Abstract
Background
The CC-chemokines CCL3, CCL4 and CCL5 have been found to block the entry of CCR5-tropic HIV into host cells and to suppress the viral replication in vitro, but the in vivo role of endogenous CC-chemokines in HIV-1 infection is still incompletely understood.
Methodology/Principle Findings
In this study, the primate host CCL3, CCL4 and CCL5 gene expression was evaluated in response to simian-human immunodeficiency virus (SHIV) infection in rhesus macaque model. Five rhesus macaques were inoculated with CCR5-tropic SHIVSF162P4. The mRNA levels of CCL3, CCL4 and CCL5 were measured by real-time PCR at post inoculation day (PID) 0, 7, 14, 21, 35, 56 and 180 in peripheral blood. In addition, a selected subset of samples from CXCR4-tropic SHIVKu1-infected macaques was included with objective to compare the differences in CC-chemokine down-regulation caused by the two SHIVs. Gut-associated lymphoid tissues (GALT) collected from SHIVSF162P4-infected animals were also tested by flow cytometry and confocal microscopy to corroborate the gene expression results. Predictably, higher viral loads and CD4+ T cell losses were observed at PID 14 in macaques infected with SHIVKu1 than with SHIVSF162P4. A decline in CC-chemokine gene expression was also found during primary (PID 7-21), but not chronic (PID 180) stage of infection.
Conclusions
It was determined that A) SHIVSF162P4 down-regulated the CC-chemokine gene expression during acute stage of infection to a greater extent (p<0.05) than SHIVKu1, and B) such down-regulation was not paralleled with the CD4+ T cell depletion. Evaluation of CC-chemokine enhancing immunomodulators such as synthetic CpG-oligonucleotides could be explored in future HIV vaccine studies.
Introduction
Chemokines play a critical role in the host defense against viruses by mobilizing leukocytes to sites of infection, and they also play a homeostatic role in secondary lymphoid tissues [1]. Structurally, chemokines are divided into the C, CC, CXC, and CX3C subclasses on the basis of their N-terminal cysteine residues spacing [2]. The majority of known chemokines belong to CC or CXC category [2]. Since CC chemokine receptor 5 (CCR5) and CXC chemokine receptor 4 (CXCR4) were identified as major co-receptors for HIV-1 entry into target cells, it was suggested that these chemokine receptors and their ligands are involved in the transmission and replication of HIV-1 [3], [4]. HIV-1 isolates that use CCR5 as co-receptor (R5 viruses) are predominantly transmitted and persist throughout the infection. By contrast, HIV-1 isolates that use CXCR4 (X4 viruses) commonly emerge in infected individuals at later stages of infection [3]. The importance of CCR5 for HIV-1 transmission was underscored by observation that certain individuals who had been repeatedly exposed to HIV-1 but remained uninfected had a defect in CCR5 expression [5]. It has been documented that mucosa-associated CD4+ T cells, macrophages, and dendritic cells express CCR5 which makes them prime targets for the virus [6]–[8].
Members of the CC-chemokine family, such as macrophage inflammatory protein (MIP)-1α (CCL3), MIP-1β (CCL4) and RANTES (CCL5) are the natural ligands for CCR5. They have been shown to inhibit HIV-1 entry into host cells in vitro by competing with viral Env protein for binding [9]–[11], and by down-regulating CCR5 surface expression [12]. However, the in vivo role of endogenous CC-chemokines in the pathogenesis of HIV-1 infection is still incompletely understood. A number of studies have analyzed levels of CCR5 ligands in the HIV-1/SIV-infected individuals or monkeys with conflicting results [13]–[18]. Some of these studies concluded that CCR5 ligands may exert protective role in HIV-1/SIV infection [13], [16]–[18], while others suggested an association between elevated levels of CCR5 ligands and HIV disease progression [14], [15]. Most of these reports were based on data that correspond to chronic stages of HIV-1/SIV infection, while the relationship between host CC-chemokine expression and HIV-1 acute infection still needs to be studied.
In this study, we evaluated the host in vivo CCL3, CCL4 and CCL5 gene expression in response to CCR5-tropic SHIV infection. We utilized SHIVSF162P4/rhesus macaque model and focused predominantly on acute stage of infection to measure the CC-chemokine gene expression changes in peripheral blood and GALT. In addition, we included a selected subset of samples from SHIVKu1-infected macaques in order to compare the differences between the extent of CC-chemokine down-regulation caused by CCR5-tropic SHIVSF162P4 and more pathogenic CXCR4-tropic SHIVKu1 at the acute stage of infection.
Results
Viral loads and CD4+ T cell counts in peripheral blood of SHIVSF162P4-infected macaques
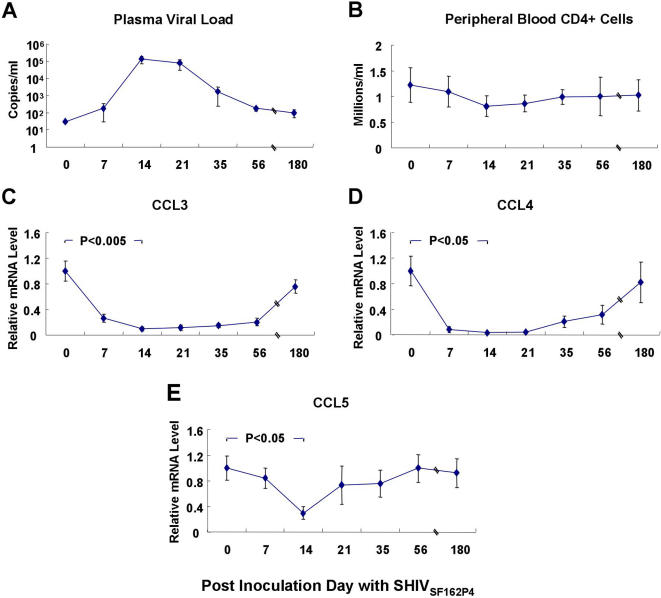
All five SHIVSF162P4-inoculated macaques became infected as determined by individual viral load measurements. Highest viral loads were detected at PID 14 with an average of ∼105 of viral copies (Fig. 1A). The lowest average peripheral CD4+ T cell counts were detected between PID 14 and 21 (Fig. 1B).
Figure 1. SHIVSF162P4 loads in plasma (A), CD4+ T cell counts in peripheral blood (B), relative CCL3 (C), CCL4 (D) and CCL5 (E) gene expression levels in PBMCs at PID 0-180 with SHIVSF162P4.
Each point on the graph represents the mean fold change in gene expression relative to pre-infection level±SE (n = 5).
Down-regulation of CC-chemokine gene expression in peripheral blood of SHIVSF162P4-infected macaques
The changes of CCL3, CCL4 and CCL5 gene expression levels were studied in peripheral blood over the period of half year following the SHIVSF162P4 inoculation. Decreased expression of all three CC-chemokines was found upon infection. The lowest gene expression was detected at PID 14, coinciding with the peak of primary SHIVSF162P4 infection (Fig. 1A, C, D and E). By PID 14, CCL3, CCL4 and CCL5 mRNA levels decreased 5.9–13.6, 3.5–27.5, and 2.3–5.4-folds, respectively. These levels were significantly lower (p<0.005 for CCL3; p<0.05 for CCL4 and CCL5) than those prior to infection (Fig. 1C, D and E). However, such a decline of CC-chemokine gene expression occurred only temporarily, because starting from PID 21 and at later time points these gene expression values showed relative increase. By PID 180, expressions of all three chemokine genes were no longer significantly different from those prior to infection.
Down-regulation of CC-chemokine gene expression in GALT of SHIVSF162P4-infected macaques
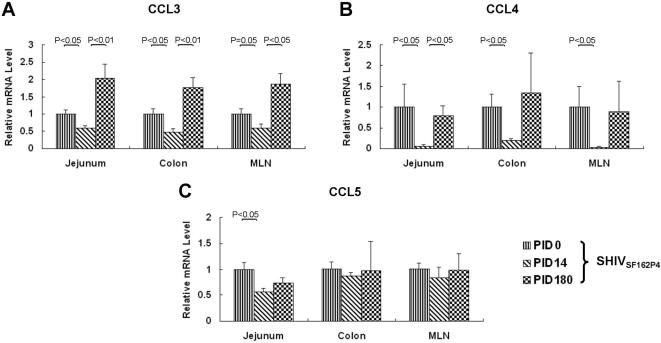
To determine whether the decline of CC-chemokine gene expression occurred also in GALT of SHIVSF162P4-infected macaques, biopsy samples of colon, jejunum and MLN were obtained at PID 0, 14 and 180. At PID 14, CCL3 and CCL4 mRNA levels in colon, jejunum and MLN were lower (p≤0.05) than those at PID 0 (Fig. 2A and B). CCL5 mRNA decreased in jejunum (p<0.05) but not in colon and MLN. In agreement with results generated with PBMC, down-regulation of CC-chemokines occurred also in GALT at PID 14. By PID 180, expression of CC-chemokines returned to pre-infection levels (CCL4 and CCL5), or even increased (CCL3).
Figure 2. Relative CCL3 (A), CCL4 (B) and CCL5 (C) expression levels in GALT tissues at different stages of SHIVSF162P4 infection (n = 5).
PID 0 was used as the calibrator (value = 1).
In situ enumeration of CCL4+ cells in SHIVSF162P4-infected macaques
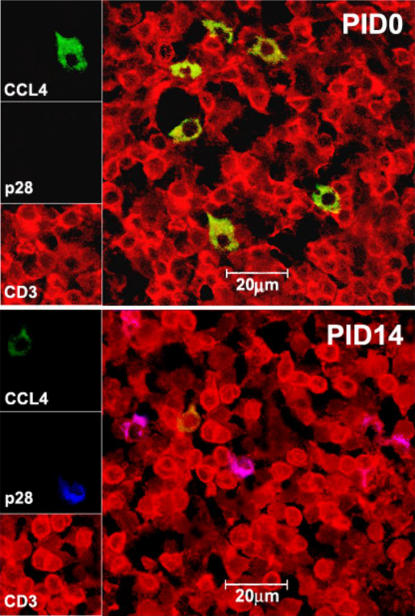
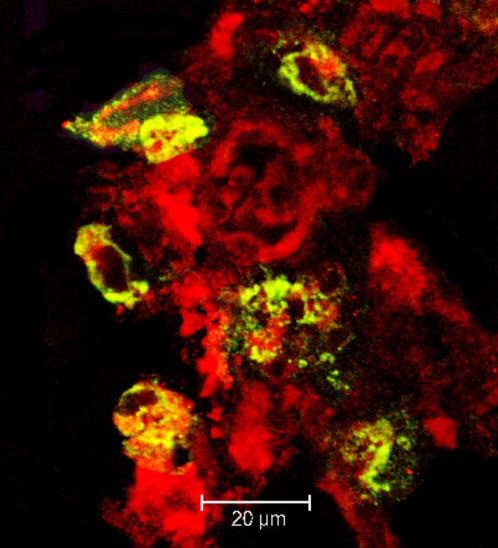
To corroborate the mRNA results, and to determine the origin and number of CC-chemokine-producing cells in SHIVSF162P4-infected macaques, confocal microscopy and MLN tissues were used. The CCL4 was selected as the CC-chemokine representative. The T lymphocytes (CD3+), macrophages (CD68+), and CCL4+ cells were identified directly in situ by tri-color staining (Figs. 3 and 4). Higher number of CD3+CCL4+ cells was seen at PID 0 than at PID 14 (Table 1) while CD3+p28+ cells were seen at PID 14 but not at PID 0 indicating the rapid spread of virus into GALTs following mucosal inoculation (Fig. 3). In addition to CD3+ cells, CD68+ macrophages were also identified as CCL4+ cells (Fig. 4). In agreement with real-time PCR data, the counts of CD3+CCL4+ and CD68+CCL4+ cells were lower (p<0.05) at PID 14 than they were at PID 0 (Table 1).
Figure 3. Triple-label confocal microscopy of CCL4+ cells in mesenteric lymph nodes obtained from control (PID 0) and SHIVSF162P4-inoculated macaques at PID 14.
T lymphocytes (CD3+) are red, CCL4+ cells are green, SHIVSF162P4-infected cells (p28+) are blue. Overlap of red and green fluorescence is seen as yellow, overlap of red and blue as pink. Higher number of CD3+CCL4+ cells was seen at PID 0 than at PID 14 (Table 1) while CD3+p28+ cells could be seen at PID 14 but not at PID 0 indicating the successful spread of virus into GALTs following mucosal inoculation.
Figure 4. Macrophages were also identified as CCL4+ cells.
Macrophages (CD68+) are red, CCL4+ cells are green, overlap of red and green fluorescence is seen as yellow.
Table 1. Absolute (counts) and relative (%) levels of CCL4+ cells in MLN following SHIVSF162P4 inoculation.
| Sample Date | Animal | CCL4+CD3+ (count)a | % CCL4+CD3+ over total CD3+ | CCL4+CD68+ (count)a | % CCL4+CD68+ over total CD68+ |
| PID 0 | Animal 1 | 12 | 0.89 | 7 | 1.92 |
| Animal 2 | 7 | 0.76 | 8 | 2.24 | |
| Animal 3 | 8 | 0.78 | 6 | 2.11 | |
| Mean±SEM | 9±2 | 0.81±0.04 | 7±1 | 2.09±0.09 | |
| PID 14 | Animal 1 | 4 | 0.65 | 3 | 0.82 |
| Animal 2 | 2 | 0.26 | 4 | 1.12 | |
| Animal 3 | 3 | 0.55 | 3 | 1.03 | |
| Mean±SEM | 3±1 | 0.49±0.12 | 3±1 | 0.99±0.09 |
Number of cells/0.5 mm2 section.
Flow cytometry of CCL4+ cells in peripheral blood of SHIVSF162P4–infected macaques
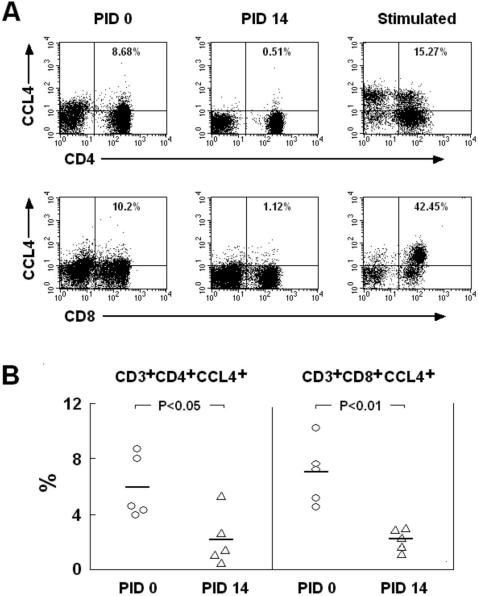
Four-color flow cytometry was used to corroborate the gene expression results. At PID 0, the average proportions of CD3+CD4+CCL4+ and CD3+CD8+CCL4+ cells over CD3+ T cells were 5.9% and 6.9%, respectively (Fig. 5B). By PID 14, both of these proportions decreased (p<0.05 and p<0.01) to 2.2% and 2.3% (Fig. 5B). In order to be able to differentiate between CCL4+ and CCL4- cells, mitogen-stimulated cells were included as controls (Fig. 5A).
Figure 5. The proportions of peripheral blood CD3+CD4+CCL4+ and CD3+CD8+CCL4+ T lymphocytes were compared at PID 0 and PID 14 with SHIVSF162P4 by flow cytometry (A).
Gating was performed via CD3+ lymphocyte population. Statistically significant differences between CD3+CD4+CCL4+ and CD3+CD8+CCL4+ cells over CD3+ lymphocytes at PID 0 vs. PID 14 (n = 5) are shown (B).
The extent of CC-chemokine gene down-regulation in blood of SHIV-infected macaques is independent from the level of CD4+ T cell depletion
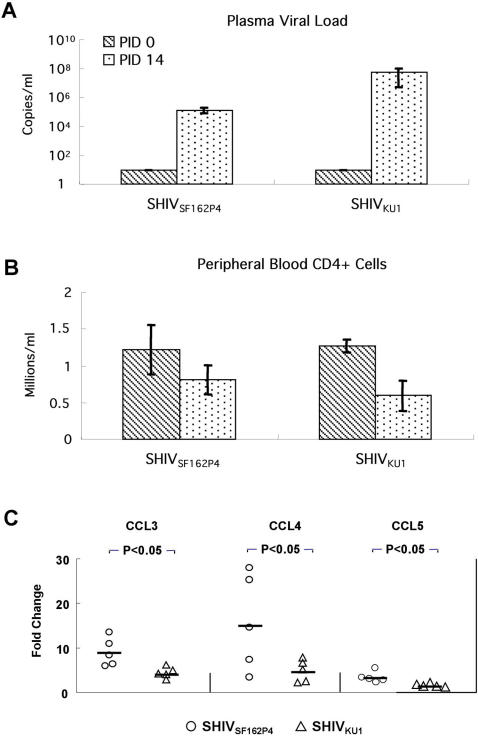
We hypothesized that SHIVSF162P4–induced CC-chemokine down-regulation at acute stage of infection was not proportionately related to peripheral CD4+ T cell depletion as a result of viral target cell destruction. Therefore, we included in this study a subset of samples obtained from SHIVKu1-infected macaques (PID 0 and 14). In accord with previous reports [19], [20], it was confirmed that SHIVKu1 infection was at PID 14 associated with greater plasma viral loads and more profound loss of peripheral CD4+ T cells than that caused by SHIVSF162P4 (Fig. 6A and B). Interestingly, SHIVSF162P4 caused by PID 14 5.9–13.6, 3.5–27.5, and 2.3–5.4-fold CCL3, CCL4 and CCL5 gene down-regulation respectively, while only 2.8–6.2, 2.3–7.8, and 1.2–2.2-fold down-regulation was measured in SHIVKu1-infected macaques (Fig. 6C). These differences in CC-chemokine down-regulation between SHIVSF162P4- and SHIVKu1-infected macaques were significant (p<0.05).
Figure 6. Comparison of SHIVSF162P4 and SHIVKu1 primary infection in peripheral blood.
Viral loads in plasma (A), CD4+ T cell counts (B), Fold-changes of CCL3, CCL4 and CCL5 gene expression levels in SHIVSF162P4- and SHIVKu1–infected macaques at PID 0 vs. PID 14 (C) (n = 5 for each group). Negative sample cut-off for viral load measurements in plasma was <30 copies/ml e.g. PID 0 value.
Discussion
Main objective of our study was to evaluate the CC-chemokine gene expression changes in SHIVSF162P4-infected macaques. We found that at primary/acute stage of infection, CC-chemokine genes were markedly down-regulated, which coincided with the peak of viremia. Gene expression results were consistent with direct measurements of chemokine production–corroborated by confocal microscopy and flow cytometry. Results generated with peripheral blood and GALT samples were also consistent. In agreement with our results, a decline in CCL4 gene expression by CD8+ intraepithelial lymphocytes (IEL) was observed at the acute stage of SIV infection [21]. Study by Wen and colleagues [22] also showed that expression of CCL3, CCL4 and CCL5 decreased in U937 promonocytes in response to HIV-1 infection. In addition, several studies reported up-regulation of CC-chemokines during chronic phase of HIV/SIV infection [14], [15], [23]. These reports are not in conflict with our results. Results shown here indicate however that down-regulation of CCR5 ligands occurred only at primary (PID 14) but not at chronic stage of SHIVSF164P4 infection (PID 180). At the chronic stage of SHIVSF164P4 infection, expression of the CCR5 ligands returned to pre-infection levels (CCL4 and CCL5), or even increased (CCL3) in all three GALTs.
There are several possible mechanisms involved in decline of CC-chemokines during primary SHIV infection. Since lymphocytes are CC-chemokine producing cells, the depletion of CD4+ T lymphocytes could be one of the reasons. Based on measurable and significant CC-chemokine gene down-regulation caused by SHIVSF162P4 that is known to be less pathogenic in term of its ability to deplete the peripheral CD4+ T cells than other SHIVs, SIV or HIV, a subset of samples obtained from SHIVKu1-infected macaques (PID 0 and 14) was included in this study. It was hypothesized that SHIVSF162P4–induced CC-chemokine down-regulation is not directly related to peripheral CD4+ T cell depletion-as a result of viral target cell destruction. This hypothesis was conclusively corroborated by measurements of CC-chemokine gene expression at PID 14 when SHIVSF162P4 was found to cause significantly greater gene down-regulation than SHIVKu1. Such result suggests that decline of CC-chemokines cannot be solely attributed to CD4+ T cell depletion, but it is more complex and likely linked to other pathways triggered by acute SHIV infection. One of them might be related to SHIV gene products, which modulate transcriptional levels of host response genes [24]. For example, HIV accessory gene product Vpr has been reported to suppress the host CC-chemokine gene expression [25]. Another candidate pathway is the interaction between the HIV/SIV gp120 and the CCR5 chemokine co-receptors, which trigger not only viral entry but also other signal transduction cascades that are impacting host CC-chemokine gene expression [26], [27]. Elucidating such pathways in context of CC-chemokine production can be subject of future studies.
CCL3, CCL4 and CCL5 function as natural ligands for CCR5, the major HIV/SIV co-receptor [3]. CC-chemokines are known to inhibit the CCR5-mediated HIV/SIV infection by blocking the viral entry into host cells [9], [10]. In addition, CC-chemokines play a role in directing the cell movements necessary for the initiation of T cell immune responses [1], co-stimulate the T cell proliferation, and augment the cytolytic capacity of T and NK cells [28], [29]. Thus, suppression of CC-chemokines during primary/acute stage of infection may be an event that facilitates the evasion of HIV/SIV from the host immune response. It was demonstrated by several authors that production of CC-chemokines was associated with vaccine-mediated protection from SIV challenge [13], [16], [30]. Furthermore, systemic therapy with CC-chemokine homologues decreased SIVmac251 replication in rhesus monkeys [31]. These reports are in agreement with our findings by corroborating the notion that CC-chemokine down-regulation may facilitate virus spread during acute stage of infection.
It may be unexpected that CCL3, CCL4 and CCL5 are down-regulated at the acute phase of SHIV infection, since most of the CC-chemokines belong to proinflammatory chemokines and are generally considered to be produced only in response to pathological conditions [32]. However, the constitutive expression of CCL3, CCL4 and CCL5 was detected in monocytes [33] and CD8+ intraepithelial lymphocytes [21]. In this study, highly sensitive real-time PCR was used to measure the expression of the above CC-chemokines in PBMC and GALTs before and after SHIVSF162P4 infection, and it was concluded that these chemokines are down-regulated during primary infection.
In summary, we hypothesize that an interventional up-regulation of CC-chemokines might lead to better outcome of HIV/SIV primary infection. Evaluation of CC-chemokine enhancing immunomodulators such as synthetic CpG-oligonucleotides could be explored in future SIV/HIV vaccine studies.
Materials and methods
Animals and virus
Five adult rhesus macaques (Macaca mulatta) of Indian origin were inoculated with 5,000 TCID50 of CCR5-tropic SHIVSF162P4 via intrarectal route [19]. SHIVSF162P4 was obtained from the Simian Retrovirus Core Laboratory at the TNPRC (Tulane National Primate Research Center). Animals were housed at the TNPRC under biosafety level 2 conditions in accordance with the regulations of the American Association for Assessment and Accreditation of Laboratory Animal Care standards. In addition, five adult rhesus macaques of Indian origin were inoculated with CXCR4-tropic SHIVKu1 by using the same route and dose as described above for SHIVSF162P4. SHIVKu1 was obtained from NIH (www.aidsreagent.org). Both SHIVs were expanded and titrated in vitro as described elsewhere [34], [35].
Sample collection and storage
From the 5 animals inoculated with SHIVSF162P4, EDTA blood was collected prior to inoculation (PID 0), and at PID 7, 14, 21, 35, 56 and 180. GALT samples (jejunum, colon and MLN) were collected via laparotomy biopsies at PID 0, 14 and 180. The peripheral blood mononuclear cells (PBMC) were isolated from EDTA blood using the lymphocyte separation medium (Cappel, Aurora, OH) according to instructions of manufacturer, and stored in lysis buffer (Qiagen, Valencia, CA) (10×106 cells/600 µl of lysis buffer) at −80°C until RNA isolation. The GALT samples were cut into 20–30 mg pieces, placed in 5 volumes of RNAlater (Ambion, Austin, TX), incubated at 4°C overnight and stored at −20°C until RNA extraction. From the 5 animals inoculated with SHIVKu1, samples of EDTA blood were obtained at PID 0 and 14 in order to compare measurements of a) plasma viral load, b) peripheral CD4+ T cell counts and c) CC-chemokine gene expression between SHIVSF162P4- and SHIVKu1–infected macaques.
RNA isolation and cDNA preparation
Total RNA was isolated from 20–30 mg of GALT tissue or 10×106 of PBMC using the RNeasy mini kit (Qiagen, Valencia, CA). Prior to RNA isolation, tissue was homogenized by 3 pulses of sonication, each at 60 W for 3 sec. All samples were treated with RNase-free DNase (Qiagen). cDNA was prepared using the random hexamer primers (Integrated DNA Technologies, Skokie, IL) and M-MLV reverse transcriptase (Promega, Madison, WI) according to instructions of manufacturer.
Evaluation of chemokine gene expression by real-time PCR
Real-time PCR was carried out using the ABI Prism 7700 Sequence Detection System (Applied Biosystem, Foster City, CA). Each PCR reaction included 12.5 µl of master mix (DyNAmo HS SYBR Green qPCR Kit, MJ research, Waltham, MA). It contained modified DNA polymerase, SYBR Green I, 5 mM MgCl2, dNTP mix, 0.5 µM of forward and reverse primers, 1×ROX reference dye, and 100 ng of the first strand cDNA in deionized water. After heating each sample to 95°C for 15 min, 40 cycles of 94°C for 10 sec, 55°C for 30 sec, and 72°C for 30 sec followed. After the thermal cycles, melting curves of the amplicons were generated by heating the reaction mixture to 95°C for 15 sec, then to 60°C for 20 sec, and slowly increasing the temperature to 95°C over a period of 20 min. The fluorescence was measured as a function of temperature. All samples were run in duplicates and the PCRs for the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the target genes (Table 2) were run in parallel for each sample. PCR reactions were carried out on a 96-well plate (Applied Biosystems, Foster City, CA) using the 25 µl/reaction volumes. Results were analyzed by SDS 1.9.1 Software (Applied Biosystems). The melting curves were obtained with ABI Prism 7700 software, version 1.7(http://www.appliedbiosystems.com/support/software/7700pdates.cfm, ABI). The specificity of real-time PCR was verified by melting curve analysis. Housekeeping gene GAPDH was used to normalize the RNA in tested samples. The variation in GAPDH Ct value among the RNA samples was ≤3%. 2−ΔΔCt method [36] was used in calculating the fold-relationships in gene expression between the time points. Pre-infection (PID 0) was used as calibrator to generate the graphs shown in Fig 1 and 2.
Table 2. Rhesus macaque-specific primers for real-time PCR.
| Gene | GenBank # | Sequences (5′-3′) | Start-End Position | Amplicon Length (bp) |
| CCL3 | AF449266 | F: CAACATTTGCTGCTGACACC | 68–87 | 103 |
| R: CACTGGCTGTTGGTCTCAAA | 151–170 | |||
| CCL4 | AF449267 | F: CCAGACCAAAAGAGGCAAGC | 195–214 | 76 |
| R: TTCCAGGTCATTAACATACTCC | 249–270 | |||
| CCL5 | AF449268 | F: TACACCAGTGGCAAGTGCTC | 154–173 | 100 |
| R: TGTACTCCCGAACCCATTTC | 234–253 | |||
| GAPDH | CO774505 | F: GGGAGCCAAAAGGGTCATCA | 378–397 | 91 |
| R: GAGGCTGTTGTCATACTTCTC | 448–468 |
Evaluation of viral loads by real-time PCR
Quantitative assessment of viral RNA load in plasma samples obtained from peripheral blood of SHIV-inoculated macaques was determined by real-time reverse transcriptase PCR as described in detail elsewhere [37]. The virus used in this study was accurately and equivalently quantified in this assay. The values were expressed as viral RNA copies per ml of plasma.
Enumeration of CCL4-producing cells in MLN by confocal microscopy
MLN biopsies obtained from 3 animals prior to infection (PID 0) and at PID 14 were cryosectioned to 10–16 µm and subjected to immunofluorescence staining as previously described [38]. Briefly, the tissues were blocked with 10% donkey serum (Sigma, St. Louis, MO) in PBS containing 0.2% fish skin gelatin (Sigma, St. Louis, MO). The sections were subsequently stained with primary antibodies to detect CCL4+ cells, SHIV-infected cells, T cells and macrophages (anti-CCL4, SIV gag p28, CD3, and CD68 antibodies were purchased from R&D Systems, Microbix, Dako and BD Pharmingen, respectively). Negative-control samples were stained with matched isotype control antibodies and no positive cells were identified (not shown). Primary staining was followed by incubation with secondary antibodies conjugated to fluorochromes. Images were captured by a Leica TCS SP2 laser-scanning microscope (Leica Microsystems, Exton, PA) and viewed using the Leica imaging software [38].
Flow cytometry analysis of CCL4 expression in CD3+CD4+/CD8+ T cells
Presence of intracellular CCL4 in PBMC was evaluated by four-color flow cytometry. The following anti-human monoclonal antibodies that cross-react with rhesus macaques were used: Phycoerythrin (PE)-conjugated anti-MIP-1β/CCL4 (clone D21-1351), Fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (clone SP34), Peridinin Chlorophyll (PerCP)-conjugated anti-CD8 (clone SK1), and Allophycocyanin (APC)-conjugated anti-CD4 (clone SK3). All antibodies were purchased from BD-Pharmingen (Pharmingen, San Diego, CA). PBMCs (5×105 per sample) were first incubated with fluorochrome-conjugated antibodies to CD3, CD4 and CD8 at 4°C, in dark, for 30 min. The cells were then washed with PBS containing 0.2% fetal bovine serum (FBS, Invitrogen, Chicago, IL), and permeabilized with 1× fixation/permeabilization solution (BD Biosciences) for 10 min at room temperature. Following two washes with PBS containing 0.2% FBS, the cells were incubated with anti-CCL4 for 30 min at 4°C. After washing, cells were resuspended in 2% paraformaldehyde/PBS. Positive control PBMCs were stimulated with 100 ng/ml of phorbol myristate acetate (PMA, Sigma) and 1 µg/ml of ionomycin (Sigma) for 6 h at 37°C, 5% CO2. Brefeldin-A (10 µg/ml, Sigma) was added for final 5 h. Mitogen-stimulated control PBMCs were stained and processed as described above for unstimulated samples. Negative-control samples were stained with matched isotype control antibodies. Stained cells were kept protected from light at 4°C and acquisition was completed within 48 h. Data acquisition was performed using the FACS-Aria Flow Cytometer (BD Biosciences), and collected data were analyzed by Cell Quest software (Becton Dickinson).
Statistical analysis
CCL3, CCL4 and CCL5 mRNA levels, CCL4+ cell numbers in MLN and percentages of CCL4+ cells in peripheral blood CD4+ and CD8+ T cell populations were compared between selected time points of SHIVSF162P4-infected macaques by Student's t test and a p<0.05 was considered statistically significant. In addition, the extent of CC-chemokine down-regulation in peripheral blood of SHIVSF162P4- and SHIVKu1-infected macaques was compared at PID 14 by Student's t test (p<0.05).
Acknowledgments
The technical assistance of Julie Bruhn, Mayra Cantu, Tessa Williams, Mike Piatak, Drs. Pyone P. Aye and Jason Dufour is greatly appreciated. We thank Dr. Preston A. Marx's laboratory for preparation of SHIVSF162P4 and SHIVKu1 inoculation stocks. SHIV viral load assays were performed by the Quantitative Molecular Diagnostic Core of the AIDS Vaccine Program, SAIC-Frederick, Inc., NCI-Frederick, MD.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants AI54146 and RR00164 from the National Institutes of Health.
References
- 1.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116:1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 3.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 4.Moser B. Chemokines and HIV: a remarkable synergism. Trends Microbiol. 1997;5:88–90. doi: 10.1016/S0966-842X(97)01019-6. [DOI] [PubMed] [Google Scholar]
- 5.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 6.Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, et al. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol. 1997;151:1341–1351. [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L, Paxton WA, Kassam N, Ruffing N, Rottman JB, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, He T, Talal A, Wang G, Frankel SS, et al. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 10.Gauduin MC, Glickman RL, Means R, Johnson RP. Inhibition of simian immunodeficiency virus (SIV) replication by CD8(+) T lymphocytes from macaques immunized with live attenuated SIV. J Virol. 1998;72:6315–6324. doi: 10.1128/jvi.72.8.6315-6324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackewicz CE, Barker E, Levy JA. Role of beta-chemokines in suppressing HIV replication. Science. 1996;274:1393–1395. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 12.Alkhatib G, Locati M, Kennedy PE, Murphy PM, Berger EA. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed RK, Makitalo B, Karlen K, Nilsson C, Biberfeld G, et al. Spontaneous production of RANTES and antigen-specific IFN-gamma production in macaques vaccinated with SHIV-4 correlates with protection against SIVsm challenge. Clin Exp Immunol. 2002;129:11–18. doi: 10.1046/j.1365-2249.2002.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennes W, Sawadogo S, Koblavi-Deme S, Vuylsteke B, Maurice C, et al. Positive association between beta-chemokine-producing T cells and HIV type 1 viral load in HIV-infected subjects in Abidjan, Cote d'Ivoire. AIDS Res Hum Retroviruses. 2002;18:171–177. doi: 10.1089/08892220252781220. [DOI] [PubMed] [Google Scholar]
- 15.LaFranco-Scheuch L, Abel K, Makori N, Rothaeusler K, Miller CJ. High beta-chemokine expression levels in lymphoid tissues of simian/human immunodeficiency virus 89.6-vaccinated rhesus macaques are associated with uncontrolled replication of simian immunodeficiency virus challenge inoculum. J Virol. 2004;78:6399–6408. doi: 10.1128/JVI.78.12.6399-6408.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehner T, Wang Y, Cranage M, Tao L, Mitchell E, et al. Up-regulation of beta-chemokines and down-modulation of CCR5 co-receptors inhibit simian immunodeficiency virus transmission in non-human primates. Immunology. 2000;99:569–577. doi: 10.1046/j.1365-2567.2000.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paxton WA, Liu R, Kang S, Wu L, Gingeras TR, et al. Reduced HIV-1 infectability of CD4+ lymphocytes from exposed-uninfected individuals: association with low expression of CCR5 and high production of beta-chemokines. Virology. 1998;244:66–73. doi: 10.1006/viro.1998.9082. [DOI] [PubMed] [Google Scholar]
- 18.Paxton WA, Kang S, Liu R, Landau NR, Gingeras TR, et al. HIV-1 infectability of CD4+ lymphocytes with relation to beta-chemokines and the CCR5 coreceptor. Immunol Lett. 1999;66:71–75. doi: 10.1016/s0165-2478(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 19.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 20.Hsu M, Harouse JM, Gettie A, Buckner C, Blanchard J, et al. Increased mucosal transmission but not enhanced pathogenicity of the CCR5-tropic, simian AIDS-inducing simian/human immunodeficiency virus SHIV(SF162P3) maps to envelope gp120. J Virol. 2003;77:989–998. doi: 10.1128/JVI.77.2.989-998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattapallil JJ, Smit-McBride Z, McChesney M, Dandekar S. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1beta expression and display antiviral cytotoxic activity despite severe CD4(+) T-cell depletion in primary simian immunodeficiency virus infection. J Virol. 1998;72:6421–6429. doi: 10.1128/jvi.72.8.6421-6429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen W, Chen S, Cao Y, Zhu Y, Yamamoto Y. HIV-1 infection initiates changes in the expression of a wide array of genes in U937 promonocytes and HUT78 T cells. Virus Res. 2005;113:26–35. doi: 10.1016/j.virusres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann-Lehmann R, Williams AL, Swenerton RK, Li PL, Rasmussen RA, et al. Quantitation of simian cytokine and beta-chemokine mRNAs, using real-time reverse transcriptase-polymerase chain reaction: variations in expression during chronic primate lentivirus infection. AIDS Res Hum Retroviruses. 2002;18:627–639. doi: 10.1089/088922202760019329. [DOI] [PubMed] [Google Scholar]
- 24.Decrion AZ, Dichamp I, Varin A, Herbein G. HIV and inflammation. Curr HIV Res. 2005;3:243–259. doi: 10.2174/1570162054368057. [DOI] [PubMed] [Google Scholar]
- 25.Muthumani K, Kudchodkar S, Papasavvas E, Montaner LJ, Weiner DB, et al. HIV-1 Vpr regulates expression of beta chemokines in human primary lymphocytes and macrophages. J Leukoc Biol. 2000;68:366–372. [PubMed] [Google Scholar]
- 26.Kinter A, Arthos J, Cicala C, Fauci AS. Chemokines, cytokines and HIV: a complex network of interactions that influence HIV pathogenesis. Immunol Rev. 2000;177:88–98. doi: 10.1034/j.1600-065x.2000.17708.x. [DOI] [PubMed] [Google Scholar]
- 27.Stantchev TS, Broder CC. Human immunodeficiency virus type-1 and chemokines: beyond competition for common cellular receptors. Cytokine Growth Factor Rev. 2001;12:219–243. doi: 10.1016/s1359-6101(00)00033-2. [DOI] [PubMed] [Google Scholar]
- 28.Dorner BG, Scheffold A, Rolph MS, Huser MB, Kaufmann SH, et al. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc Natl Acad Sci USA. 2002;99:6181–6186. doi: 10.1073/pnas.092141999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed RK, Nilsson C, Biberfeld G, Thorstensson R. Role of CD8+ cell-produced anti-viral factors in protective immunity in HIV-2-exposed but seronegative macaques resistant to intrarectal SIVsm challenge. Scand J Immunol. 2001;53:245–253. doi: 10.1046/j.1365-3083.2001.00865.x. [DOI] [PubMed] [Google Scholar]
- 31.Veazey RS, Klasse PJ, Ketas TJ, Reeves JD, Piatak M, Jr, et al. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J Exp Med. 2003;198:1551–1562. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 33.Fantuzzi L, Canini I, Belardelli F, Gessani S. IFN-beta stimulates the production of beta-chemokines in human peripheral blood monocytes. Importance of macrophage differentiation. Eur Cytokine Netw. 2001;12:597–603. [PubMed] [Google Scholar]
- 34.Harouse JM, Tan RC, Gettie A, Dailey P, Marx PA, et al. In vitro infection of primate PBMC with simian/human immunodeficiency virus, SHIV(SF33A): correlation to in vivo outcome. J Med Primatol. 1998;27:81–86. doi: 10.1111/j.1600-0684.1998.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 35.Joag SV, Li Z, Foresman L, Stephens EB, Zhao LJ, et al. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh G, Alvarez X, Borda JT, Aye PP, Lackner AA, et al. Visualizing cytokine-secreting cells in situ in the rhesus macaque model of chronic gut inflammation. Clin Diagn Lab Immunol. 2005;12:192–197. doi: 10.1128/CDLI.12.1.192-197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]