Abstract
Introduction:
Predictors of outcome in patients with metastatic colorectal cancer remain inconsistent. We aimed to identify predictors of outcome in these patients, to develop a prognostic scoring system, and to assess the general applicability of the current major risk scoring systems.
Materials and Methods:
Following IRB approval, medical records of 662 consecutive patients undergoing resection of colorectal metastases to the liver during 1960 to 1995 were reviewed. Clinicopathologic and outcome data were assessed from records and mailed questionnaire. Clinicopathologic variables were tested using univariate and multivariate analyses; best-fit models were then generated to study the effect of each independent risk factor on outcome. To validate existing scoring models, our independent data set was applied to those scores. The relative concordance probability estimates were calculated for these models and compared with that of the proposed Mayo model.
Results:
The overall and disease-specific 5-year survival rates were 37% and 42%, respectively. The probability of recurrence at any site was 65% at 5 years. Perioperative blood transfusion and positive hepatoduodenal nodes were the major determinants of survival and recurrence. To assess the general applicability of the proposed risk scoring systems, we imported the data from our patient population into 3 other scoring systems. Neither survival nor recurrence among our patients was stratified discretely by any of the scoring systems. Based on probability estimates, all models were only marginally better than chance alone in predicting outcome.
Conclusion:
Broad application of risk scoring systems for patients with metastatic colorectal cancer has limited clinical value and refinement and external validation should be undertaken before utilization.
Predictive models for assessing outcome in patients with metastatic colorectal cancer are imperfect. In this study, we aimed to identify predictors of outcome in these patients, develop a prognostic scoring system, and assess the general applicability of 3 current risk scoring systems with our data.
Hepatic resection of metastatic colorectal cancer has become the treatment of choice for selected patients after resection of the primary colorectal cancer. Despite variability in criteria for patient selection, survival outcomes have ranged consistently from 25% to 45%.1–6 These data have supported repeatedly the clinical contention that cure is achieved in some of these patients because of the long-term (>5 years) absence of recurrence7–9 and the disparate survival compared with the natural history of similar patient cohorts with unresected hepatic metastases.10–12 Although selection criteria for resection have expanded over the last 2 decades, the specific criteria for selection remain controversial.
Patient selection for resection of hepatic metastases for colorectal cancer is based primarily on documentation of resectable intrahepatic disease and exclusion of extrahepatic disease with the exception of selected pulmonary metastases. However, despite numerous studies correlating patient, tumor, and interventional factors to survival,3–6,13 reliable predictors of survival in patients with metastatic colorectal cancer remain inconsistent. To refine candidacy for selection of patient for resection and for adjuvant therapy, several prognostic scoring systems have been proposed to stratify patients into risk categories for clinical management.4–6,14–16 Although these scoring systems have a sound statistical and clinical validity within referral centers, general applicability to more heterogeneous patient populations has not been confirmed. Predictive scoring models calculated on quantitative data have numerous benefits. Such models permit accurate comparisons of patient populations among studies, provide proper prognostic information to the patient, and, if distinct stratification levels are evident, guide treatment strategies and stratification of patients among institutions. Scoring models should be validated independently before generalized application and acceptance. Although prospective validation of scoring systems is preferable, the actual frequency of patient accrual for hepatic resection of colorectal metastases and duration of follow-up required to assess survival (5 years) limit this approach. As an alternative for validation and to determine whether currently proposed risk scoring systems were applicable to our independent patient population, we have updated our experience with hepatic resection of metastatic colorectal cancer and have imported our findings into 3 proposed scoring systems4–6 to determine whether outcomes for risk stratification were similar.
Specifically, we assessed the mortality, survival, and recurrence patterns among our patients with hepatic resection for colorectal metastases. We attempted to identify predictors of outcome from our patient population and develop a scoring system. The issue of general applicability of several currently proposed risk scores was addressed by calculating survival based on the number of specific risk factors cited in the stratification schemes of those risk scoring systems. We evaluated patients managed within the time frame during which those scoring systems were developed to provide a similar patient population because current outcomes following resection of metastatic colorectal cancer to the liver may differ from earlier reports.
MATERIALS AND METHODS
Following approval of the Mayo Clinic Institutional Review Board, the medical records of 662 consecutive patients who underwent resection of colorectal metastases to the liver during the period 1960 to 1995 were reviewed. Histopathology of each metastasis confirmed metastatic colorectal cancer. Patients who had initial hepatic resection elsewhere (n = 10) or had only local ablative therapy (n = 7) were excluded.
Definitions
The primary colorectal cancer was staged using UICC/AJCC staging system for colorectal cancer.17 Synchronous liver metastases were defined as those detected within 3 months of diagnosis of primary colorectal cancer. The anatomic distribution of liver lesions was defined by Couinaud nomenclature.18 Size of the liver lesion was measured by the pathologist, in centimeters, before fixation of the specimen. The Broder's system17 was used to histologically grade metastases. Surgical margins were defined by histology as either cancer negative or positive. Margin of resection was measured in centimeters by the pathologist, before fixation of the specimen. Blood transfusions 1 week prior to and/or 2 weeks following hepatic resection were defined as perioperative. Postoperative mortality was defined as death occurring in the hospital or within 60 days of resection.
Follow-up
Questionnaires were sent to all patients who had an incomplete follow-up. Two mailings were done for nonresponders, and after that the patients were labeled as lost to follow-up. The results are reported.
The follow-up was complete, until death or within 1 year of data collection, in 93% of the patients.
Endpoints
The primary endpoints of this study were overall survival, disease-specific survival, and recurrence at the most recent follow-up evaluation. Overall survival was defined as the time interval between the date of hepatic resection and the date of death or most recent date of follow-up if the patient was alive. Disease-specific survival was defined as the time from hepatic resection to death from primary cancer. Patients who died in the postoperative period or during hospitalization were excluded from the survival analysis. Recurrence was defined as the time from hepatic resection to first documented disease recurrence. The probability of recurrence represents the chance of developing the first recurrence at 5 years. The criteria for establishing recurrent disease were histologic confirmation, radiologic evidence of progression with subsequent clinical progression, and supportive biochemical data (eg, rising serum level of carcinoembryonic antigen, CEA). Patients with recurrence were defined in terms of local, hepatic, or distant recurrence. Local recurrence was confined to the colon, rectum, pelvis, or adjacent organs. Recurrence in the liver was defined as a new lesion detected in the liver more than 1 month after hepatic resection; other extrahepatic recurrences were labeled as distant.
Surgical Procedures
Major liver resection was defined as right hepatectomy (segments V, VI, VII, and VIII), extended right hepatectomy (segments IV, V, VI, VII, and VIII), left hepatectomy (segments II, III, IV ± I), extended left hepatectomy (segments II, III, IV, V, VIII ± I) or resection of more than 2 liver segments.19 Liver resections were defined as minor if subsegmental, unisegmental, or bisegmental.
Statistical Analysis
Data are presented as median (range) or mean (standard deviation). The Kaplan-Meier method was used to analyze survival and recurrence patterns. The study period provided potential follow-up of a minimum of 5 years for patients surviving operation; thus, survival was actual. Two-tailed log-rank test was used to assess differences between numbers. Sixteen clinicopathologic variables were tested for their effect on overall survival, disease-specific survival, and probability of recurrence using univariate analysis. The statistically significant variables were used to construct a multivariable model using the Cox proportional hazards method (backward elimination method). Using parameter estimates, best-fit models were generated to study the effect of each independent risk factor on outcome. A 2-sided P value of <0.05 was considered statistically significant. Patients with missing variables were excluded from multivariable analysis and model building.
The predictive accuracy of our model was evaluated by calculating the concordance probabilities. The concordance probability is defined as the proportion of all possible pairs of observations in the data in which the ordering of that patient pair as predicted by the model agrees with the observed outcome (ignoring tied survival times).20 Values range from 0 to 1, with values close to 1 indicating that the model almost perfectly discriminated between patients with higher and lower risks of death (or recurrence), while values close to 0.5 suggested that the predictive ability of the model was no better than chance alone. Ninety-five percent confidence intervals (CIs) for the estimated concordance were calculated using bootstrap resampling with 1000 replications.
Evaluation of Proposed Risk Scores
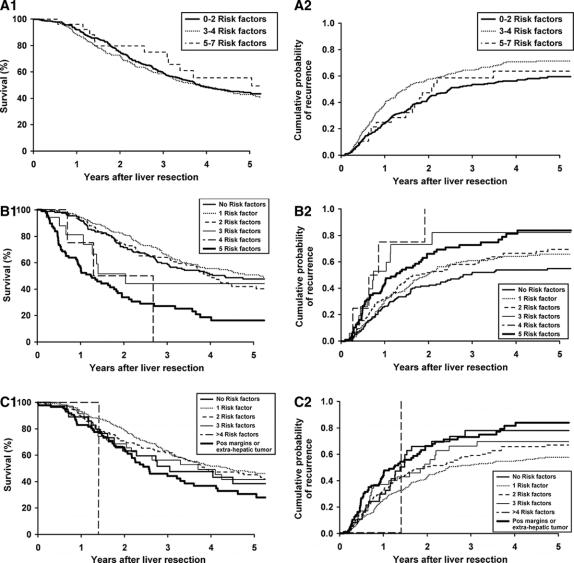
Several institutions have proposed risk scoring systems with the aim of optimizing patient selection for hepatic resection and to stratify patients for the need of adjuvant therapies. Scoring systems proposed by Schindl et al16 (n = 131, includes stage of colorectal cancer, number of liver metastases, CEA levels, albumin and alkaline phosphatase levels), Ueno et al14 et al (n = 85, includes aggressiveness of primary tumor, early liver metastases, number of liver metastases), and Lise et al15 (n = 135, includes percentage of liver invasion, metastases to lymph nodes at the primary tumor site, number of liver metastases, preoperative glutamic pyruvic transaminase levels, and type of liver resection) have been developed on small study populations and their clinical utility has not been established. Three other scoring systems were developed on large patient populations. Nordlinger et al,4 through a national collective registry of 1513 patients, developed a prognostic scoring system based on 7 identified risk factors: age >60 years; primary cancer extending into serosa; positive regional lymph nodes; liver metastases confirmed within 24 months of the primary cancer; CEA levels; size of metastasis greater than 5 cm and <1 cm resection margin of the metastases. Three risk groups were defined: low risk (0–2 risk factors), intermediate risk (3 or 4 risk factors), and high risk (5–7 risk factors). Fong et al,6 through a single institution study of 1001 patients, similarly devised a system based on node positive primary cancer, hepatic metastases confirmed within 12 months of the primary cancer, >1 metastases, size of metastasis >5 cm, and CEA level >200 ng/mL. Six risk groups were stratified by the sum of the individual prognostic variables. Iwatsuki et al,5 also through a single institution study of 230 patients, proposed a risk-score based on >3 hepatic metastases, size of metastasis >8 cm, hepatic metastases confirmed within ≤30 months of the primary cancer, and bilobar hepatic metastases. Five risk grades were stratified based on the sum of the individual prognostic variables: grade 1 being no risk factor present to grade 5, including patients with 4 risk factors. These 3 scoring systems were chosen for validation utilizing data from our institution.
Statistical methods used to construct these risk scoring systems, though similar, varied by method. To validate these scoring models, our independent data set was applied to each of the 3 major risk scoring systems. We used each described scoring criteria to stratify our patients to determine the general applicability of each scoring system. The predictive accuracy of these models was determined using relative concordance probability estimates which were then compared with that of the proposed Mayo model.
RESULTS
Patient Population
Between 1960 and 1995, 662 consecutive patients underwent resection of hepatic metastases from colorectal cancer. There were 404 men and 258 women. Mean age was 60 ± 11 years and did not differ by gender.
Primary Tumor Characteristics
The primary cancer was located in the colon in 497 patients (right, 154; transverse, 39; left, 57; sigmoid, 247) and in the rectum in 166. At the time of initial presentation, 49 (7.7%) patients had stage I and 193 (30.3%) had stage II colon cancer. Stage III represented the largest group with 291 patients (45.7%), while 104 (16.3%) patients had stage IV disease. Staging of the primary cancer was unavailable for 25 patients. Two percent of tumors were Broder's grade 1, 67.8% grade 2, 28.7% grade 3, and 1.5% grade 4. Data on tumor grade were absent for 119 patients.
Liver Metastases
Hepatic metastases were identified synchronously (ie, within 3 months of primary operation) in 221 (33.4%) patients. A total of 396 patients (59.9%) had a solitary metastasis, 198 (30%) had 2 or 3 metastases, and 68 patients (10%) had 4 or more metastases. The median size of the metastases was 4.5 cm (range, 0.2–26 cm). The hepatic metastases were unilobar in 502 (75.8%) patients and bilobar in 160 (24.2%).
The metastases were histologically grade 2 in 380 (58.3%), grade 3 in 259 (39.7%), and grade 4 in 13 (2%) patients. Tumor grade was not assessable for 10 patients. The frequency distribution of tumor grade for the hepatic metastases showed significant dedifferentiation compared with the distribution of the primary cancer (P < 0.0001).
Surgical Resections
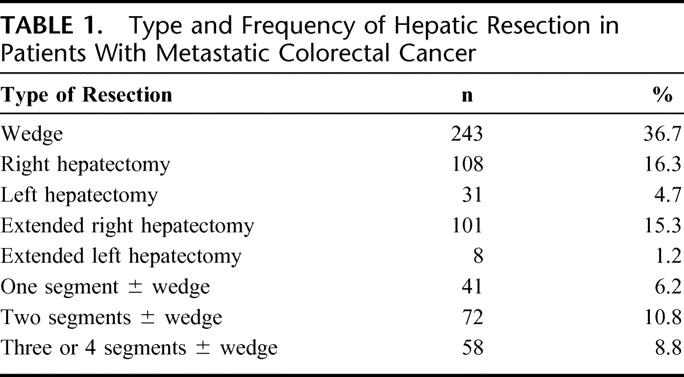
The frequency of minor resections (356; 54%) and major resections (306; 46%) was similar (Table 1). A total of 350 patients (56%) received blood transfusions perioperatively, but the frequency of blood transfusions has decreased over time.
TABLE 1. Type and Frequency of Hepatic Resection in Patients With Metastatic Colorectal Cancer
Although each patient had complete resection of the metastases macroscopically, 62 (9.4%) patients had microscopically positive pathologic surgical margins. Pathologic margins were microscopically negative in 478 (72.1%) and were not assessable by pathology report in 122 (18.4%) patients.
Of 662 patients undergoing hepatic resection, 100 also had extrahepatic disease. The majority (44) had involvement of other organs by direct extension: diaphragm (n = 21), perinephric fascia (n = 3), extrahepatic portal vein (n = 9), extrahepatic biliary tree (n = 6), and abdominal wall (n = 5). Hepatic resection was performed in the setting of discontiguous extrahepatic metastases in 56 cases: portal nodal disease (n = 36), pulmonary metastases (n = 10), peritoneal disease (n = 7), and pelvic metastases (n = 3). Adjuvant chemotherapy (primarily 5-fluorouracil based) was administered to 207 (33.3%) patients.
Surgical Mortality
The 30- and 60-day mortality was 2% and 3%, respectively. Of the 19 deaths, 5 patients had minor and 14 patients had major liver resection. Causes of death were abdominal sepsis (4), hepatic failure (4), bleeding from liver parenchyma (2), unknown (4), abdominal wound dehiscence (2), myocardial infarction (1), pulmonary embolism (1), and brainstem infarction (1).
Long-Term Outcomes
Overall median follow-up for the study period was 3 years (range, 5 days to 37 years). At last follow-up, 234 (35%) patients were alive and 428 (65%) were dead. Recurrence at any site was diagnosed in 414 (62.5%) patients; 215 (32.5%) patients remained free of disease. The disease status was uncertain in 33 (5%) patients. Recurrent hepatic metastases after initial hepatic resection developed in 208 (31%) patients and were removed surgically in 28 (12%) patients.
Survival and Recurrence
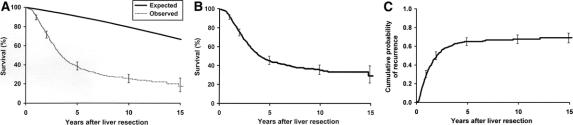
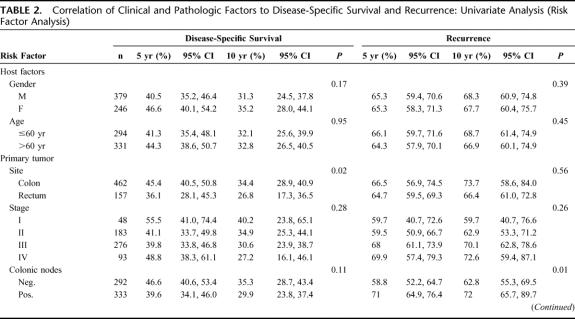
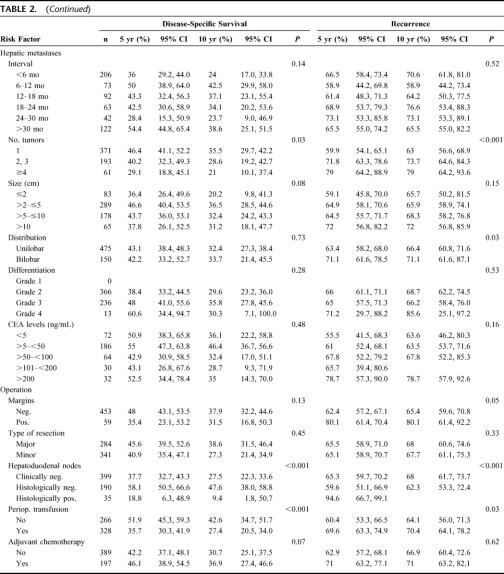
The overall and disease-specific 5-year survival were 37% and 42%, respectively (Fig. 1A, B). The probability of tumor recurrence after hepatic resection is shown in Fig. 1C. The cumulative probability of developing a recurrence at any site was 65% at 5 years. Eighty percent of patients developed recurrence within 3 years of the hepatic resection. The distribution sites of recurrence or metastases was in the liver in 224 (34%), lung in 171 (26%), and other sites in 18 (28%) patients.
FIGURE 1. Overall expected and observed survival (A) and disease-specific survival (B) of patients undergoing hepatic resection for colorectal metastases. C, Recurrence of patients undergoing hepatic resection for colorectal metastases.
Resection of recurrent hepatic metastases was possible in 28 patients and was associated with an actuarial 5-year survival rate of 73%.
Analysis of Risk Factors
All host and tumor factors were correlated to overall survival, disease-specific survival, and recurrence (Table 2). Patients who died postoperatively were excluded from risk analysis.
TABLE 2. Correlation of Clinical and Pathologic Factors to Disease-Specific Survival and Recurrence: Univariate Analysis (Risk Factor Analysis)
TABLE 2. (Continued)
Univariate Analysis
Disease-specific survival was significantly reduced for the following factors: site of primary cancer in the rectum, hepatic metastases confirmed within 30 months of the primary colorectal cancer, >1 hepatic metastasis, size of metastases >8 cm, hepatoduodenal lymph node metastases, perioperative blood transfusion, and adjuvant chemotherapy (Table 2).
Disease recurrence was significantly associated with the following factors: stage III and IV colorectal cancer at initial presentation, >1 hepatic metastasis, bilobar hepatic metastases, positive hepatoduodenal lymph nodes, positive margins after hepatic resection, and perioperative blood transfusions.
Multivariable Analysis
Multivariable analysis was performed on those factors correlating significantly to disease-specific survival and recurrence by univariate analysis (Table 3). Disease-specific survival was significantly associated with metastases confirmed in an interval of <30 months after the diagnosis of primary colorectal cancer and size of metastases >8 cm. The survival hazard ratio was 1.4 in the presence of either of these factors. Perioperative blood transfusion and positive hepatoduodenal lymph nodes were associated with 1.5 and 2.8 times the risk of death, respectively. For recurrence, stage III colorectal cancer, >1 hepatic metastasis, perioperative blood transfusions, and positive hepatoduodenal nodes were associated with hazard ratio of 1.3 to 2.5.
TABLE 3. Correlation of Clinical and Pathologic Factors to Disease-Specific Survival and Recurrence: Multivariable Analysis
Clinical Risk Score
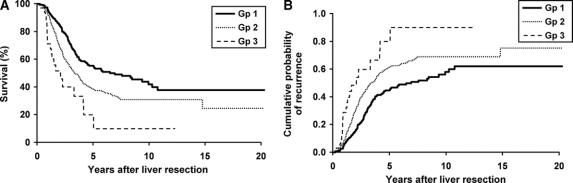
To study the effect of each statistically significant factor from multivariate analysis on long-term outcome, best-fit models were generated. These variables were organized into 3 groups, using disease-specific survival as the outcome variable (Table 4). Patients with any risk factors except for perioperative blood transfusion and positive hepatoduodenal lymph nodes comprised group I. Patients having any risk factors except for positive hepatoduodenal lymph nodes comprised group II. Patients with positive hepatoduodenal lymph nodes, with or without any other risk factor, comprised group III. The disease-specific survival of these 3 groups is shown in Figure 2A. Five-year disease-related survival was 55% in group I, 39% in group II, and only 20% in group III.
TABLE 4. Best-Fit Models
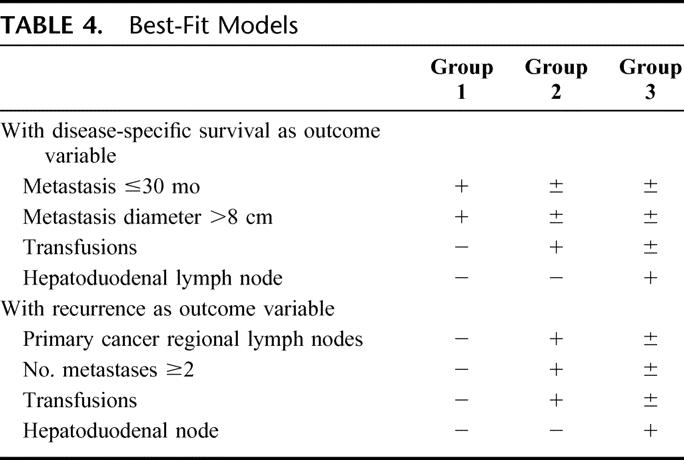
FIGURE 2. Mayo Risk Groups. (A) Disease-specific survival (A) and recurrence (B) of patients undergoing hepatic resection for colorectal metastases using best fit models.
Recurrence as the outcome variable was analyzed similarly (Table 4). Patients with no risk factors comprised group I, those with any risk factor except for positive hepatoduodenal lymph nodes comprised group II, and patients with positive hepatoduodenal lymph nodes regardless of the presence of any other risk factor comprised group III. At 5 years, 46% of patients develop recurrence in group I, 67% in group II, and 95% in group III (Fig. 2B). This risk analysis showed that perioperative blood transfusion and positive hepatoduodenal lymph nodes were the 2 most important determinants of long term survival and recurrence.
Evaluation of Proposed Risk Scores
To assess the general applicability of the proposed risk scoring systems, we imported the data from our patient population into the 3 scoring systems (Material and Methods).4–6 We used the same inclusion and exclusion criteria detailed in the methodology for those respective scoring systems. For the systems of Nordlinger et al4 and Fong et al,6 648 and 536 patients, respectively, met the outlined criteria. No patients were excluded from risk analysis proposed by the Iwatsuki et al system.5 Figure 3 (A1, B1, C1) depicts the disease-specific survival of our patients based on the criteria of the 3 risk scoring systems. Figure 3 (A2, B2, C2) depicts the probability of recurrence for the 3 scoring systems. Neither survival nor recurrence among our patients was stratified discretely by any of the scoring systems. Survival was stratified best by the risk scoring system of Fong et al6 and least by that of Nordlinger.4 However, overlap of survival for various risk scores from each system is evident. Although survival between low- and high-risk scores for the systems of Fong and Iwatsuki diverged, only a risk score of 4 from the Fong scoring system identified a patient subset without 5-year survival among our patients. Similar limitations in stratifying disease recurrence for patients with resected hepatic metastases were evident for each of the scoring systems (Fig. 3 A2, B2, C2). Recurrence was again stratified best by the risk scoring system of Fong et al6 and least by that of Iwatsuki et al.5
FIGURE 3. Disease-specific survival (A1, B1, C1) and recurrence (A2, B2, C2) using existent scoring systems where A, B, and C represent Nordlinger, Fong, and Iwatsuki scores, respectively.
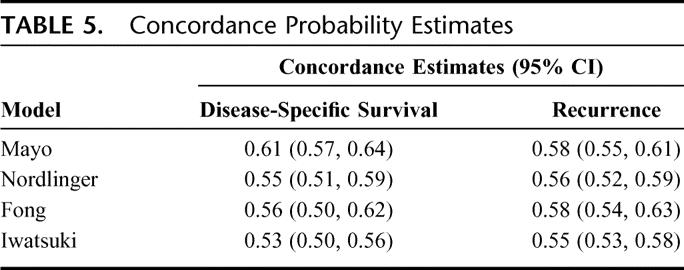
Concordance probability estimates for disease-specific survival and recurrence for all the models are shown in Table 5. Although the proposed Mayo model performed marginally better in discriminating patients at high/low risk of death from disease, all models were only marginally better than chance alone in predicting disease-specific survival and recurrence.
TABLE 5. Concordance Probability Estimates
DISCUSSION
We report a large, single-institution experience of hepatic resection for colorectal metastases with a 5-year follow-up of 93%. Our study period spanned over 35 years. Although there are few large series reporting actual 5-year survival, disease-specific survival herein was 42% at 5 years, 32% at 10 years, and 26% at 15 years, which is consistent with the predicted survival of other large series.1–8 We correlated various clinical, pathologic, and interventional factors with survival and recurrence and developed a risk scoring system from factors that were significant by multivariable analysis. However, our risk scoring system had limited utility and had only an average predictive accuracy based on concordance probability estimates. Finally, we examined other risk scoring systems to validate the usefulness for selecting patients for hepatic resection or subsequent adjuvant treatment but similarly found limited utility and suboptimal discriminatory ability. Although our findings further confirm the efficacy of hepatic resection of colorectal metastases, the broad application of risk scoring systems currently has limited clinical value.
Our study and numerous others4–6,10,14–16 have correlated a number of clinical, pathologic, and interventional factors with overall survival, disease-specific survival, and recurrence for patients with resected hepatic metastases from colorectal cancer by univariate and multivariable analyses. Many of the correlates to survival have been reported previously, but there remains inconsistency interinstitutionally. Indeed, even intrainstitutionally, correlates of survival have varied.2,8 Selection of patients for hepatic resection of colorectal metastases has likely affected the consistency of the correlates to survival. Most reports have spanned many years to decades to accumulate a substantive experience. Selection of patients for operation initially was stringent because operative mortality was significant, the accuracy of imaging for staging metastatic disease was limited, and the survival benefits for resection were unknown. Selection currently has expanded because accumulated evidence supporting survival benefit for resection has emerged, perioperative risk has decreased, and the accuracy of imaging has improved. Indeed, hepatic resection is the current treatment of choice for metastatic colorectal cancer if all disease grossly is resectable. Indeed, even staged resections of hepatic metastases are undertaken with or without neoadjuvant chemotherapy to accomplish complete resection.21
Our univariate analysis identified a number of variables affecting outcome. However, multivariable logistic regression analysis identified only perioperative blood transfusion and positive hepatoduodenal lymph nodes as independent and clinically significant correlates of both survival and recurrence. Unfortunately, neither variable is identifiable preoperatively. Thus, the “Mayo Scoring System” has limited clinical utility for selection of patients for operation or for further preoperative imaging or staging but may be used to counsel patients postoperatively regarding prognosis and for selection of various adjuvant therapies. Indeed, more aggressive adjuvant therapy may be warranted, particularly for those patients at high risk as proposed by others.6 Interestingly, we have observed that significant predictors of survival and recurrence have changed between reports from our center. Previously, satellite configuration of hepatic metastases and clinical detection of metastases were significant correlates.8 These observations coupled with the differences in risk factors observed by others2,4–6,8,10,14–16 and the low concordance probability estimates suggest that stratification of patients by clinical and pathologic factors, although statistically sound, may be clinically unreliable and are not widely applicable for selection of patients for operation or a basis for comparison of patient cohorts between institutions unless validated.
The utility of a prognostic model or scoring system for hepatic metastases from colorectal cancer requires internal and external validation before general use.22 Methods of internal validation or reproducibility of a prognostic system are based on data resampling techniques, such as bootstrapping. Although bootstrapping is widely accepted for internal validation, it is computer and labor intensive. Because we had only 2 independently significant variables, we did not perform bootstrapping for internal validation. Moreover, internal statistical validation techniques do not address the issue of generalizability or general use. Generalizability or external validity of a prognostic model can be tested by different forms of validations, including prospective, independent, multi-institutional, and multiple independent validations with varying follow-up periods.23 We attempted to address the generalizability of 3 current clinical scoring systems by use of our patient population. These risk scoring systems failed to confirm predictive stratification for our patients with metastatic colorectal cancer undergoing hepatic resection. This phenomenon or lack of validation has been observed and reported by others. Indeed, Iwatsuki et al5 failed to identify 60% of their study population with the best prognosis based on their scoring system after applying the French scoring system.4 Indeed, in repeat analysis of the French risk scoring system, only 3 of 7 risk factors significantly correlated with outcome.7 These results confirm the necessity of external validation of models of risk scoring between institutions before general use for selection or comparison of data sets. Although statistical methods can ensure model reliability, it does not mean that data from 1 institution will fit exactly into another model addressing the same disease, but the clear stratification should be evident. Unrecognized selection biases, population differences, risk of overfitting through too few events per variable, changing diagnostic modalities, and modifications in surgical techniques or other therapy may all adversely influence the transportability of risk scores between institutions. Finally, the actual duration and completeness of follow-up are particularly relevant to predictive models of survival. Although the models4–6 addressed in this study had similar mean duration of patient follow-up with appropriately complete follow-up, the actual follow-up herein was substantially longer by design. It is unlikely that our prolonged follow-up did not provide the opportunity for event of interest to occur as predicted by the other models. In other words, models developed on shorter durations of follow-up tend to less accurately predict survival and recurrences.
The remarkably consistent finding of this study, previous reports from our institution, and other studies is that long-term survival occurs after potentially curative liver resection.2,4–8,12 Despite extensive worldwide experience with liver resection for metastatic colorectal cancer, the issue of patient selection remains controversial. Neither the previously proposed risk scores nor our Mayo scoring system can be applied preoperatively for patient selection or interinstitutional comparison of patient populations. Risk scoring may be appropriate for postoperative counseling of patients regarding prognosis. Although current data suggest that the 5-year survival of patients with a solitary hepatic metastasis exceeds 50% to 60% after hepatic resection,24 the overall survival of all patients undergoing hepatic resection for colorectal metastases is not great enough to obviate adjuvant therapy on the basis of risk scoring to date.
CONCLUSION
Currently, there are no reliable scoring systems for selection of patients with hepatic metastases for resection. Hepatic resection should be undertaken if all gross disease can be addressed. The future development of risk scoring models for patients with colorectal cancer, while potentially important to patient and physician, will likely require larger patient populations and analyses of additional clinicopathologic factors, such as genetic markers, to provide accurate stratification of patients and external confirmation for validity for uses other than counseling of patients.
Footnotes
Reprints: David M. Nagorney, MD, Mayo Clinic, 200 First Street SW, Rochester, MN 55905. E-mail: nagorney.david@mayo.edu.
REFERENCES
- 1.Registry of Hepatic Metastases. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Surgery. 1988;103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheele J, Rudroff C, Altendorf-Hofmann A. Resection of colorectal liver metastases revisited. J Gastrointest Surg. 1997;1:408–422. [DOI] [PubMed] [Google Scholar]
- 4.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 5.Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Br J Surg 1997;84:977–980. [DOI] [PubMed] [Google Scholar]
- 8.Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997;132:505–510. [DOI] [PubMed] [Google Scholar]
- 9.D'Angelica M, Brennan MF, Fortner JG, et al. Ninety-six five-year survivors after liver resection for metastatic colorectal cancer. J Am Coll Surg. 1997;185:554–559. [DOI] [PubMed] [Google Scholar]
- 10.Lahr CJ, Soong S-J, Cloud G, et al. A multifactorial analysis of prognostic factors in patients with liver metastases from colorectal carcinoma. J Clin Oncol. 1983;1:720–726. [DOI] [PubMed] [Google Scholar]
- 11.Wagner JS, Adson MA, van Heerden JA, et al. The natural history of hepatic metastases from colorectal cancer. Ann Surg. 1984;199:502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. [DOI] [PubMed] [Google Scholar]
- 13.Rosen CB, Donohue JH, Nagorney DM. Liver resection for metastatic colonic and rectal carcinoma. In: Cohen AM, et al, eds. Cancer of the Colon, Rectum, and Anus. New York: McGraw-Hill; 1995:805–821. [Google Scholar]
- 14.Ueno H, Mochizuki H, Hatsuse K, et al. Indicators for treatment strategies of colorectal liver metastases. Ann Surg. 2000;231:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lise M, Bacchetti S, Da Pian P, et al. Patterns of recurrence after resection of colorectal liver metastases: prediction by models of outcome analysis. World J Surg. 2001;25:638–644. [DOI] [PubMed] [Google Scholar]
- 16.Schindl M, Wigmore SJ, Currie EJ, et al. Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg. 2005;140:183–189. [DOI] [PubMed] [Google Scholar]
- 17.American Joint Committee on Cancer. AJCC Cancer Staging Manual, 5th ed. Philadelphia: Lippincott-Raven; 1997:66–69. [Google Scholar]
- 18.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. [DOI] [PubMed] [Google Scholar]
- 19.Bismuth H, Houssin D, Casting D. Major and minor segmentectomies ‘reglees’ in liver surgery. World J Surg. 1982;6:10–24. [DOI] [PubMed] [Google Scholar]
- 20.Oakes DA. Concordance test for independence in the presence of censoring. Biometrics. 1982;38:451–455. [PubMed] [Google Scholar]
- 21.Meric F, Patt YZ, Curley SA, et al. Surgery after downstaging of unresectable hepatic tumors with intra-arterial chemotherapy. Ann Surg Oncol. 2000;7:490–495. [DOI] [PubMed] [Google Scholar]
- 22.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–473. [DOI] [PubMed] [Google Scholar]
- 23.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130:515–524. [DOI] [PubMed] [Google Scholar]
- 24.Abdalla EK, Vauthey J-N, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]