Abstract
Longitudinal bone growth is determined by the process of endochondral ossification in the cartilaginous growth plate, which is located at both ends of vertebrae and long bones and involves many systemic hormones and local regulators. Natriuretic peptides organize a family of three structurally related peptides: atrial natriuretic peptide, brain natriuretic peptide (BNP), and C-type natriuretic peptide. Atrial natriuretic peptide and BNP are cardiac hormones that are produced predominantly by the atrium and ventricle, respectively. C-type natriuretic peptide occurs in a wide variety of tissues, where it acts as a local regulator. These peptides can influence body fluid homeostasis and blood pressure control through the activation of two guanylyl cyclase (GC)-coupled natriuretic peptide receptor subtypes—GC-A and GC-B. We report here marked skeletal overgrowth in transgenic mice that overexpress BNP. Transgenic mice with elevated plasma BNP concentrations exhibited deformed bony skeletons characterized by kyphosis, elongated limbs and paws, and crooked tails. Bone abnormalities resulted from a high turnover of endochondral ossification accompanied by overgrowth of the growth plate. Studies using an in vitro organ culture of embryonic mouse tibias revealed that BNP increases the height of cartilaginous primordium directly, thereby stimulating the total longitudinal bone growth. The present study demonstrates that natriuretic peptides can affect the process of endochondral ossification.
Bone formation occurs through two different mechanisms, i.e., endochondral and membranous ossifications (1). Most of the craniofacial bones are developed through membranous ossification. On the other hand, endochondral ossification requires the sequential formation and degradation of cartilaginous structures that serve as molds for the developing bones, which involves the formation of vertebrae and long bones.
Natriuretic peptides organize a family of three structurally related peptides: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP) (2, 3). ANP and BNP are cardiac hormones that are produced mainly by the atrium and ventricle, respectively (4-7). CNP occurs in a wide variety of tissues (8-10), where it acts as a neuropeptide as well as a local regulator. These peptides are involved in the regulation of cardiovascular homeostasis by their potent natriuretic, diuretic, vasodilatory, and cell growth inhibitory activities.
The biological actions of natriuretic peptides are thought to be mediated by intracellular accumulation of cGMP through the activation of particulate guanylyl cyclases (GCs) (11, 12). Two GC-coupled natriuretic peptide receptor subtypes (GC-A and GC-B) have been cloned so far. The rank order of ligand selectivity for GC-A and GC-B is: ANP ≧ BNP ≫ CNP and CNP > ANP ≧ BNP, respectively (13, 14).
It has been recognized that natriuretic peptide receptors are expressed not only in the cardiovascular system but in a variety of extracardiovascular tissues (11, 12), suggesting that natriuretic peptides play roles outside the cardiovascular system. Several studies using primary cultures of osteoblast-like cells and chondrocytes, and osteoclast-containing bone marrow cultures, or cultured cell lines such as osteoblastic MC3T3-E1 cells have suggested that natriuretic peptides modulate the proliferation and differentiation of osteoblasts, chondrocytes, and osteoclasts in vitro (15–18). However, whether natriuretic peptides can regulate bone formation in vivo has remained to be elucidated. Here, we report skeletal overgrowth in transgenic mice that overexpress BNP.
MATERIALS AND METHODS
Generation of BNP-transgenic Mice.
Generation of BNP-transgenic mice under the control of the human serum amyloid P component promoter was reported previously (7). The human serum amyloid P component promoter was active only in the liver after birth (7). Transgenic mice have been maintained in heterozygous states. Transgene copy numbers were assessed by Southern blot analysis by using mouse tail DNAs. Five independent transgenic lines [54 (50–80 copies), 55 (20 copies), 67 (5–6 copies), 73 (2–3 copies), and 75 (30–40 copies)] and nontransgenic littermates were used in the present study. Plasma BNP concentrations were determined by using an RIA specific for mouse BNP (7). At 4 mo of age, plasma BNP concentrations (the mean ± SD) were 12.2 ± 1.2 pmol/ml, 1.8 ± 1.1 pmol/ml, <0.06 pmol/ml, <0.06 pmol/ml, and 2.4 ± 0.2 pmol/ml in lines 54, 55, 67, 73, and 75, respectively, and <0.06 pmol/ml in nontransgenic controls (n = 4–6).
Soft X-Ray Analysis and Computed X-Ray Densitometry Analysis.
Mice were killed, skinned, eviscerated, and subjected to soft x-ray analysis (27 KVp, 5 mA for 1 min, Softron Type SRO-M5, Softron, Tokyo). The femurs were dissected out and exposed separately to x-ray film. The femur bone marrow width, cortical thickness, and bone mineral density were measured by using a computed x-ray densitometer (Bonalyzer; Teijin, Tokyo) as described (19).
Skeletal Preparations and Histology.
Mice aged 5 days postnatally were killed, skinned, eviscerated, and fixed by 95% ethanol, followed by double-staining with 0.03% alcian blue and 0.01% alizarin red S in 70% ethanol and 5% acetic acid. After washing in water, samples were immersed in 1% KOH and dehydrated with 20% aqueous glycerin in 1% KOH and 50% and 80% glycerin and stored in 100% glycerin.
Carcasses were fixed in 4% paraformaldehyde in PBS and decalcified in 10% EDTA, and samples from different parts of the skeleton were embedded in paraffin. Five μm-thick sections were cut from paraffin-embedded specimens and stained with alcian blue (pH 2.5) and hematoxylin/eosin (H&E).
For alkaline phosphatase staining, fresh specimens were fixed in 70% ethanol and embedded by using glycol methacrylate semimer. Five μm-thick sections were cut from undecalcified block and stained with naphtol AS-TR phosphate and fast blue BB salt (Sigma). After the alkaline phosphatase staining, l-tartrate-resistant acid phosphatase staining was performed as described (20).
Eight-week-old mice were injected s.c. with tetracycline hydrochloride (20 mg/kg) and 3 days later with calcein at the same dose. Mice were killed 24 hr after the second injection, and their lumbar vertebrae were dissected, fixed in 70% ethanol, soaked in Villanueva staining solution (21), and embedded in methylmethacrylate. Histomorphometric parameters followed the recommended nomenclature (22). Histomorphometry was performed 500 μm apart from the growth plate and 150 μm from the cortical periosteum by using a computer-based image analyzing system, Osteoplan II (Zeiss).
Organ Culture of Embryonic Mouse Tibias.
Organ culture of fetal mouse tibias was performed by the suspension culture technique in a chemically defined medium (Bigger’s BJG medium) as described (23). Tibial explants from 16-day-old normal ICR mouse embryos were cultured for 6 days with or without 10−8–10−6 M mouse BNP [77-121] and 10−6 M CNP. Before and after the 6-day culture, the total longitudinal bone length and maximal longitudinal length of the proximal and distal cartilaginous primordia and osteogenic center were measured by using a linear ocular scale mounted on an inverted microscope. Alcian blue and H&E staining were performed as described above.
The intracellular cGMP concentrations in embryonic mouse tibias treated with or without 10−8–10−6 M mouse BNP and CNP for 45 min were determined by using an RIA for cGMP as described (24). The effects of 50 mg/liter HS-142-1, a nonpeptide GC-coupled natriuretic peptide receptor antagonist (24), on the total longitudinal bone growth also were examined.
Northern Blot Analysis of GC-A and GC-B mRNAs.
Total RNA was extracted from 8-day-old normal mouse bones (tibias, tails, and vertebrae), rat pheochromocytoma cell line PC12 cells, and cultured rat aortic smooth muscle cells by the acid guanidinium phenol chloroform method (14). Bone was removed microscopically to avoid the contamination of surrounding tissues. The tibial epiphysis and diaphysis also were separated carefully. Northern blot analysis was performed by using the rat GC-A and GC-B cDNA probes (14) and a human β-actin genomic probe (Wako Pure Chemical, Osaka).
Statistical Analysis.
Data were expressed as the mean ± SD. The statistical significance of differences in mean values was assessed by using the Student’s t test.
RESULTS
Skeletal Phenotypes of BNP-Transgenic Mice.
Transgenic mice with overexpression of BNP exhibited skeletal abnormalities of variable severity, depending on the lines examined. No gross skeletal defects were observed at birth. In line 54 with the highest plasma BNP concentrations, crooked tails were first observed 1-2 days postpartum. The mice developed kyphosis, starting as early as 2-3 days after birth, and became progressively hump-backed (Fig. 1A). They also had elongated limbs, paws, and tails. No gross abnormalities were found in the craniofacial portion of transgenic mice. During postnatal development, increased body length was observed in three independent transgenic lines, 54, 55, and 75, in proportion to plasma BNP concentrations (Fig. 1B). No significant differences in body weight were noted between transgenic and nontransgenic littermates. Mice with less than five copies (lines 67 and 73) had no apparent skeletal abnormalities during development.
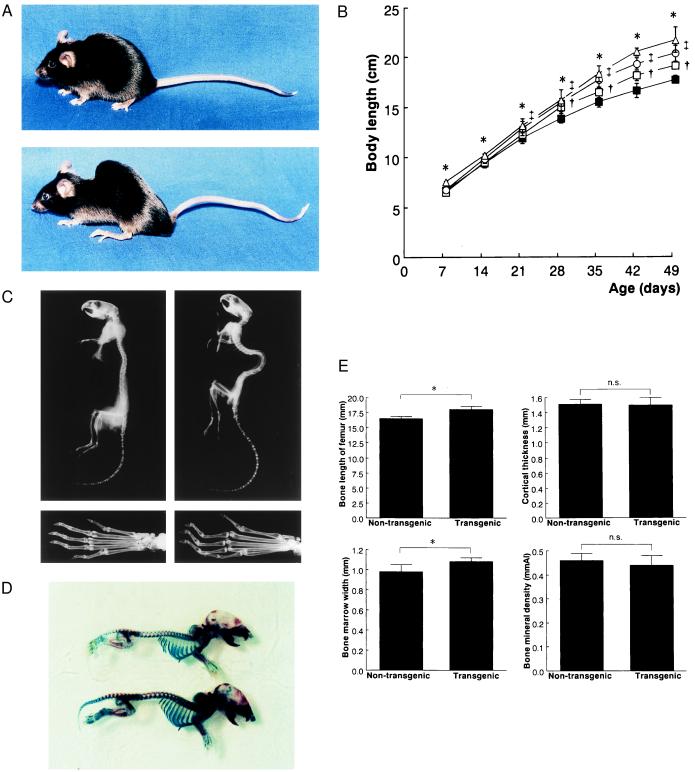
Figure 1.
Skeletal phenotypes of BNP-transgenic mice. (A) Gross appearance of female nontransgenic (Upper) and BNP-transgenic (Lower) mice (line 54) at 4 mo of age. (B) Growth curves of male BNP-transgenic mice and nontransgenic controls. Body length, defined as the distance between the incisor and the tail end, was measured every 7 days after birth before the tail was cut. The body lengths vs. time of three transgenic lines (54, 55, and 75) and nontransgenic controls are plotted. ▵, line 54; □, line 55; ○, line 75; and ▪, age-matched control. ∗, †, ‡, P < 0.01; lines 54, 55, and 75 vs. age-matched controls by Student’s t test. (C) Soft x-ray analysis of the entire skeleton (Upper) and phalanges (Lower) of male nontransgenic (Left) and BNP transgenic (Right) mice (line 54) at 7 months of age. (D) Alcian blue and alizarin red S staining of skeletons of 5-day-old nontransgenic (Upper) and BNP-transgenic (Lower) mice (line 54). (E) Computed x-ray densitometry analysis of mouse femurs. The longitudinal length, cortical thickness, bone marrow width, and bone mineral density of the femur from 7-mo-old male nontransgenic and BNP-transgenic mice (line 75 n = 12). ∗, P < 0.01; line 75 vs. age-matched controls by Student’s t test. n.s., not significant.
Soft x-ray analysis revealed that BNP-transgenic mice have deformed bony skeletons (Fig. 1C). Transgenic mice had larger vertebral bodies in length and had elongated femurs, tibias, and metatarsal bones compared with nontransgenic controls. Double-staining with alcian blue and alizarin red S showed that vertebral bodies and limbs were elongated even neonatally in BNP-transgenic mice (Fig. 1D).
The longitudinal femur length was increased in BNP-transgenic mice compared with nontransgenic littermates (P < 0.01) (Fig. 1E). The femur bone marrow width in BNP-transgenic mice also was increased significantly (P < 0.01). No significant differences in the femur cortical thickness and bone mineral density were observed between BNP-transgenic and nontransgenic littermates.
Histological Analysis.
Light microscopic examination revealed a consistent increase in the height of the growth plate cartilage in vertebrae and long bones from BNP-transgenic mice. In 7-day-old transgenic mice, the heights of both hypertrophic and nonhypertrophic zones of the growth plate cartilage were increased and the sizes of hypertrophic chondrocytes were enlarged (Fig. 2A). In the intervertebral discs from transgenic mice, cells were condensed at day 7 (Fig. 2A) and became round-shaped by day 21 (data not shown). In 4-mo-old BNP-transgenic mice, overgrowth of the growth plate cartilage also was observed and hyaline-like cartilage occurred in the intervertebral disk, where fibrous cartilage is formed normally (Fig. 2B). In the intervertebral ligament, hyaline-like cartilage also was formed in transgenic mice (Fig. 2B). Disorganized columnar array of the growth plate chondrocytes was observed in tails from BNP-transgenic mice (Fig. 2C). The growth plate cartilage sometimes was invaded into the intervertebral region, and pockets of chondrocytes were observed embedded in trabecular bone near the growth plate (Fig. 2C). Because such areas were observed only near the growth plate, this result is probably not caused by de novo chondrocyte formation in trabecular bone but by disregulation at the growth plate.
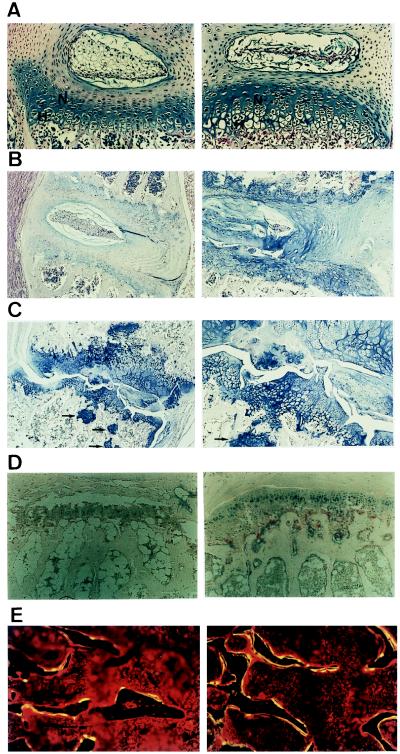
Figure 2.
Histologic analyses of bone abnormalities of BNP-transgenic mice (line 54) and nontransgenic controls. (A) Alcian blue and H&E staining of lumbar vertebral growth plate from nontransgenic (Left) and transgenic (Right) mice at 7 days of age. Magnification: ×200. N, nonhypertrophic zone; H, hypertrophic zone. (B) Alcian blue and H&E staining of lumbar vertebral growth plate from nontransgenic (Left) and transgenic (Right) mice at 4 mo of age. Magnification: ×40. (C) Lower (×40, Left) and higher (×100, Right) magnifications of alcian blue and H&E staining of the tail growth plate from transgenic mice at 4 mo of age. Arrows denote pockets of chondrocytes in trabecular bone. (D) Alkaline phosphatase (stained in blue) and l-tartrate-resistant acid phosphatase (stained in red) staining of vertebral growth plate from nontransgenic (Left) and transgenic (Right) mice at 7 mo of age. Magnification: ×100. (E) Double-labeling with tetracycline and calcein of lumbar vertebra from nontransgenic (Left) and transgenic (Right) mice at 8 wk of age. Magnification: ×100.
The vertebrae and long bones from BNP-transgenic mice were stained for alkaline phosphatase and l-tartrate-resistant acid phosphatase. The transgenic mice showed an increase in alkaline phosphatase-positive hypertrophic chondrocytes in number compared with nontransgenic littermates. l-tartrate-resistant acid phosphatase-positive osteoclasts also were increased in number (Fig. 2D).
Bone Histomorphometry Analysis.
Double-labeled lines with tetracycline and calcein were increased in BNP-transgenic mice (Fig. 2E). Bone histomorphometry analysis revealed that no significant differences in bone volume normalized to tissue volume (BV/TV) are noted between transgenic and nontransgenic littermates (Table 1). In BNP-transgenic mice, osteoid volume normalized to bone volume (OV/BV) tended to be increased and bone formation rate normalized to bone volume and bone surface (BFR/BV and BFR/BS) was increased significantly compared with that of nontransgenic littermates. Osteoclast number (N.Oc/B.Ar) also was increased in BNP-transgenic mice.
Table 1.
Histomorphometry analysis
| Nontransgenic | BNP-transgenic | |
|---|---|---|
| BV/TV, % | 19.3 ± 2.6 | 19.9 ± 2.8 |
| BS/TV, mm2/cm3 | 10248.0 ± 380.9 | 10520.9 ± 821.7 |
| OV/BV, % | 4.5 ± 1.6 | 6.1 ± 1.8 |
| N.Oc/B.Ar/cm2 | 198.9 ± 44.8 | 315.3 ± 42.9* |
| BFR/BV, mm3/cm3/year | 22.6 ± 7.8 | 40.7 ± 4.3* |
| BFR/BS, mm3/cm2/year | 1.9 ± 0.6 | 3.5 ± 0.4* |
BV, bone volume; TV, tissue volume; BS, bone surface; OV, osteoid volume; N.Oc, osteoclast number; B.Ar, bone area; and BFR, bone formation rate. Values are the mean ± SD (n = 4).
P < 0.01 by Student’s t test.
Effects of Natriuretic Peptides on Cultured Embryonic Mouse Tibias.
To examine whether BNP acts directly on the long bone and stimulates the longitudinal bone growth in BNP-transgenic mice, the effects of BNP on the long bone growth were examined by using an in vitro organ culture of normal embryonic mouse tibias. Mouse BNP significantly increased the total longitudinal bone growth compared with that of vehicle-treated groups (Fig. 3A). Treatment of cultured tibias with 10−6 M mouse BNP resulted in an increase in the length of cartilaginous primordia compared with vehicle-treated groups (P < 0.01). No significant differences in the length of osteogenic center were observed between BNP- and vehicle-treated groups. Histological examinations revealed an increase in the height of both hypertrophic and nonhypertrophic zones from BNP-treated groups compared with vehicle-treated groups (Fig. 3B). The size of hypertrophic chondrocytes also was increased in BNP-treated groups. These findings are consistent with those obtained from BNP-transgenic mice (Fig. 2). HS-142-1 completely abolished the BNP-induced increase in the length of embryonic mouse tibias. CNP at a dose of 10−6 M also significantly increased the total longitudinal bone growth compared with that of vehicle-treated groups (P < 0.01). CNP was more potent than BNP in increasing the total longitudinal length of cultured embryonic mouse tibias (Fig. 3C). Histological examinations also confirmed an increase in the height of both hypertrophic and nonhypertrophic zones in CNP-treated groups, which was more pronounced than those observed in BNP-treated groups.
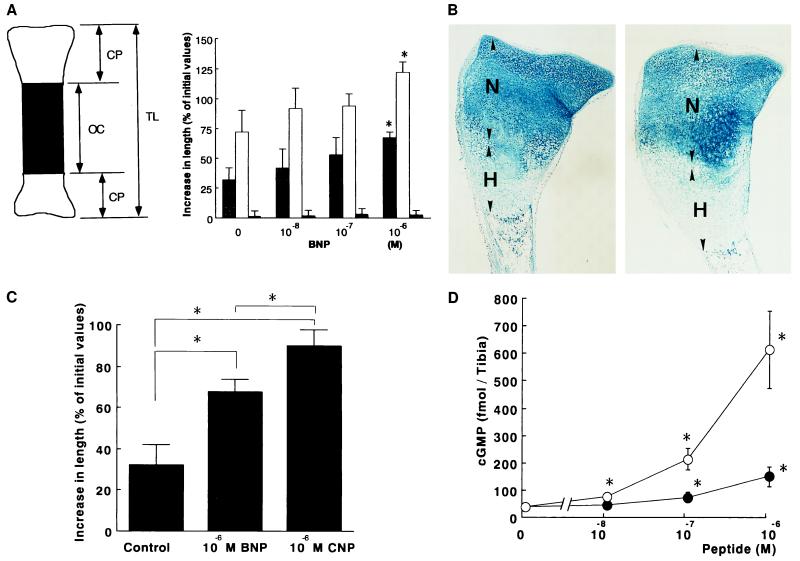
Figure 3.
Effects of BNP on cultured embryonic mouse tibias. (A) Effects of BNP on bone growth. (Left) Schematic representation of cultured mouse tibias. TL, total length; CP, cartilaginous primordium; and OC, osteogenic center. (Right) Dose-related effects of mouse BNP on the total length and length of cartilaginous primordium and osteogenic center of mouse tibias after the 6-day culture. Data are expressed as increases in length from the initial values and are from five independent determinations. Shaded bars, TL; open bars, CP; and closed bars, OC. ∗, P < 0.01 vs. vehicle-treated groups. (B) Alcian blue and H&E staining of cultured mouse tibias treated with 10−6 M mouse BNP. N, nonhypertrophic zone; H, hypertrophic zone. (C) Comparisons of effects of BNP and CNP on bone growth. Data are expressed as increases in length from the initial values and are from five independent determinations. ∗, P < 0.01 vs. vehicle-treated groups. (D) Dose-related effects of mouse BNP and CNP on cGMP production in fetal mouse tibias. Data were expressed as the mean ± SD of four independent determinations. •, BNP; ○, CNP. ∗, P < 0.01 vs. vehicle-treated groups.
Treatment with BNP or CNP significantly and dose-dependently increased the cGMP production in cultured embryonic mouse tibias (Fig. 3D). Treatment with 10−6 M BNP increased the cGMP production by fourfold compared with that of vehicle-treated groups (P < 0.01). On the other hand, CNP at the same dose increased the cGMP production by more than sixteenfold (P < 0.01). The effect of BNP on cGMP production was inhibited by 50 mg/liter HS-142-1 (≈70% inhibition, n = 4).
Expression of GC-A and GC-B mRNAs in the Bone.
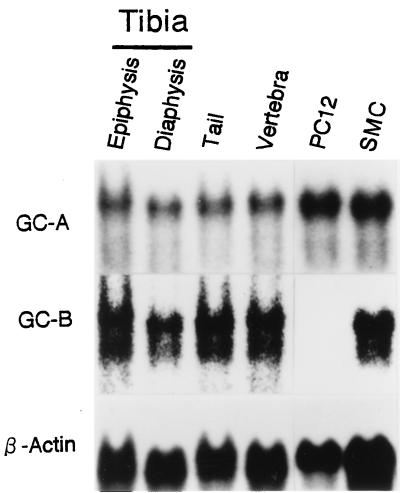
Northern blot analysis revealed that GC-A and GC-B mRNAs are expressed abundantly in 8-day-old normal mouse tibias, tails, and vertebrae (Fig. 4). Of particular note was a large amount of GC-B mRNA in long bones. The two mRNA species were detected in both the mouse tibial epiphysis and diaphysis. The amounts of GC-A and GC-B mRNAs in mouse long bones and vertebrae were comparable to those in PC12 cells and rat SMC, in which GC-A and GC-B mRNAs are expressed abundantly, respectively (14).
Figure 4.
Northern blot analysis of GC-A and GC-B mRNAs in the tibial epiphysis and diaphysis, tails, and vertebrae from 8-day-old normal mice and in PC12 cells and rat smooth muscle cells (SMC).
DISCUSSION
The present study demonstrates that BNP-transgenic mice exhibit overgrowth of the growth plate cartilage with marked longitudinal growth of vertebrae and long bones. By contrast, no significant changes in the craniofacial bones were observed between transgenic and nontransgenic littermates. Longitudinal growth of vertebrae and long bones is determined by the process of endochondral ossification, whereas most of the craniofacial bones are developed through membranous ossification (1). These observations, taken together, indicate that, in BNP-transgenic mice, BNP is involved in the process of endochondral ossification but not in the process of membranous ossification. This notion is supported by our data that show that, in BNP-transgenic mice, the femur bone marrow width is increased, which is attributable to endochondral ossification. On the other hand, the cortical thickness is unchanged, which is increased through membranous ossification.
Studies using an in vitro organ culture of mouse tibias demonstrated that BNP acts directly on the long bone, thereby increasing the longitudinal bone growth. The increase in the total bone length mostly is caused by the increased height of cartilaginous bone ends. In the present study, with an increase in cGMP production, mouse BNP increased the total longitudinal bone growth significantly, which was abolished by HS-142-1. Furthermore, GC-A and GC-B mRNAs were expressed abundantly in the normal mouse tibial epiphysis. No significant amount of C-receptor mRNA was detected (A.Y., Y.O., M.S., N.T., K.T., and K.N., unpublished results), suggesting no involvement of C-receptor in the effects of BNP on bone growth. These findings, taken together, suggest that BNP directly activates the proliferation and differentiation of the growth plate chondrocytes via GC-coupled natriuretic peptide receptors. Pfeifer and colleagues (25) reported recently that mice deficient in type II cGMP-dependent protein kinase (cGK) develop impaired endochondral ossification and showed that type I and II cGK mRNAs are expressed in the growth plate chondrocytes from long bones. Because cGK is one of the major signal transduction mechanisms of cGMP, these findings also support the idea that natriuretic peptides modulate cellular functions in these cell types through the cGMP-dependent mechanisms in vivo.
In the present study, we also observed that CNP, a selective activator of GC-B (13, 14), increases the total longitudinal bone growth and cGMP production in cultured embryonic mouse tibias more potently than BNP, an activator of GC-A. These findings suggest strongly that activation of chondrogenesis by natriuretic peptides is mediated primarily via GC-B. Because BNP is overproduced specifically by the liver and not in the bone from BNP-transgenic mice (7), we postulate that BNP secreted into the circulation in a large quantity cross-reacts with GC-B in the growth plate chondrocytes, thereby causing skeletal overgrowth of vertebrae and long bones in these animals. We and others previously reported that CNP occurs in cultured osteoblast-like cells, chondrocytes, and osteoclasts and acts on its cognate receptor GC-B, thereby regulating bone metabolism in vitro (15–18). It is tempting, therefore, to speculate that CNP is the endogenous ligand for GC-B in the bone in vivo and is involved in the process of endochondral ossification.
It is noteworthy that no skeletal defects are reported in transgenic mice overexpressing ANP (26), although ANP and BNP have similar affinity to known GC-coupled natriuretic peptide receptors (13, 14). Plasma ANP concentrations reported in ANP-transgenic mice (26) are roughly equivalent to plasma BNP concentrations in BNP-transgenic line 55, which has mild skeletal phenotypes. The ANP-transgenic mice might have mild skeletal abnormalities similar to those of BNP-transgenic line 55. The discussion above, however, does not rule out the possible existence of an as-yet-unidentified receptor specific for BNP in the bone. Therefore, BNP-transgenic mice provide the useful in vivo model system with which to assess the functional roles of natriuretic peptides in bone formation.
In the present study, bone histomorphometry analysis revealed that both osteogenic and osteoclastic activities are increased and balanced, indicating a high turnover of endochondral ossification in BNP-transgenic mice. Increased osteoclastic activity could be secondary to overgrowth of growth plate. Alternatively, BNP might increase directly bone resorption in BNP-transgenic mice because CNP has been shown to increase bone resorption via GC-B in vitro (16).
Plasma BNP concentrations in patients with congestive heart failure (4, 5) are elevated markedly and are comparable to those in BNP-transgenic mice. Skeletal overgrowth may be observed exaggerated in BNP-transgenic mice because, in rodents, growth plates remain open for a long time (1). Evidence has accumulated suggesting a significant association between scoliosis and congenital heart diseases (27, 28). It is tempting to speculate that BNP and/or ANP overproduced by the heart (4, 5, 29) might increase bone growth in patients with congenital heart defects, while growth plates are open.
In conclusion, the present study demonstrates that natriuretic peptides can affect the process of endochondral ossification. Our results provide insight into the molecular mechanisms underlying bone formation.
Acknowledgments
We thank N. Amizuka, Y. Hiraki, A. Yamaguchi, H. Kanetani, and S. Fukuda for discussions and S. Nakanishi for providing HS-142-1. This work was supported by research grants from the Japanese Ministry of Education, Science and Culture, the Japanese Ministry of Health and Welfare, the Yamanouchi Foundation for Research on Metabolic Disorders, the Smoking Research Foundation, the Salt Science Research Foundation, the Uehara Memorial Foundation, Takeda Science Foundation, Japanese Society for the Promotion of Science “Research for the Future” program (JSPS-RFTF 96100204), and the Research Society for Metabolic Bone Disorders.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CNP, C-type natriuretic peptide; GC, guanylyl cyclase; H&E, hematoxylin and eosin.
References
- 1.Howell D S, Dean D D. In: Disorders of Bone and Mineral Metabolism. Coe F L, Favus M J, editors. New York: Raven; 1992. pp. 313–353. [Google Scholar]
- 2.Rosenzweig A, Seidman C E. Annu Rev Biochem. 1991;60:229–255. doi: 10.1146/annurev.bi.60.070191.001305. [DOI] [PubMed] [Google Scholar]
- 3.Nakao K, Ogawa Y, Suga S, Imura H. J Hypertension. 1992;10:907–912. [PubMed] [Google Scholar]
- 4.Ogawa Y, Nakao K. In: Hypertension: Pathophysiology, Diagnosis, and Management. Laragh J H, Brenner B M, editors. New York: Raven; 1995. pp. 833–840. [Google Scholar]
- 5.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagnino L, Drouin J, Nemer M. Mol Endocrinol. 1991;5:1292–1300. doi: 10.1210/mend-5-9-1292. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, Matsuda S, Shiono S, Nishimoto H, Nakao K. J Clin Invest. 1994;93:1911–1921. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollmar A M, Gerbes A L, Nemer M, Schulz R. Endocrinology. 1993;132:1872–1874. doi: 10.1210/endo.132.4.8462485. [DOI] [PubMed] [Google Scholar]
- 10.Chrisman T D, Schulz S, Potter L R, Garbers D L. J Biol Chem. 1993;268:3698–3703. [PubMed] [Google Scholar]
- 11.Chinkers M, Garbers D L. Annu Rev Biochem. 1991;60:553–575. doi: 10.1146/annurev.bi.60.070191.003005. [DOI] [PubMed] [Google Scholar]
- 12.Nakao K, Ogawa Y, Suga S, Imura H. J Hypertens. 1992;10:1111–1114. doi: 10.1097/00004872-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Koller K J, Lowe D G, Bennett G L, Minamino N, Kangawa K, Matsuo H, Goeddel D V. Science. 1991;252:120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 14.Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kanbayashi Y, Inouye K, et al. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 15.Hagiwara H, Sakaguchi H, Itakura M, Yoshimoto T, Furuya M, Tanaka S, Hirose S. J Biol Chem. 1994;269:10729–10733. [PubMed] [Google Scholar]
- 16.Holliday L S, Dean A D, Greenwald J E, Gluck S L. J Biol Chem. 1995;270:18983–18989. doi: 10.1074/jbc.270.32.18983. [DOI] [PubMed] [Google Scholar]
- 17.Inoue A, Hiruma Y, Hirose S, Yamaguchi A, Hagiwara H. Biochem Biophys Res Commun. 1995;215:1104–1110. doi: 10.1006/bbrc.1995.2577. [DOI] [PubMed] [Google Scholar]
- 18.Suda M, Tanaka K, Yasoda A, Ogawa Y, Itoh H, Nakao K. Biochem Biophys Res Commun. 1996;223:1–6. doi: 10.1006/bbrc.1996.0836. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto C, Kushida K, Yamazaki K, Imose K, Inoue T. Calcif Tissue Int. 1994;55:324–329. doi: 10.1007/BF00299308. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Sanghvi R, Burnell J M, Howard G A. Histochemistry. 1987;86:559–565. doi: 10.1007/BF00489547. [DOI] [PubMed] [Google Scholar]
- 21.Villanueva A R, Lundin K D. Stain Technol. 1989;64:129–138. doi: 10.3109/10520298909106985. [DOI] [PubMed] [Google Scholar]
- 22.Parfitt A M, Drezner M K, Glorieux F H, Kanis J A, Malluche H, Meunier P J, Ott S M, Recker R R. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 23.Shiota K, Kosazuma T, Klug S, Neubert D. Acta Anat. 1990;137:59–64. doi: 10.1159/000146859. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu Y, Itoh H, Suga S, Ogawa Y, Hama N, Kishimoto I, Nakagawa O, Igaki T, Doi K, Yamashita T, et al. Circ Res. 1996;78:606–614. doi: 10.1161/01.res.78.4.606. [DOI] [PubMed] [Google Scholar]
- 25.Pfeifer A, Aszódi A, Seidler U, Ruth P, Hofmann F, Fässler R. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- 26.Steinhelper M E, Cochrane K L, Field L J. Hypertension. 1990;16:301–307. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- 27.Jordan C E, White R I, Jr, Fischer K C, Neill C, Dorst J P. Am Heart J. 1972;84:463–469. doi: 10.1016/0002-8703(72)90468-1. [DOI] [PubMed] [Google Scholar]
- 28.Reckles, L. N., Peterson, H. A., Bianco, A. J., Jr., & Weidman, W. H. (1975) J. Bone Jt. Surg. Am. Vol. 57-A, 449–455. [PubMed]
- 29.Yoshibayashi M, Saito Y, Nakao K. Eur J Endocrinol. 1996;135:265–268. doi: 10.1530/eje.0.1350265. [DOI] [PubMed] [Google Scholar]