Abstract
Objectives
To assess the long term outcome of children born following a first trimester measurement of nuchal translucency (NT) ≥ 99th centile during routine first trimester screening in an unselected population.
Study design
162 infants were born alive. Clinical examination as well as a questionnaire to the parents (Ages and Stages Questionnaires (ASQ)) at the age of 2 were obtained in 160 children. Our study population was compared to an external control group made of the 370 full-term control children.
Results
The prevalence of abnormal clinical pediatric examination and ASQ results at 2 years were not associated with NT thickness. Comparison with an external control group did not demonstrate an increased incidence of developmental delay.
Conclusion
Parents should be informed that when the fetus is shown to be normal by ultrasound at 22–24 weeks of gestation the risk of adverse neonatal outcome or developmental delay in early childhood is not increased.
Keywords: Child, Preschool; Cohort Studies; Developmental Disabilities; epidemiology; Female; Follow-Up Studies; Humans; Infant; Infant, Newborn; Karyotyping; Nuchal Translucency Measurement; Pregnancy; Pregnancy Trimester, First; Prospective Studies; Questionnaires; Time Factors
Keywords: Unselected population, First trimester screening, Nuchal translucency, Normal karyotype, Long term follow-up
Introduction
Screening for fetal aneuploidy is routinely offered to pregnant women and nuchal translucency (NT) thickness measurement is widely used as part of this screening. Although the risk of aneuploidy increases with NT thickness and no biometric cut-off is advisable (1), increased NT above the 95th centile irrespective of fetal karyotype has been associated with adverse outcome including mainly cardiac defects and genetic syndromes (2–6). Around 1% of all fetuses should show increased nuchal translucency above the 99th centile for gestational age in an unselected population. Eight studies have addressed the issue of pediatric long-term follow up of chromosomally and anatomically normal fetuses with increased nuchal translucency (7–14). Up to 9% of them had developmental delay in early childhood (Table 1). However these figures should be considered with caution owing to the limited number of children studied from heterogeneous populations and using different cut-off values for NT. Follow-up was incomplete in up to 32% of the cases in some studies and post-natal assessment was often conducted retrospectively and only based on questionnaires to the parents.
Table I.
Review of the literature on postnatal follow-up in children that had increased nuchal translucency (NT) with normal karyotype at 11–14 weeks of gestation
| Population | Cut-off value for NT | Prevalence of increased NT and normal karyotype | Liveborn neonates | Method of assessment of child development | Postnatal follow-up (months) | Lost for follow-up | Developmental delay * n(%) and 95%CI | |
|---|---|---|---|---|---|---|---|---|
| Van Vugt (7) | NA | 3.0mm | NA | 50 | Questionnaire | 33.5 (7–75) | 32% | 1/34 (2.9%) [0%–15%] |
| Adekunle (8) | Screening | 4.0mm | 0.8% | 31 | Questionnaire | 23.0 (13–38) | 26% | 2/23 (8.7%) [l%–28%] |
| Maymon (9) | Referral | 95th centile | NA | 36 | Telephone | 24 (12–36) | 0% | 0/36 (0%) [0%–10%] |
| Brady (10) | Referral | 3.5mm | NA | 90 | Clinical examination | NA 6–42 | 1.1% | 1/89 (1.1%) [0%–6%] |
| Hippala (11) | Screening | 3.0mm | 0.8% | 59 | Clinical examination | 56 (24–84) | 15% | 1/50 (2.0%) [0%–11%] |
| Senat (12) | Referral | 4.0mm | NA | 58 | Clinical examination | 39 (12–72) | 7% | 3/54 (5.6%) [1%–15%] |
| Cheng (13) | Screening | 3.0mm | 0.74% | 14 | Clinical examination/Telephone | 21 (8–30) | 0 | 1/14 (7.1%) [0%–34%] |
| Souka (14) | Referral | 3.5mm | NA | 980 | NA | NA | 0 | 4/980 (0.4%) [0.1%–1%] |
% and 95% confidence interval are calculated from the original article
NA: not available
The relationship between isolated increased nuchal translucency thickness with normal karyotype in the first trimester and developmental delay in early childhood therefore remains questionable. Data on prospective follow-up assessment are needed to counsel couples following prenatal diagnosis. The objectives of this study were to describe the prevalence of developmental abnormalities as well as the relationship between nuchal translucency thickness and neonatal and pediatric outcome following first trimester measurement of NT ≥99th centile with normal karyotype.
Population and Methods
We conducted a cohort study in a large unselected pregnant population undergoing first trimester ultrasound screening for fetal aneuploidy in a single health authority. All patients gave oral consent to undergo follow-up and the study was approved by the local ethics committee. Results on the performance of combined first trimester screening using maternal age, NT thickness and maternal serum markers over 2 years in the first 14,934 cases have been reported elsewhere (15). NT was measured when the crown rump length (CRL) was between 45 and 84 mm. Fetuses with NT measurement ≥ 99th centile adjusted for gestational age were included in this long-term follow-up study. Cystic hygroma as defined by the presence of two paracervical cystic cavities whether associated with hydrops or not were excluded from the study (16). Patients were counselled regarding the risk of chromosomal abnormality and offered fetal karyotyping whenever appropriate. A detailed ultrasound examination was performed in all chromosomally normal fetuses at between 16 and 18 weeks of gestation to follow-up changes in nuchal translucency thickness and to rule out major fetal anatomical defects including fetal echocardiography. This was repeated at between 22 and 24 weeks of gestation. In cases with persistent increased nuchal fold, parents were counselled that the risk of worsening in utero or delivering a baby with a severe abnormality was higher than in the general population (14). In addition all cases were offered genetic counselling accounting for family history of malformation, developmental delay or consanguinity. Follow-up scans were performed monthly up until delivery. Nuchal translucency measurements, fetal karyotype, ultrasound findings and pregnancy outcome were recorded prospectively on a computer database. Post-mortem examination was systematically carried out in cases with intra-uterine death or termination of pregnancy. Adverse prenatal outcome was defined as a composite outcome including termination of pregnancy for fetal malformation, intrauterine death and miscarriage.
All children were examined by a pediatrician within 2 days after birth and then at 1, 4, 9 months and at 2 years of age. Pediatric clinical examination aimed at assessing post-natal growth, psychomotor skills and speech as well as interaction with the child. Features associated with genetic syndromes were systematically looked for. No systematic additional investigation was performed. This was completed by serial questionnaire to be answered by the parents. The Ages and Stages Questionnaires (ASQ) were developed in 1980 as a screening tool to be completed by the parents for early detection of developmental problems (17). They consist of a series of 19 questionnaires spanning the developmental period at between 4 months and 5 years of age. Each questionnaire contains a set of 30 questions representing five domains: communication, gross and fine motor activities, problem solving and personal social skills. Questionnaires are scored by adding up all domain scores and comparing each domain score with the screening cut-off score for that domain. The screening cut-off for each domain was 2 standard deviations (SD) below the mean score. If the child’s score was below 2 SD in one or more domains, further assessment of the child’s performance was recommended. Children with a NT measurement < 99th centile were not followed-up. We anticipated a high loss-for-follow-up rate in a population with no incentive for clinical and developmental follow-up after birth following an uneventful pregnancy. Our study population was therefore compared to an external control group. This control group was made of the 370 full-term control children from a French national population-based cohort study designed in 1997 to investigate at the consequences of very preterm birth (18,19). The full-term control group resulted from random recruitment in all maternity wards from nine French regions covering about one-third of all births in France. Infants were followed from birth up until the age of 5. They were assessed using the same 14 items used in our study to evaluate growth and development at 2 years of age.
Statistical analysis
The analysis focused on fetal and pediatric follow-up and outcome including adverse prenatal outcome, postnatal diagnosis of malformations, as well as development at 2 years of age. The relationship between nuchal translucency and each of these characteristics was analysed by logistic regression with adjustment for both gestational age and maternal age. The shape of the relationship was established using fractional polynomials (20). Whenever the relationship was not significantly different from linearity, it was summarized by an odds ratio (OR) corresponding to the risk variation for each 1 mm of NT. The developmental characteristics were also compared to those of the external control group by using a chi-squared test.
Results
Routine first-trimester ultrasound screening was performed in 21,149 unselected pregnant women between January 1st 2001 and December 31st 2003, including nuchal translucency measurement at 11–14 weeks’. 248 fetuses (1.2%) had NT ≥ 99th centile for CRL. Figure 1 shows the course and outcome of the 248 fetuses in relation with nuchal translucency. A normal karyotype was found in 179/248 (72.2%) fetuses. Median (25th–75th percentile) maternal age in the overall and in the study populations were 30.7 (28.0 – 33.9) and 30.7 (27.9 – 34.9) years respectively. Among cases with a normal karyotype, ten cases (5.6%) underwent TOP for fetal malformation including persistent unexplained increased NT evolving into nuchal edema or hydrops at 16–18 weeks (n=5), osteochondrodysplasia (n=1), omphalocele with cardiac malformation (n=1), fetal akinesia (n=2) and polymalformation (n=1). 5 (2.8%) cases had spontaneous intrauterine death before 22 weeks’. Two cases (1.1%) had a miscarriage and a social TOP respectively. The 162 (90.5%) other cases did not show any abnormality on follow-up ultrasound examination and increased NT resolved by 22 weeks of gestation in all cases but one. This patient was counselled that the risk of a poor outcome was increased but decided to continue with her pregnancy although ventricular septal defect (VSD) was diagnosed by fetal echocardiography. A syndrome unidentified to date and consisting of VSD, polydactyly, associated with growth retardation and developmental delay was diagnosed at 18 months of age. Neonatal outcome was completed in 162 live-born children. 2 children (1.2%) were lost for follow-up at between 12 and 24 months. The median (25th–75th percentile) gestation at delivery was 39.4 (39.0–40.3) weeks. The median (25th–75th percentile) birth-weight was 3415 (3075–3690) g. 142 (87.7%) children had no malformation and normal neurological development at the age of 2 while 18 (11.1%) children were diagnosed, at birth (10/18) or within 18 months (8/18) with 20 abnormalities missed antenatally (Tableau 2). 2 infants out of 162 (1.2%) who were born alive had developmental delay at the age of 2. This was isolated in one case and it was associated with the unidentified syndrome in the second case.
Figure 1.
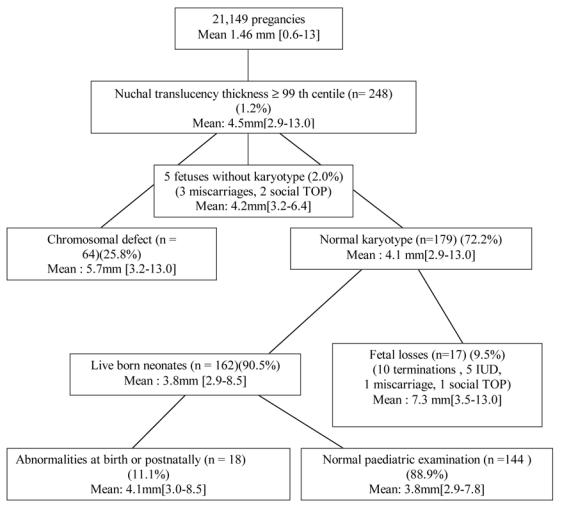
Outcome of 248 fetuses with NT ≥ 99th centile at 11–14 weeks of gestation. Mean NT thickness and ranges are given
Table II.
Fetuses with increased NT. normal karyotype and abnormalities diagnosed at birth or at 1 to 18 months of age (n=18 fetuses with 20 abnormalities)
| Abnormalities detected postnatally | NT (mm) |
|---|---|
| Cardiac defect | |
| Pulmonary stenosis and pulmonary valve dysplasia | 3.2 |
| Pulmonary valve dysplasia and atrial septal defect | 3.8 |
| Pulmonary valve stenosis | 3.2 |
| Coarctation of the aorta | 4.0 |
| Ventricular Septal Defect | 3.4 |
| Ventricular Septal Defect | 3.0 |
| Ventricular Septal Defect* | 6.6 |
| Wolf-ParkinsonWhite | 3.4 |
| Malformations | |
| Hypospadias | 3.2 |
| Macrocephaly with hydrocephaly | 3.6 |
| Cervical fistula | 4.2 |
| Hip dysplasia | 3.7 |
| Torticolis | 3.4 |
| Neurological impairment | |
| Epilepsy | 3.8 |
| Ataxia with occular motricity dysfunction | 3.2 |
| Syndromes | |
| Osmed# 163950** | 6.4 |
| Noonan# 163950** | 9.0 |
| Ventricular septum defect, polydactyly, single umbilical artery, growth retardation, unidentified syndrome * | 6.6 |
| Developmental delay | |
| Isolated | 3.2 |
| associated with the unidentified syndrome * | 6.6 |
Abnormalities diagnosed in the same infant*
OMIM number
The mean NT was higher in fetuses with an adverse prenatal outcome (7.3mm) in comparison with those born alive (3.8mm) (p<0.01). However the mean NT was similar in all fetuses born alive irrespective of the presence of abnormalities (4.1mm versus 3.8mm) (p=0.17). The prevalence of an adverse prenatal outcome in chromosomally normal fetuses increased 2.4-fold with each mm of NT thickness (OR=2.4/mm 95%CI [1.68–3.44]).
Among 160 children born alive, 29 (18.1%; 95% CI [15.4–30.5%]) had an ASQ ≤ 2SD below the mean score in at least one domain. Although close to statistical significance threshold, there was no significant association between the prevalence of an abnormality and NT thickness(OR=1.35/mm 95%CI [0.88–2.06]) or between deviant ASQ scores at 2 years of age and NT thickness (OR=1.37/mm 95 %CI [0.93–2.01]). The prevalence of children with at least one abnormal element at pediatric clinical examination was not associated with increased NT thickness (OR=1.39/mm 95%CI [0.64–2.99]) (Table 2). Furthermore development at the age of 2 was similar to that of the controls (Table 3).
Table III.
Comparison of children with increased NT ≥ 99th centile and normal karyotype with the control group of Epipage cohort study (Arch Dis Child Fetal Neonat 2004, Am J Obstet Gynecol 2005) at the age of 2
| Control group of the Epipage study | Increased NT and normal karyotype | ||||
|---|---|---|---|---|---|
| N | Mean (SD) or % | N | Mean (SD) or% | P | |
| Weight (Kg) | 370 | 12.4 (1.4) | 149 | 12.3(1.4) | 0.58 |
| Body lenght(cm) | 370 | 87.4 (3.3) | 148 | 85.3(2.9) | <0.001 |
| Cephalic circumference (cm) | 359 | 49 (2.0) | 147 | 49(1) | 0.22 |
| Able to walk | 351 | 100 | 148 | 100 | NR |
| Independant walking without support at 18 months of age | 351 | 100 | 149 | 99 | 0.98 |
| Holding the head up | 349 | 79 | 149 | 99 | 0.10 |
| Ascend and descend a set of stairs | 349 | 79 | 149 | 88 | <0.001 |
| kick a ball | 349 | 97 | 148 | 97 | 0.99 |
| drink from a glass | 349 | 99 | 148 | 99 | 0.99 |
| eat with a spoon | 355 | 99 | 149 | 98 | 0.43 |
| turn the pages of a book | 348 | 99 | 149 | 97 | 0.17 |
| Say a three word sentence | 346 | 64 | 149 | 95 | <0.001 |
| Squint | 362 | 3 | 150 | 3 | 0.72 |
| Anxiety of the mother % | 356 | 0.3 | 150 | 0.2 | 0.3 |
NR: Not Relevant
Discussion
In children born after a prenatal diagnosis of an apparently isolated increased NT with normal karyotype, there was a wide spectrum of abnormalities diagnosed postnatally in 11.1% (18/162) of the cases. Cardiac malformations accounted for about half of all these abnormalities in our population as reported in the literature (6). Our study confirms that fetuses with NT thickness above the 99th centile and normal karyotype have a high risk of adverse perinatal outcome. However among children born alive, there was no significant association between unexplained increase in first trimester NT thickness and development at 2 years as assessed by clinical examination and ASQ scores, when with a control population. These results are at odds with previous reports on the risk of developmental delay in these children. This may be partly explained by differences in study-designs. Some studies were conducted in highly biased referral populations (9,10,12,14), while others were based on unselected populations (8,11,13). We used a cut-off value of 99th centile for NT since it was reported that a cut-off value of 3.5mm was at or above the 99th centile for NT thickness throughout the first trimester of pregnancy (21). However, other studies used variable threshold definitions of abnormal NT thickness that contributed to the variability in their results. Lower cut-off led to lower risk of adverse pregnancy outcome and developmental delay (2.0% to 7.1%) (7,11,13) while higher ones reported higher risks (5.6% to 8.7%) (8,12)(Table 2). Most series included less than 40 children (8,9,13) with loss-for-follow-up rates of up to 15–32% (7,8,11). Our study is unlikely to present a selection bias and pediatric follow-up at the age of 2 was available for all children but 2. Developmental assessment was often conducted without clinical examination (7–9,13) and without comparison with a control group (7–9,11–14). The only study with few cases lost for follow-up and a control group included 89 children with NT thickness ≥ 3.5mm at 11–14 weeks and reported a prevalence of developmental delay of 1.12%. This was similar to their control group and to the rate reported in our study (10). Outcome was also poorer in series including cystic hygroma cases (22,23), a distinct condition clearly associated with a poor prognosis (24).
Souka et al (6,14) reported higher risk of adverse outcome with persistent second trimester fetal nuchal fold, including associations with cardiac defects, hydrops, intrauterine death or genetic syndrome. This is concordant with our study where increased NT evolved into nuchal edema or hydrops in the 2nd trimester of gestation in 6 of 162 fetuses (3.7%). Five of these cases underwent termination of pregnancy and one infant was diagnosed with an unrecognizable syndrome associated with developmental delay at the age of 2.
Development was assessed by pediatric clinical examination as well as by questionnaires answered by the parents at 24 months of age. The psychometric properties of ASQ including validity, inter and intra variability have been ascertained (25). Sensitivity and specificity to detect developmental delay are about 75% and 86% respectively (25). The proportion of children with ASQ < 2DS in our study was similar to that reported in normative studies (18%) (25) or in screening programs (16%) (26). ASQ results obtained in a Norwegian population were similar to American normative data suggesting few ethnic and cultural variations (27). Counselling should emphasize that when the karyotype is normal and no fetal structural malformation was missed prenatally following resolution of nuchal thickening, the prognosis is not impaired at the age of 2.
References
- 1.Snidjers RJM, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal translucency thickness at 10–14 weeks of gestation. Lancet. 1998;351:343–6. doi: 10.1016/s0140-6736(97)11280-6. [DOI] [PubMed] [Google Scholar]
- 2.Hyett J, Perdu M, Sharland G, Snidjers R, Nicolaides KH. Using fetal nuchal translucency to screen for major congenital cardiac defects at 10–14 weeks of gestation: population based cohort study. BMJ. 1999;318:81–85. doi: 10.1136/bmj.318.7176.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Souka AP, Snidjers RJM, Novakov A, Scares W, Nicolaides KH. Defects and syndromes in chromosomally normal fetuses with increased nuchal translucency thickness at 10–14 weeks of gestation. Ultrasound Obstet Gynecol. 1998;11:391–400. doi: 10.1046/j.1469-0705.1998.11060391.x. [DOI] [PubMed] [Google Scholar]
- 4.Michailidis GD, Economides DL. Nuchal translucency measurement and pregnancy outcome in karyotypically normal fetuses. Ultrasound Obstet Gynecol. 2001;17:102–105. doi: 10.1046/j.1469-0705.2001.00341.x. [DOI] [PubMed] [Google Scholar]
- 5.Malvrides E, Cobian-Sanchez F, Tekay A, Moscoso G, Campbell S, Thilaganathan B, Carvalho JS. Limitations of using first trimester nuchal translucency measurement in routine screening for major congenital heart defects. Ultrasound Obstet Gynecol. 2001;17:106–110. doi: 10.1046/j.1469-0705.2001.00342.x. [DOI] [PubMed] [Google Scholar]
- 6.Souka AP, von Kaisenberg CS, Hyett JA, Sonek JD, Nicolaides KH. Increased nuchal translucency with normal karyotype. Am J Obstet Gynecol. 2005;192:1005–1021. doi: 10.1016/j.ajog.2004.12.093. [DOI] [PubMed] [Google Scholar]
- 7.Van Vugt JMG, Tinnemans BWS, Van Zalen-Sprock Outcome and early childhood follow-up of chromosomally normal fetuses with increased nuchal translucency at 10–14 week’s gestation. Ultrasound Obstet Gynecol. 1998;11:407–409. doi: 10.1046/j.1469-0705.1998.11060407.x. [DOI] [PubMed] [Google Scholar]
- 8.Adekunle O, Goppe A, El-Sayed M, Thilaganathan B. Increased first trimester nuchal translucency: pregnancy and infant outcomes after routine screening for Down’s syndrome in an unselected antenatal population. Br J Radiol. 1999;72:457–460. doi: 10.1259/bjr.72.857.10505009. [DOI] [PubMed] [Google Scholar]
- 9.Maymon R, Jauniaux E, Cohen O, Dreazen E, Weinraub Z, Herman A. Pregnancy outcome and infant follow-up of fetuses with abnormally increased first trimester nuchal translucency. Hum Reprod. 2000;15:2023–2027. doi: 10.1093/humrep/15.9.2023. [DOI] [PubMed] [Google Scholar]
- 10.Brady AF, Pandya PP, Yuksel B, Greenough A, Patton MA, Nicolaides KH. Outcome of chromosomally normal livebirths with increased fetal nuchal translucency at 10–11’ week’s gestation. J Med Genet. 1998;35:222–24. doi: 10.1136/jmg.35.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiippala A, Eronen M, Taipale P, Salonen R, Hiilesmaa V. Fetal nuchal translucency and normal chromosomes: a long term follow-up study. Ultrasound Obstet Gynecol. 2001;18:18–22. doi: 10.1046/j.1469-0705.2001.00481.x. [DOI] [PubMed] [Google Scholar]
- 12.Senat MV, De Keersmaecker B, Audibert F, Montcharmont G, Frydman R, Ville Y. Pregnancy outcome in fetuses with increased nuchal translucency and normal karyotype. Prenat Diagn. 2002;22:345–9. doi: 10.1002/pd.321. [DOI] [PubMed] [Google Scholar]
- 13.Cheng C, Bahado-Singh RO, Chen S, Tsai M. Pregnancy outcomes with increased nuchal translucency after routine Down syndrome screening. Int J Gynaecol Obstet. 2004;84:5–9. doi: 10.1016/s0020-7292(03)00206-6. [DOI] [PubMed] [Google Scholar]
- 14.Souka AP, Krampl E, Bakalis S, Heath V, Nicolaides KH. Outcome of pregnancy in chromosomally normal fetuses with increased nuchal translucency in the first trimester. Ultrasound Obstet Gynecol. 2001;18:9–17. doi: 10.1046/j.1469-0705.2001.00454.x. [DOI] [PubMed] [Google Scholar]
- 15.Patrick Rozenberg, Laurence Bussières, Sylvie Chevret, Jean Pierre Bernard, et al. Screening for Down syndrome using first-trimester combined screening followed by second trimester ultrasound examination in an unselected population. Am J Obstet Gynecol. 2006 doi: 10.1016/j.ajog.2006.02.046. In press. [DOI] [PubMed] [Google Scholar]
- 16.Ville Y. Nuchal translucency in the first trimester of pregnancy: ten years on and still a pain in the neck? Ultrasound Obstet Gynecol. 2001;18:5–8. doi: 10.1046/j.1469-0705.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 17.Bricker D, Squires J, Mounts L. Ages and Stages Questionnaires: A parent-completed child monitoring system. 1. Baltimore: Paul H. Brookes Publishing Co; 1995. [Google Scholar]
- 18.Ancel PY, Marret S, Larroque B, Arnaud C, et al. The Epipage Study Group. Are maternal hypertension and small-for-gestational age risk factors for severe intraventricular hemorrhage and cystic periventricular leukomalacia? Results of the EPIPAGE cohort study . Am J Obstet Gynecol. 2005;193:178–84. doi: 10.1016/j.ajog.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 19.Larroque B, Breart G, Kaminski M, Dehan M, et al. Epipage study Group. Survival of very preterm infants: Epipage, a population based cohort study . Arch Dis Child Fetal Neonatal. 2004;89:139–44. doi: 10.1136/adc.2002.020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–74. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 21.Pandya PP, Snidjers RJM, Johnson SP, Brizot M, Nicolaides KH. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10–14 weeks of gestation. Br J Obstet Gynecol. 1995;102:957–62. doi: 10.1111/j.1471-0528.1995.tb10902.x. [DOI] [PubMed] [Google Scholar]
- 22.Boyd PA, Anthony MY, Manning N, Rodriguez CL, Wellesley DG, Chamberlain P. Antental diagnosis of cystic hygroma or nuchal pad-report of 92 cases with follow up of survivors. Arch Dis Child. 1996;74:F38–F42. doi: 10.1136/fn.74.1.f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumann C, Delagarde R, Vuillard E, Oury JF. Long-term follow-up of children with increased nuchal translucency and normal karyotype. J Gynecol Obstet Biol Reprod. 2005;34:2897–2898. doi: 10.1016/s0368-2315(05)82695-x. [DOI] [PubMed] [Google Scholar]
- 24.Malone F, Ball R, Nyberg D, Comstock C, Saade G, Berkowitz R, Gross S, Dugoff L, Craigo S, Timor-Tritsch I, Carr S, Wolfe H, Dukes K, Canick J, Bianchi D, D’Alton M. First-trimester septated cystic hygroma . Obstet Gynecol. 2005;106:288–294. doi: 10.1097/01.AOG.0000173318.54978.1f. [DOI] [PubMed] [Google Scholar]
- 25.Squires J, Potter L, Bricker D. Child monitoring System. 2. Baltimore: Paul H. Brookes Publishing Co; 1999. The ASQ User’s Guide for the Ages and Stages Questionnaires: A parent completed. [Google Scholar]
- 26.Squires J, Katzev A, Jenkins F. Early screening for developmental delays: use of parent-completed questionnaires in Oregon’s healthy start program. Early Child development and care. 2002;172:275–282. [Google Scholar]
- 27.Janson H, Squires J. Parent-completed developmental screening in a Norwegian population sample: a comparison with US normative data. Acta Paediatr. 2004;93:1525–29. doi: 10.1080/08035250410033051. [DOI] [PubMed] [Google Scholar]


