Abstract
Objective:
We evaluated the role of type I interferons (IFNs) and IFN receptors in the regulation of cell growth in 3 human pancreatic adenocarcinoma cell lines (BxPC-3, MiaPaCa-2, and Panc-1).
Background:
Chemotherapy and radiotherapy have a marginal role in the management of pancreatic adenocarcinoma. The addition of IFN-α showed promising results in early clinical trials.
Methods:
Cell proliferation and apoptosis were evaluated by DNA measurement and DNA fragmentation, respectively. Type I IFN receptor (IFNAR-1 and IFNAR-2 subunits) was determined by quantitative RT-PCR and immunocytochemistry. Cell cycle distribution was evaluated by propidium iodide staining and flow-cytometric analysis.
Results:
The incubation with IFN-β for 6 days showed a potent inhibitory effect on the proliferation of BxPC-3 (IC50, 14 IU/mL) and MiaPaCa-2 (IC50, 64 IU/mL). The inhibitory effect of IFN-β was stronger than IFN-α in all 3 cell lines and mainly modulated by the stimulation of apoptosis, although cell cycle arrest was induced as well. The expression of the type I IFN receptors was significantly higher in BxPC-3 (the most sensitive cell line to IFN) and mainly localized on the membrane, whereas in Panc-1 (the most resistant cell line) about 60% to 70% of cells were negative for IFNAR-2c with a mainly cytoplasmic staining for IFNAR-2c.
Conclusion:
The antitumor activity of IFN-β is more potent than IFN-α in pancreatic cancer cell lines through the induction of apoptosis. Further studies should investigate in vivo whether the intensity and distribution of IFNAR-1 and IFNAR-2c may predict the response to therapy with IFN-α and IFN-β in pancreatic cancer.
Treatment with IFN-β showed a potent inhibitory effect on the proliferation of pancreatic cancer cell lines. The expression, distribution, and localization of type I IFN receptor subtypes (IFNAR-1 and IFNAR-2c) seem to predict the response to IFN treatment in these cells. However, further studies will need to confirm this observation in vivo.
Pancreatic adenocarcinoma is a highly aggressive malignancy.1 Surgery is the only curative therapy. Unfortunately, only 5% to 15% of patients are surgical candidates at the time of the diagnosis due to a lack of specific symptoms, limitations in diagnostic methods, and the biologically aggressive nature of this tumor.1 In this selected group of patients, adjuvant chemotherapy has a survival benefit but the 5-year survival of 21%, as described by the European Study Group for Pancreatic Cancer, remains poor.2 The role of chemoradiotherapy in the management of pancreatic adenocarcinoma is unclear.3 However, it has been recently described4 that interferon (IFN)-α in combination with adjuvant chemoradiotherapy improved 5-year survival to 55%.
In vitro and in vivo studies have demonstrated the efficacy of type I IFNs (eg, IFN-α, IFN-β, IFN-ω, IFN-κ, and IFN-τ), in the treatment of several tumors.5–9 Although the antitumor effects of IFN-α have been studied in detail, those of IFN-β are not well clarified. IFN-β is a multifunctional cytokine that binds the same receptor of IFN-α, but with higher affinity.10 It seems to be an essential mediator not only for the innate immune responses against microbial infections, but also for a host defense system against oncogenesis.6,11 Moreover, several studies showed that IFN-β has greater antitumor effects than IFN-α.10,12–16 On the basis of these observations, IFN-β represents a promising drug in the treatment of cancer.
Importantly, several chromosomal aberrations have been detected in pancreatic adenocarcinoma, including a frequent loss of chromosome arm 9p, observed in more than 80% of human pancreatic cancer.17 Together with the tumor-suppressor genes p16INK4a, p15INK4b, and p14ARF also the IFN-α and IFN-β genes are located on chromosome 9p.18 Therefore, in relation to the defensive role of IFNs against tumors,11 the absence of the expression of IFNs may have an important role in the pathogenesis and probably in the treatment of pancreatic adenocarcinoma.
To further explore the possibilities of new medical treatments in pancreatic cancer, we evaluated in the present study the antitumor activity of IFN-α and IFN-β in 3 human pancreatic adenocarcinoma cell lines (BxPC-3, MiaPaCa-2, and Panc-1), as well as the role of IFN receptors in the responsiveness to type I IFNs.
METHODS
Cell Lines and Culture Conditions
The human pancreatic cell lines, BxPC-3, MiaPaCa-2, and Panc-1 were purchased from the American Type Culture Collection. The cells were cultured in a humidified incubator containing 5% CO2 at 37°C. The culture medium consisted of RPMI 1640 supplemented with 10% FCS, penicillin (1 × 105 U/L) and l-glutamine (2 mmol/l). Periodically, the cells were confirmed as Mycoplasma-free. Cells were harvested with trypsin (0.05%), EDTA (0.02%), and resuspended in medium. Before plating, the cells were counted microscopically using a standard hemocytometer. Trypan Blue staining was used to assess cell viability and always exceeded 95%. Media and supplements were obtained from GIBCO Bio-cult Europe (Invitrogen, Breda, The Netherlands).
Drugs and Reagents
Human recombinant IFN-α-2b (Intron-A) was obtained from Schering-Plough Corporation (Utrecht, The Netherlands), while human recombinant IFN-β-1a was acquired from Serono Inc. (Rebif, Rockland, MA). All compounds were stored at −20°C, and the stock solution was constituted in distilled water according to the manufacturer instructions.
Cell Proliferation Assay
After trypsinization the cells were plated in 1 mL of medium in 48-well plates at a density of 5 × 103 to 4 × 104 cells/well, depending on the length of the incubation period. The plates were then placed in a 37°C, 5% CO2 incubator overnight. The next day the cell culture medium was replaced with 1 mL/well medium containing increasing concentrations (0–10,000 IU/mL) of IFN-α or IFN-β. Each treatment was performed in quadruplicate. After 1, 3, and 6 days of treatment, the cells were harvested for DNA measurement, at an approximately 70% to 80% confluence. For 6-day experiments, the medium was refreshed after 3 days and compounds were added again. Measurement of total DNA contents, representative for the number of cells, was performed using the bisbenzimide fluorescent dye (Hoechst 33258) (Boehring Diagnostics, La Jolla, CA), as previously described.19
Measurement of DNA Fragmentation (Apoptosis)
Cells (104 to 4 × 104)/well, depending on the length ofthe incubation period, were plated on a 48-well plate andthe cells were allowed to adhere overnight. The next day, the cell culture medium was replaced with 1 mL/well medium containing increasing concentrations (0–10,000 IU/mL) of IFN-α or IFN-β. Each treatment was performed in quadruplicate. After an additional incubation of 1 and 3 days, apoptosis was assessed using a commercially available ELISA kit (Cell Death Detection ELISAPlus, Roche Diagnostic GmbH, Penzberg, Germany). The standard protocol supplied by the manufacturer was used, as previously described.20 Relative apoptosis was determined by calculating the ratio of the average absorbance of the treatment wells to the average absorbance of the control wells. The data were corrected for the effect on cell number after 1 and 3 days of treatment.
Cell Cycle Analysis
Cells (1 to 4 × 106) depending on the length of the incubation period, were plated in 75-cm2 culture flasks (Corning Costar, Amsterdam, The Netherlands). After 1 day medium was changed with fresh medium (control group) and with fresh medium plus IFN-α or IFN-β at the concentration of 1000 IU/mL. Each treatment was performed in duplicate. After 1, 2, and 3 days of incubation, the cells were harvested by gentle trypsinization and prepared for cell cycle determination using propidium iodide for DNA staining, as previously described.16 The stained cells were analyzed by FACS-calibur flow cytometer (Becton Dickinson, Erembodegem, Belgium) and CellQuest Pro Software. Cell cycle progression was measured with corresponding absorbances for G0/G1, S and G2-M phases, whereas apoptosis was measured by quantifying the sub-G0 peak.
Quantitative RT-PCR
The expression of type I IFN receptors (IFNAR-1, IFNAR-2 total, the short form IFNAR-2b, and the long form IFNAR-2c) and housekeeping gene hypoxanthine-phosphoribosyl-transferase (HPRT) mRNA was evaluated by quantitative RT-PCR in all 3 pancreatic cancer cell lines, as previously described.16 Briefly, poly A+ mRNA was isolated using Dynabeads Oligo (dT)25 (Dynal AS, Oslo, Norway) from cell pellets containing approximately 5 × 105 cells. Complementary DNA (cDNA) was synthesized using the poly A+ mRNA in a Tris-buffer together with 1 mmol/L of each deoxynucleotide triphosphate, 10 U RNAse inhibitor, and 2 U AMV Super Reverse Transcriptase (HT Biotechnology Ltd., Cambridge, UK) in a final volume of 40 μL. This mixture was incubated for 1 hour at 42°C. One fifth of the cDNA library was used for quantification of IFN receptors and HPRT mRNA levels.
A quantitative PCR was performed by AmpliTaq Gold DNA Polymerase and the ABI PRISM 7700 sequence detection system (Perkin-Elmer Applied Biosystems, Groningen, The Netherlands) for real-time amplifications, according to the manufacturer's protocol. Each sample was assayed in duplicate. The assay was performed using 15 μL TaqMan Universal PCR Master Mix (Applied Biosystems, Capelle aan de Ijssel, The Netherlands), forward primer, reverse primer, probe, and 10 μL cDNA template, in a total reaction volume of 25 μL. PCR amplification started with a first step for 2 minutes at 50°C, followed by an initial heating at 95°C for 10 minutes, samples were subjected to 40 cycles of denaturation at 95°C for 15 seconds and annealing for 1 minute at 60°C.
The primer and probe sequences that were used for the detection of IFNAR-1, IFNAR-2 total, IFNAR-2b, IFNAR-2c, and HPRT have been previously described.16 All the primer and probe sequences were purchased from Biosource (Nivelles, Belgium).
The detection of HPRT mRNA was used for normalization of IFN receptor mRNA levels. Expression of IFNAR-2a mRNA, the soluble form of IFNAR-2 subunit, was determined indirectly by subtracting IFNAR-2b and IFNAR-2c from IFNAR-2 total. To exclude contamination of the PCR reaction mixtures, the reactions were performed in the absence of DNA template in parallel with cDNA samples. As a positive control for the PCR reactions of HPRT and type I IFN receptors human cDNA was amplified in parallel with the cDNA samples.
Immunocytochemistry
Cytospin preparations of BxPC-3, MiaPaCa-2, and Panc-1 cells were fixed with acetone for 10 minutes. After washing 2 times with PBS, the cells were incubated for 30 minutes at room temperature with antibodies to human IFNAR-1 (rabbit polyclonal antibody, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and IFNAR-2c (monoclonal antibody, Dr E. Croze, Berlex Biosciences, Richmond, CA) subunits, and for overnight with antibodies to IFNAR-2b (rabbit polyclonal antibody, Santa Cruz Biotechnology, Inc.). Finally, a peroxidase complex for IFNAR-1 and IFNAR-2b, or standard streptavidin-biotinylated alkaline phosphatase (both from IL Immunologic, Duiven, The Netherlands) for IFNAR-2c, were used according to the manufacturer's recommendations to visualize the bound antibodies.
Negative controls for the immunohistochemistry included: 1) omission of the primary antibody; and 2) preabsorption of the antibody for IFNAR-2b with the respective immunizing receptor peptide.
Statistical Analyses
All experiments were carried out at least 3 times and gave comparable results. For statistical analysis GraphPad Prism 3.0 (GraphPad Software, San Diego, CA) was used. Fifty percent growth-inhibitory concentrations (IC50) and maximal inhibitory effects were calculated using nonlinear regression curve-fitting program. The comparative statistical evaluation among groups was first performed by the ANOVA test. When significant differences were found, a comparison between groups was made using the Newman-Keuls test. The unpaired Student t test was used to analyze the differences in concentration-effect curves (IC50 and maximal inhibitory effect) and effects in cell cycle modulation between different types of IFNs, and the differences of the growth inhibitory effects of IFNs after 3 and 6 days of treatment. Correlation analyses were performed using Pearson's coefficients.
In all analyses, values of P < 0.05 were considered statistically significant. Data are reported as mean ± SEM. Statistical analysis was made after logarithmic transformation.
RESULTS
Antiproliferative Effects of Type I IFNs
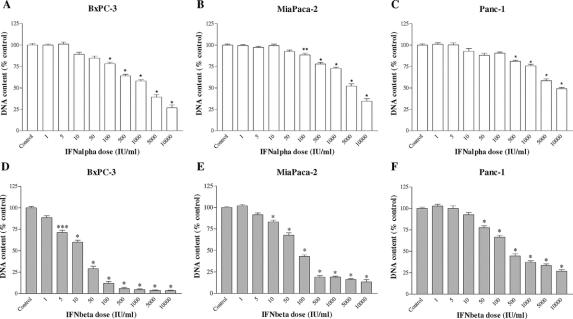
After 6 days of incubation, IFN-α and IFN-β significantly suppressed the growth of all 3 pancreatic cancer cell lines in a dose-dependent manner (Fig. 1), with a mean IC50 of 606 IU/mL and 14 IU/mL in BxPC-3, respectively; 1531 IU/mL and 64 IU/mL in MiaPaCa-2, respectively; and 1250 IU/mL and 112 IU/mL in Panc-1, respectively.
FIGURE 1. Effects of IFN-α (A–C) and IFN-β (D–F) treatment on cell proliferation, as measured by total DNA content, using Hoechst 33258. Pancreatic cancer cell lines were incubated for 6 days without (control) or with the drugs indicated at different concentrations. Values are expressed as the percentage of control (untreated cells) and represent the mean ± SEM of at least 3 independent experiments in quadruplicate. The mean DNA content in controls were: 2260 ng/well (IFN-α, BxPC-3), 2430 ng/well (IFN-β, BxPC-3), 8562 ng/well (IFN-α, MiaPaCa-2), 8803 ng/well (IFN-β, MiaPaCa-2), 4224 ng/well (IFN-α, Panc-1), and 4172 ng/well (IFN-β, Panc-1). *P < 0.001; **P < 0.01; ***P < 0.05 versus control.
The growth-inhibitory effect of IFN-β was significantly more potent than that of IFN-α, as shown by the higher maximal inhibition of proliferation induced by IFN-β compared with IFN-α (96.7% ± 2% and 72% ± 5.7%, respectively, P < 0.0001 in BxPC-3; 87.5% ± 3.2% and 69.1% ± 6.1%, respectively, P < 0.0001 in MiaPaCa-2; 70.7% ± 1.4% and 53% ± 5.7%, respectively, P < 0.0001 in Panc-1) after 6 days of treatment, as well as by the lower logIC50 of IFN-β compared with IFN-α (1.15 ± 0.06 and 2.78 ± 0.15, respectively, P < 0.00001 in BxPC-3; 1.8 ± 0.07 and 3.18 ± 0.13, respectively, P < 0.00001 in MiaPaCa-2; 2.05 ± 0.05 and 3.1 ± 0.17, respectively, P < 0.0001 in Panc-1). In BxPC-3 and MiaPaCa-2, IFN-β induced a statistically significant cell growth inhibition already at very low concentrations (5–10 IU/mL).
In all 3 pancreatic cell lines, the effects of IFN-α and IFN-β were time-dependent. Indeed, the maximal inhibition of cell proliferation, induced by both cytokines, was higher after 6 days compared with 3 days of incubation (both P < 0.0001 in BxPC-3; P < 0.005 and P < 0.0001, respectively, for IFN-α and IFN-β in MiaPaCa-2; both P < 0.0001 in Panc-1). In addition, there was no difference in IC50 values after 3 and 6 days of incubation with IFN-α or IFN-β in the 3 cell lines (data not shown).
The cell lines exhibited different sensitivities to the treatment, particularly with IFN-β. BxPC-3 resulted to be the most sensitive and Panc-1 the most resistant. The maximal inhibition of proliferation for IFN-β was higher in BxPC-3 compared with MiaPaCa-2 (P < 0.05) and Panc-1 (P < 0.001), while it was lower in Panc-1 compared with MiaPaCa-2 (P < 0.01). Similarly, the IC50 of IFN-β was significantly lower in BxPC-3 than in MiaPaCa-2 and Panc-1 (both P < 0.001), and higher in Panc-1 compared with MiaPaCa-2 (P < 0.05). The maximal inhibition of proliferation for IFN-α was higher in BxPC-3 compared with Panc-1 (P < 0.05), while no difference in IC50 values of IFN-α was observed between the 3 cell lines.
Effects of Type I IFNs on Apoptosis
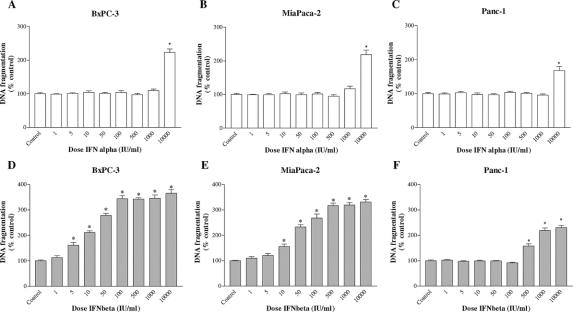
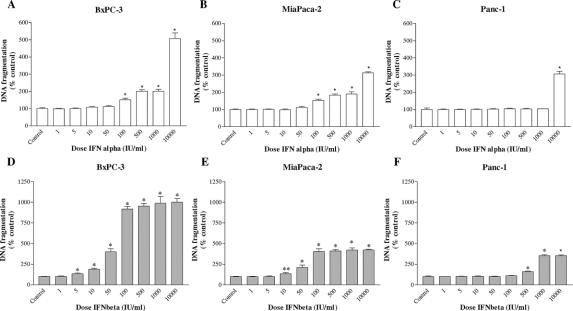
A crucial step in apoptosis is DNA fragmentation, a process that results from the activation of endonucleases, which degrade chromatin into smaller fragments. The measurement of DNA fragmentation was used to investigate the effect of treatment with IFN-α and IFN-β on apoptosis (Figs. 2 and 3).
FIGURE 2. Effects of IFN-α (A–C) and IFN-β (D–F) treatment on apoptosis (DNA fragmentation) in BxPC-3, MiaPaCa-2, and Panc-1 cell lines. The cells were incubated for 1 day without (control) or with the drugs indicated at different concentrations. Values are absorbance units and are expressed as percent of the control. Data are the mean ± SEM. *P < 0.001 versus control.
FIGURE 3. Effects of IFN-α (A–C) and IFN-β (D–F) on the apoptosis (DNA fragmentation) in BxPC-3, MiaPaCa-2, and Panc-1 cell lines. Pancreatic cancer cell lines were incubated for 3 days without (control) or with the drugs indicated at different concentrations. Values are absorbance units and are expressed as percent of the control. Data are the mean ± SEM. *P < 0.001; **P < 0.01.
After 1 day of incubation, IFN-α had no remarkable stimulatory effects on DNA fragmentation at any concentration up to 1000 IU/mL in all 3 cell lines, only at the very high dose of 10,000 IU/mL IFN-α induced a significant increase in DNA fragmentation (Fig. 2A–C). On the other hand, a dose-dependent induction of apoptosis was observed after IFN-β treatment in BxPC-3 and MiaPaCa-2, with a maximal increase of DNA fragmentation of about 3.5 times compared with the untreated control (Fig. 2D, E). This effect was already statistically significant at very low concentrations (5–10 IU/mL). In Panc-1, a stimulating effect on apoptosis was observed only for very high concentrations of IFN-β (≥500 IU/mL) (Fig. 2F).
After 3 days of treatment with IFN-α, an increase in DNA fragmentation was detected in BxPC-3 and MiaPaCa-2 at a moderate to high dose (Fig. 3A, B). Moreover, the induction of apoptosis by IFN-β remained high, with a maximal stimulation of about 4- and 10-fold, compared with the control, respectively, in MiaPaCa-2 and BxPC-3 (Fig. 3D, E). In addition, after 3 days of incubation, the stimulatory effects on apoptosis persisted in Panc-1 only at high doses of IFN-α (10,000 IU/mL, Fig. 3C) and IFN-β (≥500 IU/mL, Fig. 3F).
These data were also confirmed by morphologic observations. In all 3 cell lines, the treatment with IFN-β induced clear structural alterations consistent with apoptosis, such as cell shrinkage, pyknotic nucleus, and detachment from the plate after 1 to 3 days, also at very low doses in BxPC-3 cell line (not shown). These morphologic changes were evident only at high doses of IFN-α treatment.
The inhibitory effects of IFN-β on the cell growth of BxPC-3 and MiaPaCa-2 cell lines appeared to be mainly due to an early pro-apoptotic activity, as shown by the highly significant positive correlation between cell proliferation inhibition after 6 days of treatment and DNA fragmentation induction after 1 day (r2 = 0.95, P < 0.0001, both for BxPC-3 and MiaPaCa-2) and 3 days of incubation (r2 = 0.95, P < 0.0001, for BxPC-3; r2 = 0.90, P < 0.0001, for MiaPaCa-2). At this early time point, apoptosis seems to be not involved in the antiproliferative effect of IFN-α on pancreatic cancer cells. Indeed, no significant correlation has been observed between cell proliferation inhibition after 6 days and DNA fragmentation variation after 1 day of treatment with IFN-α. Only after 3 days of treatment with IFN-α, we observed a positive correlation between DNA fragmentation variation and the 6 days cell proliferation inhibition (r2 = 0.74, P < 0.0001, for BxPC-3; r2 = 0.71, P < 0.0001, for MiaPaCa-2).
Effects of Type I IFNs on the Cell Cycle
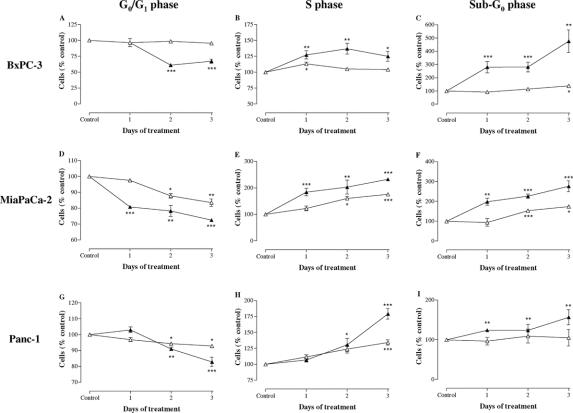
We also evaluated the effect of treatment with IFN-α (1000 IU/mL) and IFN-β (1000 IU/mL) on cell cycle phase distribution after 1, 2, and 3 days of incubation in BxPC-3, MiaPaCa-2, and Panc-1 (Fig. 4A–I).
FIGURE 4. Cell cycle distribution after 1, 2, and 3 days of incubation with 1000 IU/mL IFN-α and 1000 IU/mL IFN-β in BxPC-3 (A–C), MiaPaCa-2 (D–F), and Panc-1 (G–I) cells. Data are expressed as mean ± SEM of the percentage of cells in the different phases of the cell cycle, as compared with untreated control cells. Control values have been set to 100%. Δ, IFN-α; ▴, IFN-β. *P < 0.05; **P < 0.01; ***P < 0.001 versus control.
IFN-α treatment induced a significant accumulation in S phase compared with the control in all 3 cell lines and a decrease in the proportion of cells in G0/G1 phase in MiaPaCa-2 and Panc-1. In addition, the histograms of cell cycle revealed a late and slight increase in cells with subdiploid DNA content (sub-G0 phase) only in BxPC-3 and MiaPaCa-2, confirming the induction of apoptosis after IFN-α treatment, as previously shown by the analysis of DNA fragmentation. In a comparable manner, the incubation with IFN-β increased the fraction of all 3 cell lines in the S phase of the cell cycle, whereas the proportion of cells in G0/G1 phase decreased in comparison with the control. IFN-β induced a variable accumulation of cells in sub-G0 phase in all cell lines (BxPC-3>MiaPaCa-2>Panc-1).
These data suggest that pancreatic cancer cells in S phase fail to transit into G2 and M phases efficiently and exhibit a prolonged stay in S phase after treatment with type I IFNs.
The cell cycle arrest induced by IFN-β was more potent than that of IFN-α, considering that the percentage of cells in S phase compared with the control was significantly higher after 3 days of incubation with IFN-β than after IFN-α (BxPC-3: P < 0.05, MiaPaCa-2: P < 0.001, Panc-1: P < 0.001).
Expression of Type I IFN Receptor mRNA
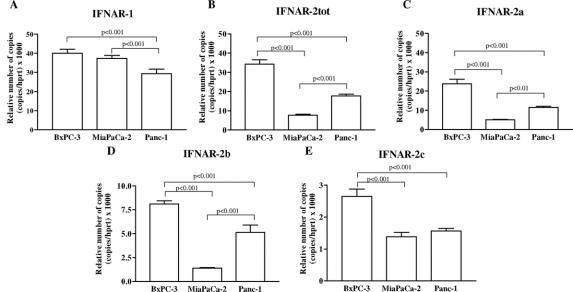
Since the susceptibility of cells to IFNs could reflect the different amount of corresponding receptors, we analyzed the expression of type I IFN receptors (IFNAR-1 and IFNAR-2, short and long form) mRNA by real-time quantitative RT-PCR in the BxPC-3, MiaPaCa-2, and Panc-1 cell lines. Using sequence specific primers against the type I IFN receptor subunits, we detected the presence of IFNAR-1, IFNAR-2 total, IFNAR-2b and IFNAR-2c mRNA, normalized for the amount of the housekeeping gene HPRT. As shown in Figure 5, the expression of IFNAR-1 mRNA was significantly higher in BxPC-3 and MiaPaCa-2 compared with Panc-1 (both P < 0.001), whereas no statistically significant difference was observed between BxPC-3 and MiaPaCa-2. In addition, the mRNA expression level of IFNAR-2a, IFNAR-2b, and IFNAR-2c mRNA was higher in BxPC-3 compared with MiaPaCa-2 and Panc-1 (P < 0.001).
FIGURE 5. A–E, Relative expression level of type I IFN receptor (IFNAR-1, IFNAR-2 total, IFNAR-2a, IFNAR-2b, IFNAR-2c) mRNA normalized to HPRT mRNA in human pancreatic cancer cell lines (BxPC-3, MiaPaCa-2, Panc-1), evaluated by quantitative RT-PCR. Values represent the mean ± SEM.
Immunocytochemistry
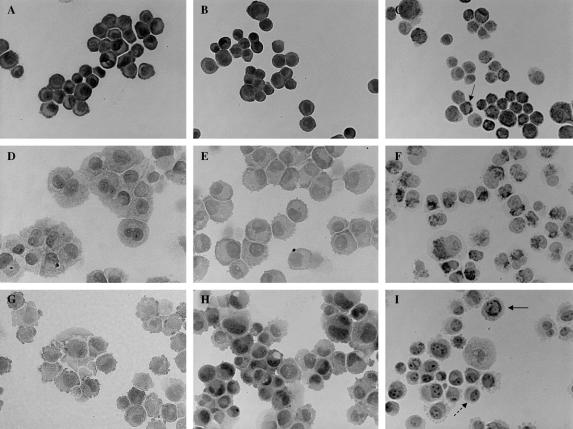
Specific immunoreactivity for IFN receptor subunits (IFNAR-1, IFNAR-2b, and IFNAR-2c) was found in all 3 pancreatic cancer cell lines (Fig. 6A–I). It was strongly positive for IFNAR-1 in BxPC-3 (Fig. 6A) and the staining was predominantly at the plasma membrane. On the other hand, in MiaPaCa-2 (Fig. 6D) and in Panc-1 (Fig. 6G), the expression of IFNAR-1 was lower and particularly distributed in the cytoplasm, although a proportion of Panc-1 cells resulted to be mildly to moderately positive for IFNAR-1 at the plasma membrane as well. IFNAR-2b showed a comparable expression in BxPC-3 (Fig. 6B) and Panc-1 (Fig. 6H), while in MiaPaCa-2 (Fig. 6E) the expression of this subunit was lower compared with the other 2 cell lines. In BxPC-3 and in MiaPaCa-2, the immunostaining of IFNAR-2b was localized in the cytoplasm and on the membrane, while in Panc-1 the expression of IFNAR-2b was preferentially on the cytoplasm. IFNAR-2c is mainly expressed on the plasma membrane and in the cytoplasm in BxPC-3 (Fig. 6C) and in MiaPaCa-2 (Fig. 6F), respectively. Finally, in Panc-1 (Fig. 6I) this receptor subunit is primarily expressed in cytoplasm, the IFNAR-2c pattern is heterogeneous and the staining is negative in about 60% to 70% of the cells.
FIGURE 6. Immunocytochemical detection of IFNAR-1 (A, D, G), IFNAR-2b (B, E, H), and IFNAR-2c (C, F, I) receptors in BxPC-3 (A–C), MiaPaCa-2 (D–F), and Panc-1 (G–I). Original magnification, ×400. The expressions of IFNAR-1 and IFNAR-2c are mainly membranous in BxPC-3 (A and C, solid arrow). In Panc-1, IFNAR-2c is localized in the cytoplasm (I, solid arrow) and 60% to 70% of the cells are negative for IFNAR-2c (I, dashed arrow).
DISCUSSION
Although few trials criticized the intense toxicity of IFNs,21,22 Traverso's group showed that combination of IFN-α with adjuvant chemoradiation therapy may increase response rates and survival in patients with pancreatic cancer.4,23 In addition, the administration of IFN-α in combination with 13-cis retinoic acid or with 5-fluorouracil, leucovorin, and cisplatin increased antitumor effect in advanced pancreatic carcinoma.24–26
Whereas the role of IFN-α has been extensively studied, the effect of other type I IFNs on pancreatic cancer has been evaluated less extensively. Preliminary reports suggested the possibility to use IFN-β in the treatment of pancreatic cancer.27–30 A high local production of IFN-β induced a strong antitumor effect on PANC02-H7 cells, a highly metastatic mouse pancreatic carcinoma cell line successfully transfected with a vector containing a murine IFN-β gene.27 A recent paper showed that the treatment of human pancreatic cancer cell lines with gemcitabine and human IFN-β gene entrapped in liposomes was more effective than either treatment alone.28 Busch et al described the stabilization of the disease in a patient with incomplete resection of a pancreatic cancer, treated with IFN-β in combination with gemcitabine, cisplatinum, and radiotherapy.29 On the other hand, few long-lasting responses and disease stabilization have been achieved in patients with advanced pancreatic cancer by combining IFN-β with chemotherapy and retinoids.30 However, the efficacy of IFN-β in the treatment of pancreatic cancer, the potential differences in antitumor activity with IFN-α, and the mechanisms of action that are involved are still poorly understood. Moreover, in clinical practice, one of the main limits of therapy with type I IFNs is the scanty availability of molecular predictors, potentially useful in deciding whether a patient should be treated. This is a crucial point, considering that several tumors are completely or partially resistant to IFNs. A recent in vitro study showed that IFNs have antiproliferative effects on pancreatic cancer cell lines expressing the IFNAR-2 subunit.31 Besides, patients with pancreatic cancer who expressed IFNAR-2 represent about 25% of cases32,33 and have better survival compared with patients who did not express this receptor.33 However, IFNAR-2 receptor is not the only component modulating the antitumor activity of type I IFNs. These cytokines activate a common receptor complex composed of 2 major subunits, IFNAR-1 and IFNAR-2.34,35 IFNAR-1 is considered the signaling subunit, as it is absolutely required for signal transduction. There are 3 forms of IFNAR-2, which are differentially spliced products of the same gene, eg, the soluble (IFNAR-2a), short (IFNAR-2b), and long (IFNAR-2c) form.6,36–38 The IFNAR-2c and IFNAR-1 subunits constitute the predominantly active form of the type I IFN receptor complex. IFNAR-2c is capable of binding ligand, but with a lower affinity (20-fold less) than the dimeric IFN receptor complex itself.39 Therefore, both receptor chains are required to form a high affinity-binding site and initiate signal transduction leading to the induction of IFN-responsive genes. The short form is able to bind type I IFNs but does not couple to signal transduction.40 The soluble form may act as a regulator of free IFNs and, depending on concentration, leads to the neutralization or even enhancement of IFN bioactivity.41,42
In the present study, we compared the antitumor effects of IFN-α and IFN-β, as well as the mechanisms that are involved in the growth inhibition of 3 human pancreatic cancer cell lines (BxPC-3, MiaPaCa-2, and Panc-1). Moreover, for the first time, we evaluated the expression and the subcellular distribution of type I IFN-receptor subtypes in these cells. We found that IFN-β potently inhibits cell proliferation already at very low concentrations (5–10 IU/mL) in BxPC-3 and MiaPaCa-2. These concentrations can be achieved in vivo after subcutaneous administration of IFN-β.43,44
The direct antitumor effects of IFN-α and -β are associated with the induction of apoptosis and cell cycle arrest. In BxPC-3 and MiaPaCa-2, both cytokines are able to induce apoptosis, but the increase in DNA fragmentation after IFN-β treatment occurred earlier and was considerably more potent than after IFN-α treatment. Panc-1 was the most resistant cell line to both IFNs, showing a stimulation of apoptosis only at very high doses (IFN-α ≥10,000 IU/mL, IFN-β ≥500 IU/mL). In all 3 pancreatic cancer cell lines, both IFNs induce a significant accumulation of cells in S phase compared with the untreated control, suggestive of a cell cycle arrest in the late S phase. The S-phase block induced by IFN-β is more potent and earlier than that of IFN-α.
Quantitative RT-PCR study and immunocytochemical analysis demonstrate the presence of all type I IFN receptor subunit transcripts and proteins in BxPC-3, MiaPaCa-2, and Panc-1 cells. The high expression of IFNAR-1 and IFNAR-2c subunits in BxPC-3 could explain the major sensitivity of this cell line to IFN treatment. Indeed, as shown by Wagner et al,45 increasing the cell surface levels of IFNAR2c in cancer cells enhances their sensitivity to the antiproliferative and apoptotic effects of type I IFNs. Moreover, long-term cultures of IFNAR1-deficient mouse embryonic fibroblasts, as well as IFN-β deficient cells, resulted in the formation of transformed colonies in vitro and the formation of tumors in nude mice.5 It is interesting to observe striking differences in the subcellular localization and distribution of IFNs receptor subunits, as determined by immunocytochemistry. In BxPC-3, the staining for the active subunits of IFN receptor (IFNAR-1 and IFNAR-2c) is mainly membranous, whereas in Panc-1 the expression of IFNAR-2c is preferentially in the cytoplasm. A potential explanation to clarify the antitumor activity of type I IFNs at high doses in PANC-1, where IFNAR-2c subunits is mainly detected in the cytoplasm, may be that immunohistochemistry is not sensitive enough to demonstrate very low quantities of IFN membrane receptors. In addition, it has been recently observed that IFNAR-1, which is mildly to moderately expressed in the plasma membrane of several PANC-1 cells, has an important role in antiproliferative activity modulated by type I IFNs.46 These arguments may explain why, even in the presence of low membrane expression of IFN receptors, antiproliferative and pro-apoptotic effects of high concentrations of type I IFNs are observed in Panc-1 cells. In Panc-1, about 60% to 70% of the cells exhibit no detectable levels of IFNAR-2c. This heterogeneity in IFNAR-2c expression may provide an additional explanation for the low sensitivity of these cells to IFN-α and IFN-β treatment. In Panc-1, it is possible a selection of cell type during type I IFN treatment, with higher possibility to survive for IFNAR-2c negative cells. Summarizing, these data suggest that the high sensitivity of BxPC-3 to IFNs treatment could be related to the strong expression of IFNAR-1 and IFNAR-2c and the main membranous localization, whereas the low expression, cytoplasmic localization, and heterogeneous staining of IFNAR-2c in Panc-1 could explain the relative resistance of these cells to IFN treatment. This is the first study, as far as we know, showing the importance of expression, distribution, and localization of type I IFN receptor subtypes in the modulation of response to IFN treatment in pancreatic cancer. Our data also suggest that a careful evaluation of both active IFNAR subtypes in pancreatic cancer is required before treatment with type I IFNs is considered.
Although IFN-α and IFN-β interact with the same receptor, the induction of a differential response can be explained by the diversity in the structure between both cytokines,47,48 generating different interactions and affinities for the related receptor. Indeed, IFN-β has a higher binding affinity (10-fold) than IFN-α.10 However, this cannot completely explain the difference in potency of cell growth inhibition between both cytokines, particularly in BxPC-3, where the IC50 for IFN-β is 40 times lower than that of IFN-α. Differences in the interaction of these IFNs with their receptors could be also involved. Both IFNs induce tyrosine phosphorylation of the receptor subunits; IFN-β, but not IFN-α, induces the association of IFNAR-1 and IFNAR-2c chains, indicating that the specificity of signaling for distinct type I IFN subtypes is established by differential conformation of the receptor complex.40,49
CONCLUSION
This study shows that IFN-β is significantly more effective than IFN-α in inducing cell growth inhibition in pancreatic cancer because it induces a more potent and early cell cycle arrest and apoptosis activation compared with IFN-α. Considering that IFN-β stimulates apoptosis already at very low dose, this cytokine could be a more promising agent than IFN-α for the treatment of human pancreatic cancer, particularly in tumors with a high expression of IFNAR-1 and IFNAR-2c, which is supporting its use in future clinical investigation. In addition, there is clear in vitro evidence that differential expression levels and distribution of the IFNs receptor subunits play a role in the regulation of the response to type I IFNs therapy in pancreatic cancer. Future studies should investigate in vivo whether the intensity, subcellular localization, and distribution of IFNAR-1 and IFNAR-2c at immunohistochemistry may predict the response to therapy with both IFN-α and IFN-β in pancreatic cancer.
ACKNOWLEDGMENTS
The authors thank Claessen Sandra, MH, for technical assistance.
Footnotes
Supported by study grants from the Yamagiwa-Yoshida Memorial UICC International Cancer.
Reprints: Casper H. J. van Eijck, MD, PhD, Department of Surgery, Erasmus Medical Center, Room H 911, Dr. Molewaterplein 50, 3015 GE Rotterdam, The Netherlands. E-mail: c.vaneijck@erasmusmc.nl.
REFERENCES
- 1.Sohn T, Yeo C, Cameron J, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. [DOI] [PubMed] [Google Scholar]
- 3.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–480. [DOI] [PubMed] [Google Scholar]
- 5.Lindner DJ, Borden EC, Kalvakolanu DV. Synergistic antitumor effects of a combination of interferons and retinoic acid on human tumor cells in vitro and in vivo. Clin Cancer Res. 1997;3:931–937. [PubMed] [Google Scholar]
- 6.Takaoka A, Taniguchi T. New aspects of IFN-α/β signalling in immunity, oncogenesis and bone metabolism. Cancer Sci. 2003;94:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell MS. Combination of anticancer drugs and immunotherapy. Cancer Immunol Immunother. 2003;52:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitale G, Tagliaferri P, Caraglia M, et al. Slow release lanreotide in combination with interferon-α2b in the treatment of symptomatic advanced medullary thyroid carcinoma. J Clin Endocrinol Metab. 2000;85:983–988. [DOI] [PubMed] [Google Scholar]
- 9.Vitale G, Caraglia M, Ciccarelli A, et al. Current approaches in the therapy of medullary thyroid carcinoma. Cancer. 2001;91:1797–1808. [DOI] [PubMed] [Google Scholar]
- 10.Johns TG, Mackay IR, Callister KA, et al. Antiproliferative potencies of interferons on melanoma cell lines and xenografts: higher efficacy of interferon beta. J Natl Cancer Inst. 1992;84:1185–1190. [DOI] [PubMed] [Google Scholar]
- 11.Xie K, Bielenberg D, Huang S, et al. Abrogation of tumorigenicity and metastasis of murine and human tumor cells by transfection with the murine IFN-beta gene: possible role of nitric oxide. Clin Cancer Res. 1997;3:2283–2294. [PubMed] [Google Scholar]
- 12.Giandomenico V, Vaccari G, Fiorucci G, et al. Apoptosis and growth inhibition of squamous carcinoma cells treated with interferon-α, interferon-β and retinoic acid are associated with induction of the cyclin-dependent kinase inhibitor p21. Eur Cytokine Netw. 1998;9:619–631. [PubMed] [Google Scholar]
- 13.Rosenblum MG, Yung WKA, Kelleher PJ, et al. Growth inhibitory effects of interferon-β but not interferon-α on human glioma cells: correlation of receptor binding 2′,5′–oligoadenylate synthetase and protein kinase activity. J Interferon Res. 1990;10:141–151. [DOI] [PubMed] [Google Scholar]
- 14.Coradini D, Biffi A, Pirronello E, et al. The effect of α-, β- and λ-interferon on the growth of breast cancer cell lines. Anticancer Res. 1994;14:1779–1784. [PubMed] [Google Scholar]
- 15.Damdinsuren B, Nagano H, Sakon M, et al. Interferon-β is more potent than interferon-α in inhibition of human hepatocellular carcinoma cell growth when used alone and in combination with anticancer drugs. Ann Surg Oncol. 2003;10:1184–1190. [DOI] [PubMed] [Google Scholar]
- 16.Vitale G, de Herder WW, van Koetsveld PM, et al. Interferon-beta is a highly potent inhibitor of gastroenteropancreatic neuroendocrine tumor cell growth in vitro. Cancer Res. 2006;66:554–562. [DOI] [PubMed] [Google Scholar]
- 17.Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 18.Chen ZH, Zhang H, Savarese TM. Gene deletion chemoselectivity: codelection of the genes for 16INK4, methylthioadenosine phosphorylase, and the α- and β-interferons in human pancreatic cell carcinoma lines and its implications for chemotherapy. Cancer Res. 1996;56:1083–1090. [PubMed] [Google Scholar]
- 19.Hofland LJ, van Koetsveld PM, Lamberts SW. Percoll density gradient centrifugation of rat pituitary tumor cells: a study of functional heterogeneity within and between tumors with respect to growth rates, prolactin production and responsiveness to the somatostatin analog SMS 201–995. Eur J Cancer. 1990;26:37–44. [DOI] [PubMed] [Google Scholar]
- 20.Ferone D, Pivonello R, van Hagen PM, et al. Quantitative and functional expression of somatostatin receptor subtypes in human thymocytes. Am J Physiol Endocrinol Metab. 2002;283:E1056–E1066. [DOI] [PubMed] [Google Scholar]
- 21.John WJ, Flett MO. Continuous venous infusion of 5-fluorouracil and interferon-alpha in pancreatic carcinoma. Am J Clin Oncol. 1998;21:147–150. [DOI] [PubMed] [Google Scholar]
- 22.Sparano J, Lipsitz S, Wadler S, et al. Phase II trial of prolonged continuous infusion of 5-fluorouracil and interferon-alpha in patients with advanced pancreatic cancer: Eastern Cooperative Oncology Group protocol 3292. Am J Clin Oncol. 1996;19:546–551. [DOI] [PubMed] [Google Scholar]
- 23.Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000;179:367–371. [DOI] [PubMed] [Google Scholar]
- 24.Brembeck FH, Schoppmeyer K, Leupold U, et al. A phase II pilot trial of 13-cis retinoic acid and interferon-α in patients with advanced pancreatic carcinoma. Cancer. 1998;83:2317–2323. [DOI] [PubMed] [Google Scholar]
- 25.Sporn JR, Buzaid AC, Slater D, et al. Treatment of advanced pancreatic adenocarcinoma with 5-FU, leucovorin, interferon-alpha-2b, and cisplatin. Am J Clin Oncol. 1997;20:81–83. [DOI] [PubMed] [Google Scholar]
- 26.Bernhard H, Jager-Arand E, Bernhard G, et al. Treatment of advanced pancreatic cancer with 5-fluorouracil, folinic acid and interferon alpha-2A: results of a phase II trial. Br J Cancer. 1995;71:102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Xiong Q, Shi Q, et al. Intact nitric oxide synthase II gene is required for interferon-β-mediated suppression of growth and metastasis of pancreatic adenocarcinoma. Cancer Res. 2001;61:71–75. [PubMed] [Google Scholar]
- 28.Endou M, Mizuno M, Nagata T, et al. Growth inhibition of human pancreatic cancer cells by human interferon-β gene combined with gemcitabine. Int J Mol Med. 2005;15:277–283. [PubMed] [Google Scholar]
- 29.Busch M, Wilkowski R, Schaffer M, et al. Combined chemotherapy, radiotherapy, and immunotherapy for pancreatic carcinoma: a case report. Adv Ther. 2000;17:133–139. [DOI] [PubMed] [Google Scholar]
- 30.Recchia F, Sica G, Casucci D, et al. Advanced carcinoma of the pancreas: phase II study of combined chemotherapy, beta-interferon, and retinoids. Am J Clin Oncol. 1998;21:275–279. [DOI] [PubMed] [Google Scholar]
- 31.Saidi RF, Williams F, Ng F, et al. Interferon receptors and the caspase cascade regulate the antitumor effects of interferons on human pancreatic cancer cell lines. Am J Surg. 2006;191:358–363. [DOI] [PubMed] [Google Scholar]
- 32.Ota H, Nagano H, Doki Y, et al. Expression of type I interferon receptor as a predictor of clinical response to interferon-α therapy of gastrointestinal cancers. Oncol Rep. 2006;16:249–255. [PubMed] [Google Scholar]
- 33.Saidi RF, Williams F, Silberberg B, et al. Expression of interferon receptors in pancreatic cancer: identification of a novel prognostic factor. Surgery. 2006;139:743–748. [DOI] [PubMed] [Google Scholar]
- 34.Mogensen KE, Lewerenz M, Reboul J, et al. The type I interferon receptor: structure, function, and evolution of a family business. J Interferon Cytokine Res. 1999;19:1069–1098. [DOI] [PubMed] [Google Scholar]
- 35.Deonarain R, Chan DCM, Platanias LC, et al. Interferon-α/β-receptor interactions: a complex story unfolding. Curr Pharm Des. 2002;8:2131–2137. [DOI] [PubMed] [Google Scholar]
- 36.Domanski P, Witte M, Kellum M, et al. Cloning and expression of a long form of the beta subunit of the interferon alpha beta receptor that is required for signalling. J Biol Chem. 1995;270:21606–21611. [DOI] [PubMed] [Google Scholar]
- 37.Domanski P, Colamonici OR. The type-I interferon receptor: the long and short of it. Cytokine Growth Factor Rev. 1996;7:143–151. [DOI] [PubMed] [Google Scholar]
- 38.Pestka S. The interferon receptors. Semin Oncol. 1997;24(suppl 9): 18–40. [PubMed] [Google Scholar]
- 39.Cohen B, Novick D, Barak S, et al. Ligand-induced association of the type I interferon receptor components. Mol Cell Biol. 1995;15:4208–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeffer LM, Basu L, Pfeffer SR, et al. The short form of the interferon alpha/beta receptor chain 2 acts as a dominant negative for type I interferon action. J Biol Chem. 1997;272:11002–11005. [DOI] [PubMed] [Google Scholar]
- 41.Hardy MP, Owczarek CM, Trajanovska S, et al. The soluble murine type I interferon receptor Ifnar-2 is present in serum, is independently regulated, and has both agonistic and antagonistic properties. Blood. 2001;97:473–482. [DOI] [PubMed] [Google Scholar]
- 42.McKenna SD, Vergilis K, Arulanandam AR, et al. Formation of human IFN-beta complex with the soluble type I interferon receptor IFNAR-2 leads to enhanced IFN stability, pharmacokinetics, and antitumor activity in xenografted SCID mice. J Interferon Cytokine Res. 2004;24:119–129. [DOI] [PubMed] [Google Scholar]
- 43.Buchwalder PA, Buclin T, Trinchard I, et al. Pharmacokinetics and pharmacodynamics of IFN-beta 1a in healthy volunteers. J Interferon Cytokine Res. 2000;20:857–866. [DOI] [PubMed] [Google Scholar]
- 44.Salmon P, Le Cotonnec JY, Galazka A, et al. Pharmacokinetics and pharmacodynamics of recombinant human interferon-beta in healthy male volunteers. J Interferon Cytokine Res. 1996;16:759–764. [DOI] [PubMed] [Google Scholar]
- 45.Wagner TC, Velichko S, Chesney SK, et al. Interferon receptor expression regulates the antiproliferative effects of interferons on cancer cells and solid tumors. Int J Cancer. 2004;111:32–42. [DOI] [PubMed] [Google Scholar]
- 46.Jaks E, Gavutis M, Uze G, et al. Differential receptor subunit affinities of type I interferons govern differential signal activation. J Mol Biol. 2006;18 (Epub ahead of print). [DOI] [PubMed]
- 47.Klaus W, Gsell B, Labhardt AM, et al. The three-dimensional high resolution structure of human interferon alpha-2a determined by heteronuclear NMR spectroscopy in solution. J Mol Biol. 1997;274:661–675. [DOI] [PubMed] [Google Scholar]
- 48.Karpusas M, Nolte M, Benton CB, et al. The crystal structure of human interferon beta at 2. 2-A resolution. Proc Natl Acad Sci USA. 1997;94:11813–11818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platanias LC, Fish EN. Signaling pathways activated by interferons. Exp Hematol. 1999;27:1583–1592. [DOI] [PubMed] [Google Scholar]