perioperative outcome but also survival.
Technical variant graft types were associated with an increased need for blood transfusion. Although speculative, it is intriguing to consider that the increased risk of systemic complications around the time of transplant may be partially related to this. Perioperative transfusions are associated with an increased risk of infection and systemic complications in adult patients27 and previous reports have demonstrated that a high blood loss correlates with poor patient outcome after liver transplantation.28 A recent report demonstrated that perioperative normovolemic anemia, with a target hemoglobin concentration between 8 and 9 g/dL, was safe in pediatric live-donor transplants.29 Such a strategy warrants further consideration in a larger trial, and this may be achievable via a multicenter network such as the SPLIT research group.
Age was not an independent predictor for graft loss on univariate analyses, and was not included in the multivariate analysis. This is in contrast to a previous report on a large series of pediatric liver transplant recipients.26 In secondary analyses, we included age for consideration in the multivariate model, as a multicategory variable, continuous variable, and dichotomous variable (< or >1 year). However, age never achieved statistical significance for inclusion in our final multivariate model. Age is closely associated with graft type as well as primary disease, both of which were included in the final model. Therefore, it is possible that age did not meet inclusion in the multivariate model because of its tight relationship to these variables. However, an alternate interpretation that may be drawn from these data is that the notion that younger children have a worse outcome has to do with the fact that younger children were more likely to receive a technical variant graft. It may not have been possible, given the small number of patients with each type of technical variant graft in the previous report,26 to statistically examine the impact of graft type. Thus, age was viewed to be the important predictor rather than graft type. A recipient's age, size, and acuity are the primary features that guide the choice of graft. Although it would be optimal to transplant only whole livers and minimize complications, the unfortunate current limited availability of organs for transplantation precludes this possibility.
This analysis based on data from a registry has a number of limitations and biases, both known and unknown. First, participation in SPLIT is voluntary, and therefore may be subject to bias. Second, it is not possible to perform 100% verification of accuracy and completeness of data submitted to the data-coordinating center. Site visits are conducted every 2 years. Third, given that centers with a wide range of experience contribute data to SPLIT, discrepancies between the outcome of a large series such as this and smaller single center series may be related to center-specific effects. However, we were not able to demonstrate any center-specific effects in interpreting the impact of graft type on the probability of graft loss. Despite the limitations afforded by registry data, our results are important both as they provide an insight into the cumulative experience in managing children undergoing liver transplantation but also in that they may be used to generate hypotheses for future studies.
In conclusion, our analysis of the differential outcomes of various graft types in the SPLIT registry clearly demonstrates that despite significant advances, there is a differential outcome for recipients of split and reduced grafts. This is in contrast to single center series, and may be related to the enhanced statistical power of this large series. The challenge from a surgical perspective is to continue to improve on the technical variant techniques and as such ultimately improve outcome. We believe the fact that children who were transplanted after 2002 had improved survival rates is evidence that technical innovations can improve outcome. As the SPLIT registry continues to accumulate data, we shall continue to actively track the outcome and advances in managing these challenging and important patients.
ACKNOWLEDGMENTS
The SPLIT Research Group are: S. Dunn, J. Menendez, L. Flynn (Alfred I Dupont Hospital for Children, Wilmington, DE); M. Jonas, L. Krawczuk, M. Christoff (Boston Children's Hospital, Boston, MA); R. Kane, E. Phillips, L. Ferrer (Cardinal Glennon Children's Hospital, St. Louis University, St. Louis, MO); T. Heffron, J. DePaolo, T. Pillen, L. Davis (Children's Healthcare of Atlanta, Atlanta, GA); J. Bucuvalas, F. Ryckman, A. Hawkins, G. Arya (Children's Hospital Cincinnati, Cincinnati, OH); M. Narkewicz, R. Sokol, F. Karrer, C. Mark, K. Orban-Eller (Children's Hospital Denver, University of Colorado School of Medicine, Denver, CO); E. Rand, K. Anderer (Children's Hospital of Philadelphia, Philadelphia, PA); G. Mazariegos, N. Chien, L. Seward (Children's Hospital of Pittsburgh, Thomas E Starzl Transplantation Institute Pittsburgh, PA); P. Atkinson (Children's Hospital Western Ontario, London, ON, Canada); G. Telega, S. Lerrett (Children's Hospital of Wisconsin, Milwaukee, WI); J. Roden, N. Mittal, L. Cutright (Children's Medical Center of Dallas, Dallas, TX); E. Alonso, J. Lokar, S. Kelly, K. Neighbors (Children's Memorial Medical Center, Transplant Center of Excellence, Chicago, IL); W. Andrews, J. Daniel, V. Fioravanti, A. Tendick (Children's Mercy Hospital, Kansas City, MO); D. Desai, S. Jarvis (Duke University Medical Center, Durham, NC); A. Fecteau, V. Ng, M. De Angelis, A. Benidir (Hospital for Sick Children Toronto, Toronto, ON, Canada); J. Tector, J. Lim, J. Molleston, J. Pearson (Indiana University Medical College, Indianapolis, IN); K. Schwarz, P. Colombani, M. Alford, M. Felix, R. Jurao (Johns Hopkins Hospital, Baltimore, MD); J. Eason, J. Eshun, S. Powell (Le Bonheur Children's Medical Center, Memphis, TN); D. Freese, J. Weckwerth, J. Greseth, L. Young (Mayo Medical School, Rochester, MN); R. Fisher, M. Akyeampong (Medical College of Virginia, Richmond, VA); S. Emre, B. Shneider, N. Kerkar, S. Cuellar (Mount Sinai Medical Center, New York, NY); S. Lobritto, L. Smith, P. Harren, K. Maseda (New York Presbyterian Hospital, New York, NY); L. Book, M. O'Gorman, C. Kawai, L. Bruschke, J. Kraus (Primary Children's Hospital Medical Center, Salt Lake City, UT); F. Alvarez, S. Martin, C. Viau (Sainte-Justine Hospital, Montreal, QC, Canada); H. Soriano, K. Falkenstein (St. Christopher's Hospital for Children, Philadelphia, PA); R. Shepherd, J. Lowell, M. Nadler (St. Louis Children's Hospital, St. Louis, MO); S. So, W. Berquest, M. Castillo, A. Bula (Stanford University Medical Center, Palo Alto, CA); S. Karpen, J. Mayo, J. Goss, D. Fishman, V. McLin, B. Carter, C. O'Mahony, T. Aloia, D. Garner (Texas Children's Hospital, Houston, TX); S. McDiarmid, S. Fiest (UCLA Medical Center, Los Angeles, CA); S. Gilmour, B. Dodd, N. Kneteman (University of Alberta, Edmonton, AB, Canada); J. Lavine, A. Khanna, R. Clawson (University of California San Diego, San Diego Medical Center, San Diego, CA); P. Rosenthal, D. Filipowski (University of California San Francisco, San Francisco, CA); J. Millis, P. Boone (University of Chicago, Chicago, IL); R. González-Peralta, A. Hemming, M. Hodik, S. McCracken (University of Florida-Shands Children's Hospital, Gainesville, FL); A. Tzakis, T. Kato, D. Weppler, L. Cooper, M. Gonzalez, A. Santiago (University of Miami, Jackson Memorial Hospital, Miami, FL); J. Lopez, J. Magee, V. Shieck (University of Michigan, Ann Arbor, MI); A. Humar, B. Durand, L. Studenski (University of Minnesota, Minneapolis, MN); A. Langnas, D. Antonson, J. Botha, W. Grant, D. Sudan, D. Andersen, B. Fleckten, K. Seipel (University of Nebraska Med Center, Omaha, NE); J. Fair, S. Lichtman, K. Andreoni, J. Prinzhorn, V. Buchholz (University of North Carolina, Chapel Hill, NC); P. Abt, T. Shisler, C. Mack (University of Rochester, Strong Memorial Hospital, Rochester, NY); G. Halff, S. Wallace (University of Texas, HSC San Antonia, San Antonio, TX); S. Horslen, M. Young (University of Washington Seattle, Seattle, WA); M. Kalayoglu, A. D'Alessandro, N. Erickson, R. Judo, S. Knechtle, E. Spaith (University of Wisconsin, Madison, WI).
The Studies of Pediatric Liver Transplantation (SPLIT) research group thanks the following people at the data coordinating center (EMMES Corporation): A. Lindblad PhD, D. Brown, G. Fraser, N. Hornbeak, J. Mitchell, L. Covington, and N. Patel.
Footnotes
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (U01-DK061693-01A1), Astellas Pharma US, (unrestricted grant), Roche Laboratories (unrestricted grant), Johnson and Johnson Medical Products Surgeon Scientist Program Fellowship, Department of Surgery, University of Toronto (I.D.), and Fellowship award, Canadian Institutes of Health Research (I.D.).
Presented in part at the 2003 American Transplant Congress in Washington, DC.
Reprints: J. Michael Millis, MD, Section of Transplantation, MC 5026, Room J 517, 5841 South Maryland Avenue, Chicago, Illinois 60637. E-mail: mmillis@surgery.bsd.uchicago.edu.
Address correspondence to: Annie Fecteau, MD, Division of General Surgery, The Hospital for Sick Children, 555 University Avenue, Room 1526, Toronto, Ontario M5M 4N8. E-mail: annie.fecteau@sickkids.ca.
REFERENCES
- 1.Abramson O, Rosenthal P. Current status of pediatric liver transplantation. 2000;4:533–552. [DOI] [PubMed] [Google Scholar]
- 2.Ghobrial RM, Farmer DG, Amersi F, et al. Advances in pediatric liver and intestinal transplantation. Am J Surg. 2000;180:328–334. [DOI] [PubMed] [Google Scholar]
- 3.Bismuth H, Houssin D. Reduced-size orthotopic liver graft for liver transplantation in children. Surgery. 1984;95:367–370. [PubMed] [Google Scholar]
- 4.Pichlmayr R, Ringe B, Gubernatis G, et al. Transplantation einer spenderbeber auf zwei empfanger (splitting-transplantation): eine neue methode in der weiterentwicklung der lebersegment transplantation. Langenbecks Arch Chir. 1988;373:127–130. [PubMed] [Google Scholar]
- 5.Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet. 1988;2:497–503. [DOI] [PubMed] [Google Scholar]
- 6.Strong RW, Lynch SV, Ong TN, et al. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322:1505–1507. [DOI] [PubMed] [Google Scholar]
- 7.Dunn SP, Haynes JH, Nicolette LA. Split liver transplantation benefits the recipient of the ‘leftover liver’. J Pediatr Surg. 1997;32:252–254. [DOI] [PubMed] [Google Scholar]
- 8.Ghobrail RM, Yersiz H, Farmer DG, et al. Predictors of survival after in vivo split liver transplantation: analysis of 110 consecutive patients. Ann Surg. 2000;232:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reding R, de Ville de Goyet J, Delbeke I, et al. Pediatric liver transplantation with cadaveric or living-related donors: comparative results in 90 elective recipients of primary grafts. J Pediatr. 1999;134:280–286. [DOI] [PubMed] [Google Scholar]
- 10.Sieders E, Peeters PMJG, Vergert EMT, et al. Analysis of survival and morbidity after pediatric liver transplantation with full-size and technical variant grafts. Transplantation. 1999;68:540–545. [DOI] [PubMed] [Google Scholar]
- 11.Burdelski MM, Rogiers X. What lessons have we learned in pediatric liver transplantation? J Hepatol. 2005;42:28–33. [DOI] [PubMed] [Google Scholar]
- 12.Abt PL, Rapaport-Kelz R, Desai NM, et al. Survival among pediatric liver transplant recipients: impact of segmental grafts. Liver Transpl. 2004;10:1287–1293. [DOI] [PubMed] [Google Scholar]
- 13.Sindhi R, Rosendale J, Mundy D, et al. Impact of segmental grafts on pediatric liver transplantation—a review of the United Network for Organ Sharing Scientific Registry data (1990–1996). J Pediatr Surg. 1999;34:107–110. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JP, Hulbert-Shearon TE, Merion RM, et al. Influence of graft type on outcomes after pediatric liver transplantation. Am J Transplant. 2004;4:373–377. [DOI] [PubMed] [Google Scholar]
- 15.Whitington PF. Living donor liver transplantation: ethical considerations. J Hepatol. 1996;24:625–627. [DOI] [PubMed] [Google Scholar]
- 16.Boering DC, Kim JS, Mueller T, et al. One hundred thirty-two consecutive pediatric liver transplants without hospital mortality: lessons learned and outlook for the future. Ann Surg. 2004;240:1002–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cronin DC, Alonso EM, Piper JB, et al. Biliary complications in living donor liver transplantation. Transplant Proc. 1997;29:419–420. [DOI] [PubMed] [Google Scholar]
- 18.Heffron TG, Pillen T, Welch D, et al. Biliary complications after pediatric liver transplantation revisited. Transplant Proc. 2003;35:1461–1462. [DOI] [PubMed] [Google Scholar]
- 19.Saad S, Tanaka K, Inomata Y, et al. Portal vein reconstruction in pediatric liver transplantation from living donors. Ann Surg. 1998;227:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millis JM, Seaman DS, Piper JB, et al. Portal vein thrombosis and stenosis in pediatric liver transplantation. Transplantation. 1996;62:748–754. [DOI] [PubMed] [Google Scholar]
- 21.Marwan IK, Fawzy AT, Egawa H, et al. Innovative techniques for and results of portal vein reconstruction in living-related liver transplantation. Surgery. 1999;125:265–270. [PubMed] [Google Scholar]
- 22.Blumhardt G, Ringe B, Lauchart W, et al. Vascular problems in liver transplantation. Transplant Proc. 1987;19:2412. [PubMed] [Google Scholar]
- 23.Tan KC, Yandza T, de Hemptinne B, et al. Hepatic artery thrombosis in pediatric liver transplantation. J Pediatr Surg. 1988;23:927–930. [DOI] [PubMed] [Google Scholar]
- 24.Millis JM, Cronin DC, Brady LM, et al. Primary living-donor liver transplantation at the University of Chicago: technical aspects of the first 104 recipients. Ann surg. 2000;232:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori K, Nagata I, Yamagata S, et al. The introduction of microvascular surgery to hepatic artery reconstruction in living-donor liver transplantation—its surgical advantages compared with conventional procedures. Transplantation. 1992;54:263–268. [DOI] [PubMed] [Google Scholar]
- 26.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg. 1998;228:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor RW, Manganaro L, O'Brien J, et al. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30:2249–2254. [DOI] [PubMed] [Google Scholar]
- 28.Sieders E, Peeters PMJG, Vergert EMT, et al. Graft loss after pediatric liver transplantation. Ann Surg. 2002;235:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jawan B, de Villa V, Luk HN, et al. Perioperative normovolemic anemia is safe in pediatric living-donor liver transplantation. Transplantation. 2004;77:1394–1398. [DOI] [PubMed] [Google Scholar]