Abstract
From an analysis of the distributions of measures of transmission rates among hosts, we identify an empirical relationship suggesting that, typically, 20% of the host population contributes at least 80% of the net transmission potential, as measured by the basic reproduction number, R0. This is an example of a statistical pattern known as the 20/80 rule. The rule applies to a variety of disease systems, including vector-borne parasites and sexually transmitted pathogens. The rule implies that control programs targeted at the “core” 20% group are potentially highly effective and, conversely, that programs that fail to reach all of this group will be much less effective than expected in reducing levels of infection in the population as a whole.
Keywords: basic reproduction number, HIV/AIDS, leishmaniasis, malaria, schistosomiasis
The transmission of infectious agents within host populations is influenced by many different sources of heterogeneity ranging from genetic via behavioral factors to spatial factors (1–6). A consequence of such heterogeneity is the commonly observed aggregated (clumped) distributions of infection and/or disease within the host population such that a few hosts are rapidly, frequently, or heavily infected, while the majority either evade infection or suffer infrequent or light infections (1, 7–10). Theoretical studies suggest that heterogeneity in exposure to infection is a key factor for the optimal design of disease control programs (11–13), and, most importantly, whether or not to target interventions to specific risk groups or stratifications of the host population (1, 14–17). This decision will depend on many factors including the cost of identifying those most at risk, but an initial step deriving from epidemiological study is to quantify the contributions of individuals within a host population to the transmission potential of a given infectious agent.
The transmission potential of an infectious agent can be quantified as the basic reproduction number, R0. For microparasites, such as viruses or parasitic protozoa, R0 represents the number of secondary cases produced in a fully susceptible host population by a single primary case over its entire infectious period. For macroparasites, such as the parasitic helminths, R0 represents the number of adult female parasites produced per adult female parasite over her reproductive lifespan in the absence of density-dependent constraints on population growth. In both cases R0 must be greater than one for endemic or epidemic infection to be possible. The value of R0 is affected by heterogeneities in transmission rates; we consider the case where the host population is divided into m subgroups, each experiencing different rates of transmission and where transmission rates to and from hosts are perfectly correlated. With all other aspects of transmission identical, R0 is lower if the host population is homogeneous (i.e., m = 1), and is higher if it is heterogeneous (m > 1). This applies to parasites transmitted by biting arthropods, such as malaria or filarial worms (11, 18), to parasites transmitted via snails and with free-living larval stages, such as schistosomes (12, 13), and to directly transmitted infections (19–21), including sexually transmitted diseases (STDs) (22–24).
Control programs will eliminate parasites from a host population if they have the effect of reducing the effective reproduction number, Rc, to below one. Some control measures can be viewed as removing the contribution of host subgroups to parasite transmission; these measures include exposure protection (e.g., by the use of condoms against STDs), regular drug treatment, and vaccination. The proportion of hosts that must be covered by such control measures for elimination, pc, depends both on R0 and on heterogeneities in the host population. An important general result (1) is that pc for untargeted (mass) control is higher when the host population is heterogeneous (m > 1) than when it is homogeneous (m = 1), but that pc for targeted control is lower when the host population is heterogeneous, provided control can be accurately directed at host subgroups contributing most to R0. The extent to which targeted control should be favored over mass control and the potential impact of control thus depend crucially on the magnitude of heterogeneities in transmission rates and on the associated costs of mass versus targeted interventions.
Various studies have shown that there are heterogeneities in host–vector contact [e.g., biting by mosquito vectors of malaria (25–27); exposure to blackfly vectors of onchocerciasis (28); contact between cattle herds and tsetse vectors of trypanosomes (29); and exposure to water containing snail intermediate hosts of schistosomes (30, 31)] and in risk behavior for STDs (32, 33). However, there are relatively few studies that quantify this variation across an entire host population or a representative sample. In this paper we analyze 10 such data sets: three studies of arthropod transmitted parasites reporting vector-to-host ratios across households (one for sandfly vectors of canine leishmaniasis in Brazil, and two for mosquito vectors of human malaria, in Papua New Guinea and Tanzania); five studies of human schistosomes reporting individual contact rates at water contact sites containing snail intermediate hosts (two from Zimbabwe and three from Mali); and two national surveys of sexual behavior relevant to STDs (one from the United Kingdom and one from France).
METHODS
Data.
Data on Leishmania chagasi transmission were obtained from Marajo Island, Para state, Brazil (34). Light trap counts of the sandfly vector Lutzomyia longipalpis (females only) were made in chicken sheds at each house on 1 night over a 2-month period at 60 households containing 1 to 7 dogs. Data on malaria [Plasmodium spp. P. falciparum, P. vivax (210 and 247 polymorphs), and P. malariae] transmission were obtained from Wosera, East Sepik, Papua New Guinea (35). All-night human biting collections of the mosquito vectors (Anopheles spp. A. koliensis, A. punctulatus, A. karwari, and A. farauti s.l.) were done on up to 9 nights over a 28-month period at 477 households containing 1–13 individuals with data adjusted for temporal trends and sampling bias. Further data on malaria (P. falciparum) transmission were obtained from Namawala in the Morogoro region of Tanzania (36). Light trap counts of the mosquito vectors Anopheles gambiae s.l. and Anopheles funestus were made on an average of more than 15 nights over a 12-month period at 50 households containing 1–29 individuals with data adjusted for temporal trends and sampling bias. There are likely to be additional heterogeneities in vector–host ratios between individuals within households, but these are not allowed for by the available data.
Data on Schistosoma haematobium and Schistosoma mansoni transmission were obtained from the villages Maniale, Medina Coura, and Dogofry Ba in the Segou region of Mali (37). Contacts with water containing Bulinus truncatus and Biomphalaria pfeifferi snails made during daylight hours on an average of more than 5 days over a 7-month period by 337, 281, and 295 individuals were recorded. Further data on S. haematobium transmission were obtained from Nyamikari farm, Burma Valley, Zimbabwe (31). Contacts with water containing Bu. globosus snails made during daylight hours over a 14-day period by 465 individuals were recorded. Additional data were obtained from Nahoon farm in the same area over 42 days during a 2-month period by 850 individuals.
Data on sexual behavior among the United Kingdom adult (16–59 years old) population giving the difference in numbers of partners reported (both heterosexual and homosexual) over the last year and the last 5 years are taken as an index of the rate of partner change over a 4-year period for a total of 18,111 individuals (38). This time scale is appropriate for the transmission of human immunodeficiency virus (HIV). Data reporting the number of new sexual partners over the last year were obtained from a similar study in France (39). This time scale is more appropriate for the transmission of bacterial STDs including gonorrhaea, chlamydia, and syphilis (33).
Mathematical Models.
Using the appropriate mathematical models, we can quantify the effects of observed heterogeneities in the host populations. For transmission by biting arthropods, R0 is calculated assuming that individual vectors bite in host household i (where i = 1 to m, and m is the number of households) at a rate proportional to the number of vectors sampled in household i. This gives:
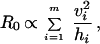 |
1 |
where vi is the proportion of vectors sampled in household i and hi is the proportion of hosts in household i.
For transmission via snails, R0 is calculated assuming transmission rates from host to snail and from snail to host are proportional to contact rates of each host individual i, ηi (where i = 1 to m, and m is the number of individuals); available evidence suggests that these transmission rates are indeed positively correlated (M.E.J.W. and S.K.C., unpublished data) but this approach should be regarded as providing upper estimates of the impact on R0 (40). This gives:
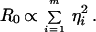 |
2 |
For sexual transmission, R0 is calculated assuming that transmission rates are proportional to the rate of change of sexual partners, ci, of each host individual i (where i = 1 to m). For heterosexual transmission it is assumed, as the simplest case, that males and females have the same mean and distribution of ci values (1). This gives:
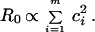 |
3 |
In each case, the effects of discounting hosts on the relative value of Rc are shown by discounting hosts in decreasing order of their observed contribution to R0 by giving zero weighting to that contribution. Individuals or households are taken as units as appropriate; both have been considered elsewhere (41). This assumes that control measures affect only the targeted hosts and do not have indirect or direct effects on the contributions of other hosts to R0. Relative Rc (as a proportion of R0) is then plotted against cumulative fraction discounted. These plots are generalized Lorenz curves; one method of quantifying the concentration of R0 in certain individuals is to calculate a Gini index (42).
To explore the relationship between the statistical distribution of transmission rates and R0, a log-uniform distribution of vector-to-host ratios was generated for m = 100 (with the same number of hosts in each subgroup). Parameters were chosen to give different values for the standardized variance (SV = variance/mean2) of vector-to-host ratios for the same mean value. Relative R0 values are then calculated according to Eq. 1 as described above.
RESULTS
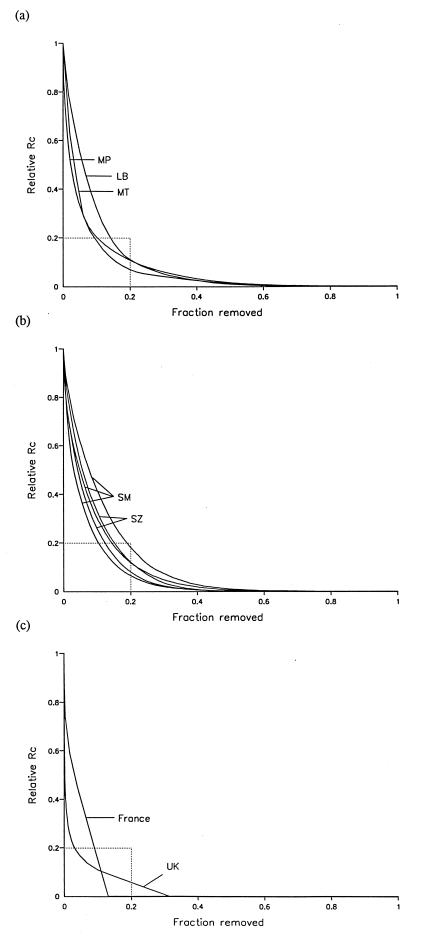
The results suggest that heterogeneities in contact rates lead to consistent and substantial increases in the value of R0, by factors in the range 2 to 4 for vector-borne infections and considerably higher for STDs (Table 1). In the case of the STD study from France (39), only 13% of the adult population had any new sexual partners during 1 year and thus made any contribution to R0 over that time scale. For all other nine data sets, Rc can reduced by more than 80% of R0 by removing 20% of households or individuals (Fig. 1); we refer to this empirical observation as the 20/80 rule (43).
Table 1.
Effects of heterogeneities in contact rates on the value of R0
| Parasite/pathogen | Vector | Host | Region | Ref. | Relative R0 | Gini index |
|---|---|---|---|---|---|---|
| Le. chagasi | Lu. longipalpis | Dog | Brazil | 34 | 3.43 | 0.817 |
| Plasmodium spp. | Anopheles spp. | Human | Papua New Guinea | 35 | 3.89 | 0.859 |
| Plasmodium spp. | Anopheles spp. | Human | Tanzania | i36 | 3.70 | 0.866 |
| Schistosoma spp. | Bu. truncatus | Human | Mali | i37 | 2.90 | 0.749 |
| and Bi. pfeifferi | 2.39 | 0.719 | ||||
| 3.75 | 0.769 | |||||
| S. haematobium | Bu. globosus | Human | Zimbabwe | i31 | 3.02 | 0.825 |
| 3.35 | 0.856 | |||||
| HIV | — | Human | United Kingdom | 38 | 13.82 | 0.938 |
| Bacterial STDs | — | Human | France | i39 | 12.01 | 0.912 |
R0 values for heterogeneous host populations are calculated according to equations given in the main text, as appropriate, relative to R0 = 1 for a homogeneous population.
Figure 1.
Effects of discounting hosts (as individuals or as households) on the relative value of Rc (see text). Relative Rc (as a proportion of R0) is plotted against cumulative fraction discounted (these are step functions over the interval 1/m). The intercept between relative Rc = 0.2 and fraction discounted = 0.2, corresponding to the 20/80 rule, is shown in each case. (a) Vector–host ratios for parasites transmitted by biting arthropods. LB, Leishmania chagasi transmission on Marajo island, Brazil; MP, malaria transmission in Wosera, Papua New Guinea; MT, malaria transmission at Namawala in Tanzania. (b) Water contact rates for parasites transmitted via snails. SM, S. haematobium and S. mansoni transmission at the villages Maniale, Medina Coura, and Dogofry Ba in Mali; SZ, S. haematobium transmission at Nyamikari and Nahoon farms, Zimbabwe. (c) Rates of sexual partner change for STDs: UK, new partners (over 4 years) among the adult population of the United Kingdom; France, new partners (over 1 year) among the adult population of France. This data set includes censored values that were taken as the lower limit of each interval. The different shapes of these curves reflects the different time scales of the observations.
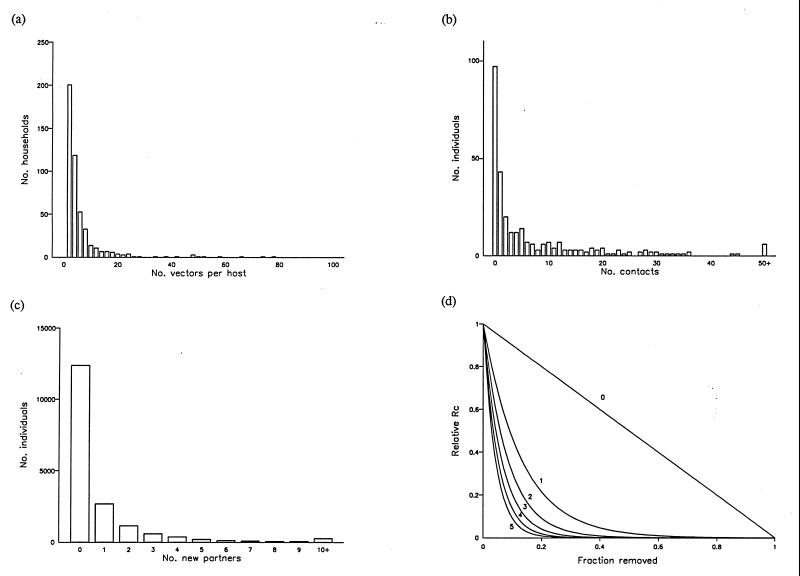
The 20/80 rule is related to the statistical distribution of transmission rates across host subgroups. These distributions are typically aggregated, that is, most households or individuals experience low or zero transmission but a few experience very high transmission (Fig. 2 a–c). For a log-uniform distribution, if the standardized variance (SV) is greater than 1.083 (giving a Gini index of 0.74), then this corresponds to the 20/80 rule (Fig. 2d). A similar result applies if the contact rate has a gamma distribution, requiring SV > 1.109 (K. Dietz, personal communication). All data sets analyzed here satisfy the condition SV > 1. Gini indices range from 0.72 to 0.94, quantifying the concentration of transmission potential within a small fraction of individuals or households (Table 1). The data could not comply with the 20/80 rule if the Gini index was below 0.60.
Figure 2.
Relationship of reduction in Rc to the distribution of contributions to transmission potential. Frequency distributions for (a) vector–host ratios for Anopheles spp. and humans in Wosera, Papua New Guinea (see Fig. 1a), standardized variance (SV) equals 4.01; (b) contacts with schistosome infective water bodies for individuals in Dogofry Ba, Mali (see Fig. 1b), SV = 2.75; (c) changes of sexual partners for individuals in the United Kingdom (see Fig. 1c), SV = 12.82. (d) Expected effects of discounting host subgroups in decreasing order of vector-to-host ratios (following a log-uniform distribution) on the relative value of Rc for microparasites, compared for values of SV from 0 to 5 (as shown). A value of SV = 1.083 corresponds to the 20/80 rule.
DISCUSSION
The results suggest that heterogeneous contact is likely to be an important determinant of the epidemiology of vector-borne diseases and STDs. This may well apply to other infectious agents where “contact” is less easy to quantify. In practice, additional heterogeneities are also likely to contribute to the effects on R0. Biting by mosquitoes of humans is not random, and attractiveness may be related to sex, skin surface area (hence age), and other factors (25, 44–48). Susceptibility to schistosome infection varies with the nature of contacts (31, 49), possibly with skin thickness and lipid content (50, 51) and may be related to genetic factors (52). Risk of STD transmission is affected by the frequency and type of sexual acts and by cofactors (53, 54). Acquired immunity may also affect host susceptibility to infection (55, 56), but this is not relevant to analyses of R0, which is defined with respect to a fully susceptible (naive) population. The magnitude of the effect of these other heterogeneities at the population level is unknown; but they will not decrease R0 unless negatively correlated with the variables analyzed here. There may also be effects of “higher order” heterogeneities, all of which may further increase R0: heterogeneities in the distribution of vectors (11, 18); differing usage of different water contact sites (12, 13, 57); patterns of contact between highly sexually active and less sexually active groups, and differences between the sexes (58). Consistency through time of the heterogeneities in transmission rates is also important. Clearly, if the relative contribution of each host subgroup to R0 is itself variable then the fraction to be targeted must be continually reappraised. For human malaria in Tanzania over 12 months and in Papua New Guinea over 28 months, and for canine leishmaniasis over consecutive years, there is little variation in the relative abundance of vectors compared across households. Such information is not available for behaviors associated with the transmission of either schistosomiasis or STDs. It is important that the time scale of the observation of contact rates is sufficient to properly characterize their distribution across the host population (see Fig. 1c).
Although the actual values of R0 for any given infectious agent will vary between locations and through time, the heterogeneities in transmission rates recorded in this study suggest that targeted interventions can, in principle, induce very substantial reductions in rates of infection. This has been suggested before (1, 13), but the novelty of the result lies in the apparent conformity with the 20/80 rule found across widely different host–parasite associations and in the relative magnitude of the contribution to the transmission potential (over 80%) made by a small fraction (20%) of the host population. In the application of this empirical rule the key issues will be the methods used and the costs entailed in identifying the “core” transmitters. Put simply, if the cost of identifying and treating the core 20% is less than the cost of treating the entire population then targeting control interventions such as vaccination, drug treatment, or exposure protection may be preferred to nontargeted interventions on economic grounds. What is lacking in public health research into disease control is a quantitative assessment of the relative costs and effects. The 20/80 rule should encourage efforts to make such assessments.
Acknowledgments
We are grateful to D. A. P. Bundy, K. Dietz, D. T. Haydon, and C. C. Lord for comments and discussion. M.E.J.W. is supported by the Royal Society. Field work in Brazil was supported by the Wellcome Trust; in Papua New Guinea by the United States Agency for International Development; in Tanzania by the Swiss Directorate for Technical Cooperation and Humanitarian Aid, the Dutch Directorate General for Technical Cooperation, and the World Health Organization Immunology Research and Training Centre; in Mali by the Caisse Francaise de Developpement, the Fonds d’Aide et de Cooperation, the Gesellschaft fur Technische Zusammenarbeit, the Commission of the European Community, and the United Nations Children’s Fund; and in Zimbabwe by the Wellcome Trust, the Commission of the European Community, and the World Health Organization Special Program for Research and Training in Tropical Diseases. Additional support was provided by the Biotechnology and Biological Sciences Research Council.
Footnotes
Abbreviation: STD, sexually transmitted disease.
References
- 1.Anderson R M, May R M. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford Scientific; 1991. [Google Scholar]
- 2.Scott M E, Smith G. Parasitic and Infectious Diseases Epidemiology and Ecology. New York: Academic; 1994. [Google Scholar]
- 3.Manning S D, Woolhouse M E J, Ndamba J. Int J Parasitol. 1995;25:37–42. doi: 10.1016/0020-7519(94)00097-8. [DOI] [PubMed] [Google Scholar]
- 4.Grenfell B T, Dobson A P. Ecology of Infectious Diseases in Natural Populations. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 5.Morand S, Manning S D, Woolhouse M E J. Proc R Soc London Ser B. 1996;263:119–128. doi: 10.1098/rspb.1996.0019. [DOI] [PubMed] [Google Scholar]
- 6.Isham V, Medley G. Models for Infectious Human Diseases: Their Structure and Relation to Data. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]
- 7.Crofton H D. Parasitology. 1971;63:343–364. doi: 10.1017/s0031182000079890. [DOI] [PubMed] [Google Scholar]
- 8.Woolhouse M E J, Chandiwana S K, Bradley M. Int J Parasitol. 1990;20:325–327. doi: 10.1016/0020-7519(90)90147-f. [DOI] [PubMed] [Google Scholar]
- 9.Hudson P J, Dobson A P. In: Ecology of Infectious Diseases in Natural Populations. Grenfell B T, Dobson A P, editors. Cambridge, U.K.: Cambridge Univ. Press; 1995. pp. 144–176. [Google Scholar]
- 10.Woolhouse M E J, McNamara J J, Hargrove J W, Bealby K. Mol Ecol. 1996;5:11–18. doi: 10.1111/j.1365-294x.1996.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 11.Dye C, Hasibeder G. Trans R Soc Trop Med Hyg. 1986;80:69–77. doi: 10.1016/0035-9203(86)90199-9. [DOI] [PubMed] [Google Scholar]
- 12.Barbour A D. Trans R Soc Trop Med Hyg. 1978;72:6–15. doi: 10.1016/0035-9203(78)90290-0. [DOI] [PubMed] [Google Scholar]
- 13.Woolhouse M E J, Watts C H, Chandiwana S K. Proc R Soc London Ser B. 1991;245:109–114. doi: 10.1098/rspb.1991.0095. [DOI] [PubMed] [Google Scholar]
- 14.Bundy D A P. Trans R Soc Trop Med Hyg. 1990;84:622–625. doi: 10.1016/0035-9203(90)90126-y. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. World Report on Tropical Diseases. Geneva: W.H.O.; 1990. [Google Scholar]
- 16.World Bank. World Development Report. Oxford: Oxford Univ. Press; 1993. [Google Scholar]
- 17.Jamison D T, Mosley W H, Measham A R, Bobadilla J L, editors. Disease Control Priorities in Developing Countries. Oxford: Oxford Medical; 1993. [Google Scholar]
- 18.Hasibeder G, Dye C. Theor Pop Biol. 1988;33:31–53. doi: 10.1016/0040-5809(88)90003-2. [DOI] [PubMed] [Google Scholar]
- 19.Hethcote H W. Theor Popul Biol. 1978;14:338–349. doi: 10.1016/0040-5809(78)90011-4. [DOI] [PubMed] [Google Scholar]
- 20.Post W M, De Angelis D L, Travis C C. Math Biosci. 1983;63:289–302. [Google Scholar]
- 21.May R M. Math Biosci. 1984;64:83–111. [Google Scholar]
- 22.Lajmanovich A, Yorke J A. Math Biosci. 1976;28:221–236. [Google Scholar]
- 23.Gupta S, Anderson R M, May R M. AIDS. 1989;3:807–817. [PubMed] [Google Scholar]
- 24.Anderson R M, May R M. Nature (London) 1988;333:514–519. doi: 10.1038/333514a0. [DOI] [PubMed] [Google Scholar]
- 25.Carnevale P, Frezil J L, Bosserio M F, Le Pont F, Lancien J. Bull W H O. 1978;56:147–154. [PMC free article] [PubMed] [Google Scholar]
- 26.Burkot T R, Graves P M, Paru R, Wirtz R A, Heywood P F. Am J Trop Med Hyg. 1988;39:135–144. doi: 10.4269/ajtmh.1988.39.135. [DOI] [PubMed] [Google Scholar]
- 27.Trape J-F, Lefebvrezante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, Salem G. Am J Trop Med Hyg. 1992;47:181–189. doi: 10.4269/ajtmh.1992.47.181. [DOI] [PubMed] [Google Scholar]
- 28.Bocharie M J, Davies J B. Ann Trop Med Parasitol. 1990;84:599–605. doi: 10.1080/00034983.1990.11812515. [DOI] [PubMed] [Google Scholar]
- 29.Wacher T J, Milligan P J M, Rawlings P, Snow W F. Parasitology. 1994;109:149–162. doi: 10.1017/s0031182000076265. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins H A, Blumenthal U J, Hagan P, Hayes R J, Tulloch S. Trans R Soc Trop Med Hyg. 1987;81:29–35. doi: 10.1016/0035-9203(87)90273-2. [DOI] [PubMed] [Google Scholar]
- 31.Chandiwana S K, Woolhouse M E J. Parasitology. 1991;103:363–370. doi: 10.1017/s0031182000059874. [DOI] [PubMed] [Google Scholar]
- 32.Brooks G F, Danow W W, Day J A. J Infect Dis. 1978;137:161–169. doi: 10.1093/infdis/137.2.161. [DOI] [PubMed] [Google Scholar]
- 33.Brunham R C, Plummer F A. Med Clin N Am. 1990;74:1339–1352. doi: 10.1016/s0025-7125(16)30484-9. [DOI] [PubMed] [Google Scholar]
- 34.Quinnell R J, Dye C. Med Vet Entomol. 1994;8:219–224. doi: 10.1111/j.1365-2915.1994.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 35.Hii, J. L. K., Smith, T., Paru, R., Mai, A., Dagoro, H., Lewis, D. & Alpers, M. P. (1997) J. Med. Entomol. in press.
- 36.Smith T, Charlwood J D, Takken W, Tanner M, Spiegelhalter D J. Acta Trop. 1995;59:1–18. doi: 10.1016/0001-706x(94)00082-c. [DOI] [PubMed] [Google Scholar]
- 37.Etard J-F, Audibert M, Dabo A. Am J Trop Med Hyg. 1995;52:549–558. doi: 10.4269/ajtmh.1995.52.549. [DOI] [PubMed] [Google Scholar]
- 38.Johnson A M, Wadsworth J, Wellings K, Field J. Sexual Attitudes and Lifestyles. Oxford: Blackwell Scientific; 1994. [Google Scholar]
- 39.Spira A, Bajos N. Sexual Behavior and AIDS. Aldershot, U.K.: Avebury; 1994. [Google Scholar]
- 40.Dietz K. Lecture Notes Biomath. 1979;39:264–277. [Google Scholar]
- 41.Becker N G, Dietz K. Math Biosci. 1995;127:207–219. doi: 10.1016/0025-5564(94)00055-5. [DOI] [PubMed] [Google Scholar]
- 42.Johnson N L, Kotz S, Balakrishnan N. Continuous Univariate Distributions. 2nd Ed. Vol. 1. New York: Wiley; 1994. [Google Scholar]
- 43.Lawrence R J. In: Lognormal Distributions: Theory and Applications. Crow E L, Shimizu K, editors. New York: Dekker; 1988. pp. 229–266. [Google Scholar]
- 44.Muirhead-Thompson R C. Br Med J. 1951;1:1114–1117. doi: 10.1136/bmj.1.4715.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas T C E. Br Med J. 1951;2:1402. doi: 10.1136/bmj.2.4744.1402-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Port G R, Boreham P F L, Bryan J H. Bull Entomol Res. 1980;70:133–144. [Google Scholar]
- 47.Gillies M T. In: Malaria: Principles and Practices of Malariology. Wernsdorfer W H, MacGregor I, editors. Edinburgh: Churchill Livingstone; 1988. pp. 453–485. [Google Scholar]
- 48.Burkot T R. Parasitol Today. 1988;4:156–162. doi: 10.1016/0169-4758(88)90151-2. [DOI] [PubMed] [Google Scholar]
- 49.Bundy D A P, Blumenthal U J. In: Parasitism and Host Behavior. Barnard C J, Behnke J M, editors. London: Taylor & Francis; 1990. pp. 264–289. [Google Scholar]
- 50.Shiff C J, Cmelik S H W, Ley H E, Kriel R L. J Parasitol. 1972;58:476–480. [PubMed] [Google Scholar]
- 51.Salafsky B, Wang Y S, Kevin M B, Hill H, Fusco A C. J Parasitol. 1984;70:584–591. [PubMed] [Google Scholar]
- 52.Abel L, Demenais F, Prata A, Souza A E, Dessein A. Am J Hum Genet. 1992;48:959–970. [PMC free article] [PubMed] [Google Scholar]
- 53.Padian N S, Shiboski S C. In: HIV Epidemiology: Models and Methods. Nicolosi A, editor. New York: Raven; 1994. pp. 87–98. [Google Scholar]
- 54.Laga, M., Diallo, M. O. & Buve, A. (1994) AIDS 8, Suppl., s119–s124.
- 55.McGregor I A. Clinics Trop Med Commun Dis. 1986;1:29–53. [Google Scholar]
- 56.Hagan P, Blumenthal U J, Dunn D, Simpson A J G, Wilkins H A. Nature (London) 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 57.Smith G, Basanez M G, Dietz K, Gemmell M A, Grenfell B T, et al. In: Ecology of Infectious Diseases in Natural Populations. Grenfell B T, Dobson A P, editors. Cambridge, U.K.: Cambridge Univ. Press; 1995. pp. 209–229. [Google Scholar]
- 58.Garnett G P, Anderson R M. Soc Trans Dis. 1993;20:181–191. doi: 10.1097/00007435-199307000-00001. [DOI] [PubMed] [Google Scholar]