Abstract
Rationale and Objectives
The rates of enrollment of volunteers for brain magnetic resonance imaging (MRI) studies vary by demographic and clinical characteristics. We use data from a large MRI study to identify factors associated with differential enrollment and to examine potential biases this may produce in study results.
Materials and Methods
Results from recruitment of 1,431 women into the MRI substudy of the Women's Health Initiative Memory Study (WHIMS-MRI) are described. A sensitivity analysis was conducted to estimate the degree of bias associated with missing data on estimates of risk factor relationships.
Results
Of 2,345 women contacted from an established cohort of women older than 70 years of age, 72% consented to undergo screening for WHIMS-MRI. Scanning was ultimately completed on 61%. Completion rates varied according to a range of sociodemographic, lifestyle, and clinical characteristics that may be related to study outcomes. Plausible levels of selective enrollment in magnetic resonance imaging studies may produce moderate biases (< ±20%) in characterizations of risk factor relationships. Adverse events, such as claustrophobia, occurred during 1.7% of the attempted scans and, in 0.8% of instances, led to lost data.
Conclusions
Enrollment of older women into brain imaging studies is feasible, although selection biases may limit how well study cohorts reflect more general populations.
Keywords: Informed consent, magnetic resonance imaging, clinical trial
Clinical trials and cohort studies increasingly are designed to include magnetic resonance imaging outcomes. Experiences with this new technology have provided successful protocols for standardized measurement, assessment, and safety (1-4). Brain magnetic resonance studies conducted in established cohorts have reported consent rates ranging from 71% to 90% and yields of completed imaging in 57%–72% of contacted individuals. However, these rates appear to vary markedly among subgroups based on important demographic and clinical factors (3,6-9). Thus, although it appears that recruitment into brain magnetic resonance imaging (MRI) studies is feasible, differential selection could have the potential to bias research findings.
We used data from the Women's Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS-MRI) to explore potential biases. We report rates of women who consented to undergo screening for the study, as a whole and by various subgroups, to identify potential barriers to recruiting participants. We also report the rates that MRI images were obtained, which are jointly affected by consent, eligibility criteria, and factors that interfere with imaging, such as claustrophobia. A sensitivity analysis was then performed to understand how selection biases inherent in enrollment may ultimately influence estimates of relationships among study outcomes. Additionally, we also describe the rate and nature of serious adverse events that were reported during the scanning procedure, some of which resulted in interruption of scanning and loss of data.
METHODS
Study Design
The WHIMS program consisted of parallel placebo-controlled randomized clinical trials of postmenopausal estrogen therapy with and without progestin therapy. The WHIMS study designs, eligibility criteria, and recruitment procedures have been described previously (10). Participants were recruited from 38 of the 40 centers participating in the Women's Health Initiative (WHI) Estrogen-Alone and Estrogen Plus Progestin clinical trials (11,12). To be eligible, women were between 65 and 79 years of age and free of dementia according to standardized study protocols involving psychometric testing, clinical evaluation, and central adjudication (10). Written informed consent was obtained; the National Institutes of Health and Institutional Review Boards for all participating institutions approved the protocols and consent forms.
Following discovery of an unfavorable risk-to-benefit ratio of its noncognitive end points, administration of study drug in the WHI Estrogen Plus Progestin trial was stopped early (July 2002) (13). The WHI Estrogen-Alone trial was also stopped early for harm from an adverse risk profile with respect to stroke and a lack of benefit for cardiovascular disease (February 2004) (14). These decisions ended the two WHIMS trials, with average follow-up of 4.0 and 5.2 years, respectively (15-18). Women continued to be seen annually after the conclusion of the WHIMS trials, with the objective to understand whether the adverse consequences of hormone therapy on cognitive outcomes demonstrated by these two trials continued after cessation of study treatment.
WHIMS-MRI was designed to contrast neuroradiologic outcomes among women older than age 70 who had been assigned to active versus placebo therapy during the WHIMS trials. It was conducted in 14 of the original 38 WHIMS clinical centers, selected based on interest, experience in conducting multicenter MRI studies, participation in an ancillary study to WHIMS (the Women's Health Initiative Study of Cognitive Aging), and availability of necessary equipment. All participants in these centers were to be solicited for potential screening to join WHIMS-MRI, regardless of their prior adherence to the WHI study protocol, on-trial use of study medications, on-study measures of cognitive function, or their willingness to continue posttrial follow-up. This contact was made by a variety of means, including telephone, mailings, and face-to-face meetings during scheduled study visits. The purposes of these contacts were to inform potential participants of the WHIMS-MRI study and to obtain informed consent for screening. A model consent form (https://www.phsapps.wfubmc.edu/whims/index.cfm) was circulated and clinical sites tailored this according to local practices and institutional review board guidance. Trained WHI clinic staff described the purpose of obtaining MRI images, steps taken to protect the participant's confidentiality and privacy in terms of how images and records would be managed and how research would be presented, a description of the MRI procedure, and a discussion of how participant safety would be maintained. The participant was allowed to read the consent form in private. Women who provided this consent underwent screening to determine if they were acceptable candidates for MRI. Exclusion criteria included the presence of pacemakers, defibrillators, neurostimulators, prohibited medical implants, and foreign bodies (eg, bullets, shrapnel, metal slivers) that would pose a hazard to the participant during the MRI procedure. Other exclusion criteria included shortness of breath or inability to lie flat and conditions that can be exacerbated by stress (eg, anxiety/panic disorders, claustrophobia, uncontrolled high blood pressure, seizure disorders) severe enough to preclude an MRI.
Baseline Data Collection, Variable Definitions, and Data Analysis Plans
We assembled a range of factors to serve as markers of health, culture, demography, and lifestyle. We wished to explore whether there was evidence that screening consent rates differed according to these attributes. Thus, although we include diabetes among the factors examined, we view it more generally as a marker of health rather than a specific medical condition. We used the same factors to examine associations with our success in ultimately obtaining an image. In some cases, these factors were associated with eligibility criteria (eg, heart disease was related to use of a pacemaker) and thus there is some degree of causality. Factors were collected by standardized questionnaires, either self- or staff-administered, or by clinical measures obtained from trained and certified technicians, as discussed in greater detail elsewhere (10,11). These included characteristics at the time of enrollment (age, education, ethnicity, and history of stroke or transient ischemic attack or coronary heart disease) and prior treatment assignment. Self-reported medical conditions were used because conditions known to the participant would be more likely to influence behavior than events adjudicated through the WHI process. Annual Modified Mini-Mental state (3MS) exams were used to measure global cognition (19). Women were classified according to whether they had experienced declines of two standard deviations (eight units) on the 3MS during follow-up. The average time between the start of WHI data collection and the start of WHIMS-MRI enrollment was 8 years.
A subset of WHIMS-MRI women were enrolled in the Women's Health Initiative Study of Cognitive Aging, (18) which included an annual assessment of depression using the Geriatric Depression Scale-Short Form (GDS-SF) to measure nonsomatic features of depressed mood (20). We grouped these women according to whether there was evidence of depressive symptoms measured within the past year (GDS-SF score >5) or not.
Univariable and multivariable associations between these participant characteristics and the rates of consent and of successful imaging were examined with logistic regression. Backward stepwise logistic regression was used to identify important sets of factors that were mutually associated with agreement. Associations were summarized with odds ratios. Model fit was assessed with influence plots (21). Because many factors were interrelated, these approaches may not have characterized the full expression of all important multivariate relationships. Sensitivity analyses were conducted to examine the potential breadth of biases in study outcomes that derive from selection biases we describe. A P value < .05 was considered significant.
RESULTS
Recruitment for the WHIMS-MRI study began in January 2005 and was completed by April 2006 (Fig 1). Over this period, 2,345 WHIMS participants were approached for enrollment in WHIMS-MRI and 1,688 (72%) consented to undergo screening for the study. Of those screened, 1,431 (61%) ultimately provided MRI scans, which was 99% of the study goal of 1,450.
Figure 1.
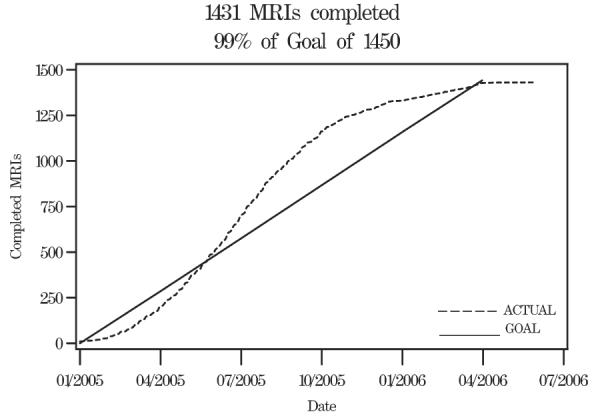
Enrollment Women's Health Initiative Memory Study Magnetic Resonance Imaging Study over time.
Rates of Consent for WHIMS-MRI Enrollment
Table 1 presents rates that consent was provided among subgroups of women defined by markers of socio-demographic, lifestyle, and clinical factors. Rate of consent varied significantly by several factors that we examined. Consent was more frequent among women who were younger than 82 years of age, who reported more formal education, and who did not have a history of heart disease. Consent was also more frequent among women with higher levels of global cognitive function, as measured by 3MS examinations, and for women who experienced less cognitive decline during the WHIMS trial. Consent did not appear to be strongly related to ethnicity, smoking, history of cerebrovascular disease, diabetes, alcohol intake, body mass index, or WHI treatment assignment.
Table 1.
Characteristics of Participants Enrolled in WHIMS Grouped by Whether They Provided at Least Some Level of Consent to Participate in the WHIMS-MRI Study
| Consent for Screening |
MRI Obtained |
||||
|---|---|---|---|---|---|
| Characteristic | n | Percent Providing Consent |
Chi-square P Value |
Percent MRI Obtained |
Chi-square P Value |
| Overall | 2,345 | 72% | 61% | ||
| Age | |||||
| 70–75 | 766 | 74% | 64% | ||
| 76–81 | 1,144 | 73% | <.001 | 63% | <.001 |
| 82–88 | 435 | 64% | 50% | ||
| Education, years | |||||
| <12 (high school) | 136 | 63% | 50% | ||
| High school | 574 | 68% | .001 | 57% | <.001 |
| Some college | 1,149 | 72% | 61% | ||
| College diploma | 486 | 77% | 67% | ||
| Race/ethnicity | |||||
| African-American | 105 | 70% | 62% | ||
| American Indian | 6 | 67% | 67% | ||
| Asian | 31 | 81% | .594 | 74% | .447 |
| White | 2,141 | 72% | 61% | ||
| Hispanic | 31 | 84% | 68% | ||
| Other/multiple | 23 | 70% | 48% | ||
| Smoking | |||||
| Never | 1,324 | 73% | 62% | ||
| Former | 877 | 71% | .123 | 61% | .025 |
| Current | 120 | 64% | 49% | ||
| History of cerebrovascular disease | |||||
| No | 2,280 | 72% | .064 | 61% | .364 |
| Yes | 65 | 62% | 55% | ||
| History of heart disease | |||||
| No | 2,103 | 73% | .008 | 62% | <.001 |
| Yes | 242 | 64% | 48% | ||
| History of diabetes | |||||
| No | 2,193 | 72% | .062 | 61% | .013 |
| Yes | 152 | 65% | 51% | ||
| Alcohol intake | |||||
| None | 1,078 | 71% | 58% | ||
| <1/day | 421 | 73% | .684 | 65% | .084 |
| 2–3/day | 422 | 73% | 64% | ||
| 4+/day | 419 | 73% | 60% | ||
| Physical activity (METS/wk) | |||||
| <3.5 | 772 | 71% | 60% | ||
| 3.6–12.5 | 801 | 70% | .183 | 59% | .081 |
| 12.6+ | 772 | 74% | 64% | ||
| Body mass index (kg/m2) | |||||
| <20.0 | 62 | 68% | 58% | ||
| 20.0–24.9 | 643 | 71% | 60% | ||
| 25.0–29.9 | 861 | 72% | .86 | 62% | .612 |
| 30.0–34.9 | 493 | 73% | 62% | ||
| 35.0+ | 275 | 73% | 57% | ||
| Drop in 3MS score from baseline | |||||
| <8 (<2 SD) | 2,138 | 73% | .005 | 62% | .003 |
| 8+ (≥2 SD) | 207 | 63% | 51% | ||
| Baseline 3MS score | |||||
| 95–100 | 1,720 | 73% | 63% | ||
| 90–94 | 451 | 69% | .025 | 57% | .005 |
| <90 | 157 | 65% | 51% | ||
| Former treatment assignment | |||||
| E+P | 719 | 72% | 62% | ||
| E+P placebo | 725 | 71% | .604 | 63% | .334 |
| E-alone | 447 | 74% | 58% | ||
| E-alone placebo | 454 | 70% | 59% | ||
METS: metabolic equivalents; SD: standard deviation; 3MS: Modified Mini-Mental state; E = estrogen; P = progestin.
Backwards stepwise logistic regression was used to select a group of these factors that appeared to have the strongest independent associations with rates of consent. These included age, education, history of heart disease, and on-trial cognitive decline (Table 2). From the multi-variable model, the odds of consent were about 40% greater among women ages 70–81 than those ages 82–88. The rates were similar for women with and without a history of heart disease. The odds of consent increased steadily with education level, such that approximately 40% more college graduates consented than did those with a high school (or less education). Consent rates were approximately 40% higher among women without marked on-trial cognitive decline compared with those with cognitive decline.
Table 2.
Factors Selected by Backward Stepwise Logistic Regression as being Strongly and Independently Associated with Agreement to Participate in Screening
| Characteristic | Odds Ratio |
95% Confidence Interval |
P Value |
|---|---|---|---|
| Age | |||
| 70–75 | 1.48 | (1.14–1.92) | |
| 76–81 | 1.47 | (1.15–1.87) | .004 |
| 82–88 | 1.00 | — | |
| Education, years | |||
| <12 (high school) | 0.51 | (0.34–0.78) | |
| High school | 0.62 | (0.47–0.82) | .001 |
| Some college | 0.78 | (0.61–1.01) | |
| College diploma | 1.00 | — | |
| Heart disease | |||
| No | 1.38 | (1.04–1.83) | .028 |
| Yes | 1.00 | — | |
| Drop in 3MS score from baseline | |||
| <8 (<2 SD) | 1.38 | (1.02–1.88) | .039 |
| 8+ (≥2 SD) | 1.00 | — |
SD: standard deviation; 3MS: Modified Mini-Mental state.
Rates of Obtaining MRIs
Of those who consented, 5.35% were excluded because of contraindications to MRI imaging, with the presence of a pacemaker (2.38%) being the most common reason for exclusion (Table 3).
Table 3.
Contraindications to Magnetic Resonance Imaging: Frequency and Prevalence Rate and Responding Participants
| Report Detailed and/or Summarized Report Contraindication |
Frequency | Percent |
|---|---|---|
| Pacemakers | 40 | 2.38 |
| Other implantable devices* | 4 | 0.24 |
| Intracranial aneurysm clip | 4 | 0.24 |
| Cochlear implant | 3 | 0.18 |
| Harrington rods | 2 | 0.12 |
| McGee stapes implant | 2 | 0.12 |
| Defibrillator | 1 | 0.06 |
| Other† | 9 | 0.54 |
| Not specified | 26 | 1.55 |
| Total | 91 | 5.41 |
Defibrillator, transcutaneous electrical nerve stimulation (TENS) unit, and pump (n = 2).
Two metal plates, three stents, two implants, one rod, one prosthetic device.
After excluding those with a contraindication, the individual factors associated with variable rates in obtaining MRI scans include all those that were associated with consent (age, education, history of heart disease, baseline cognition, and on-trial cognitive decline), as listed in Table 1. In addition, current smoking and diabetes were found to be significantly associated with reduced odds of obtaining an image.
Backwards stepwise logistic regression selected six factors as being independently associated with variable rates of obtaining scans (Table 4). The odds of completing scans were approximately 60% greater among women in the lowest two tertiles of age (e.g. ≤81 years), compared with the oldest tertile. Less education, heart disease, diabetes, current smoking, and an on-trial marked decrease of 3MS were each independently associated with lower odds of obtaining scans.
Table 4.
Factors Selected by Backward Stepwise Logistic Regression as being Strongly and Independently Associated with Ultimately Obtaining a Study Magnetic Resonance Image
| Characteristic | Odds Ratio |
95% Confidence Interval |
P Value |
|---|---|---|---|
| Age | |||
| 70–75 | 1.74 | (1.36–2.23) | |
| 76–81 | 1.66 | (1.32–2.10) | <.001 |
| 82–88 | 1.00 | — | |
| Education, years | |||
| <12 (high school) | 0.51 | (0.34–0.76) | |
| High school | 0.61 | (0.47–0.79) | <.001 |
| Some college | 0.75 | (0.60–0.95) | |
| College diploma | 1.00 | — | |
| Heart disease | |||
| No | 1.58 | (1.20–2.08) | .001 |
| Yes | 1.00 | — | |
| Diabetes | |||
| No | 1.50 | (1.07–2.11) | .020 |
| Yes | 1.00 | ||
| Smoking | |||
| Never | 1.90 | (1.29–2.78) | |
| Past | 1.78 | (1.20–2.62) | .005 |
| Current | 1.00 | — | |
| Drop in 3MS score from baseline | |||
| <8 (<2 SD) | 1.36 | (1.01–1.83) | .423 |
| 8+ (≥2 SD) | 1.00 | — |
Associations with Depression
GDS-SF scores within the last year were available for 1,646 women who were approached about enrolling in WHIMS-MRI; 1,272 provided consent for screening. Consent for screening was lower among women with depressive symptoms (GDS-SF >5) than those without: 67% versus 78% (P = .005). Among women who consented to screening, MRIs were less often obtained among these women, as well: 55% versus 68% (P = .006).
Rates of Adverse Events
Adverse events were defined to be conditions that arose during the MRI study visit or during the scanning procedure that elicited a participant complaint or injury. These may have resulted in interruption of the scanning procedure and loss of data. Overall, 25 adverse events were encountered: 12 of claustrophobia (0.8%), 6 of difficulty breathing (0.4%), 2 each of pain while lying flat, dementia-unable to cooperate, and postnasal drip/excessive swallowing (each 0.1%), and 1 recorded as fear of scanner. These adverse events results in 12 losses of data: 6 from claustrophobia, 2 from difficulty of breathing, and 1 from each of the other reasons. No serious adverse events (ie, those that required medical treatment) were noted during this study.
DISCUSSION
The failure to obtain potential study outcomes from planned individuals, whether because of nonconsent or measurement difficulties, affects costs and introduces biases. For studies of magnetic resonance imaging, because of its intrusive nature and complexity, nonconsent and missing data may be especially important issues. It may be expected that selection biases in these studies may differ from those in other research protocols.
Rates of Consent for Screening and Imaging Yields
Table 5 summarizes the rates that participants in large cohort studies have agreed to be screened for brain MRI studies and the rates that images were ultimately collected. Consent rates varied from 71% to 90% and yields varied from 57% to 72%. Although the characteristics of cohorts and consenting protocols varied among studies, the collective experience indicates that large MRI studies are quite feasible to plan and conduct using established cohorts. The rates observed in WHIMS-MRI, 72% consenting to undergo screening and 61% ultimately being scanned, align with the reports from other studies.
Table 5.
Characteristics Reported to be Associated with Lower Rates of Brain MRI Collection Across the Cohort Targeted for Enrollment
| Study | Characteristics of Targeted Cohort |
Consent Rate |
Image Collection Rate |
Characteristics Associated with Lower Rates |
Factors Not Associated with MRI Scan Rates |
|---|---|---|---|---|---|
| Cardiovascular Health Study (6) | n = 5,888 men and women aged ≥69 years | 88% | 72% | Older age, less education, lower family income, history of smoking, cardiovascular disease, cerebrovascular disease, diabetes, hypertension | Ethnicity, gender |
| Atherosclerosis Risk in Communities (3) | n = 4,474 men and women aged 55–74 years | 73% | 67% | Younger age, less education, greater body weight, lower high density lipoprotein cholesterol | Blood pressure, glucose, triglycerides |
| Rotterdam Scan Study (7) | n = 1,904 men and women aged 60–90 years | 90% | 57% | Older age, less education, lower cognitive function | Cholesterol, body weight, blood pressure |
| Honolulu-Asia Aging Study (8) | n = 845 men with mean age 82 years | 71% | 68% | Older age, cerebrovascular disease, dementia, ApoE4 genotype | |
| Framingham Scan Study (9) | n = 4,100 men and women aged 32–103 years | 80%* | 71%* | Older age, higher blood pressure, hypertension, greater body weight, diabetes, higher glucose, cardiovascular disease, cerebrovascular disease | Gender, cholesterol, high density lipoprotein-cholesterol, smoking, alcohol intake |
| WHIMS-MRI | n = 2,345 women aged 70–88 years | 72% | 61% | Older age, lower education, history of smoking, cardiovascular disease, diabetes, cognitive function, cognitive decline, depression | Ethnicity, history of cerebrovascular disease, alcohol intake, physical activity, body weight |
MRI: Magnetic resonance imaging.
Projected.
Factors Associated with Missing Data
WHIMS-MRI and other studies have identified many factors that appear to be associated with differential rates of obtaining images (Table 6). Consistently, these include demographic markers (age, education, income), markers of chronic disease (cardiovascular disease, diabetes), and cognitive status. Less consistently, other factors related to risk and health (body weight, cerebrovascular disease, hypertension) may be associated with missing data. WHIMS-MRI adds to this list cognitive decline and depression. In total, these studies suggest that the factors associated with missing data in MRI studies are varied and may be complex. Because of this, and because many of the related factors are expected to have strong associations with MRI outcomes, results from MRI studies are likely to have some bias, as discussed in the following section.
Table 6.
Expected Relative Bias Associated with Missing MRI Scans When Rates of Missing Data Depend on a Confounding Factor. The Prevalence of the Risk Factor, MRI Outcome, and Confounding Factor were Each Assumed to be 50%
| Risk Factor |
Expected Relative Bias in Estimated Odds Ratio Relating Risk Factor to Outcome |
|||
|---|---|---|---|---|
| Absent |
Present |
|||
| Confounding Factor |
Confounding Factor |
|||
| Absent | Present | Absent | Present | |
| 20% | 20% | 20% | 20% | 0% |
| 40% | −9% | |||
| 40% | 20% | 9% | ||
| 40% | −1% | |||
| 40% | 20% | 20% | 9% | |
| 40% | −1% | |||
| 40% | 20% | 19% | ||
| 40% | 8% | |||
| 40% | 20% | 20% | 20% | −9% |
| 40% | −17% | |||
| 40% | 20% | 0% | ||
| 40% | −9% | |||
| 40% | 20% | 20% | −1% | |
| 40% | −10% | |||
| 40% | 20% | 9% | ||
| 40% | 0% | |||
In general, many of relationships suggest that brain MRI studies are likely to enroll cohorts that are of relatively lower risk for neuropathology than the targeted populations, and thus may underestimate the prevalence of disease and have diminished power for detecting risk factor relationships.
Recruitment programs for MRI studies should be sensitive to potential barriers associated with older age, cognitive declines, and chronic diseases; greater commitment of resources to recruitment may be required than for other populations. It is not surprising that these factors influence enrollment in MRI studies, even among women who previously enrolled in less “invasive” studies. Anxiety about MRI scanning has been reported to be related to fear of enclosed places, pain, the unknown, and apprehension about potential findings (23). It has been reported that as many as 20% of patients may experience claustrophobia severe enough to preclude the completion of attempted MRI, (24) and many of these would be unlikely to agree to screening.
We find it of interest that among these studies, enrollment rates appeared to be fairly uniform across ethnic groups. Historically, many barriers have been described in recruiting individuals from ethnic minorities into clinical research (25-28).
Potential Impact of Nonconsent on Findings
The goal of the WHIMS-MRI is to identify relationships that hormone therapy and cognitive risk factors have with silent infarcts and other neuroradiologic outcomes. Yields across the four arms of the WHIMS trials were fairly similar, which provides some reassurance that intention-to-treat comparisons may provide balanced assessments of relative differences. Secondary goals of the WHIMS-MRI, however, include examining relationships that suspected risk factors have with MRI outcomes. For these, selection biases may complicate analyses. Consider the table in Fig 2. MRI data are not available for the lower panel; however, within the context of an established cohort, data on the risk factor would be expected to be available.
Figure 2.
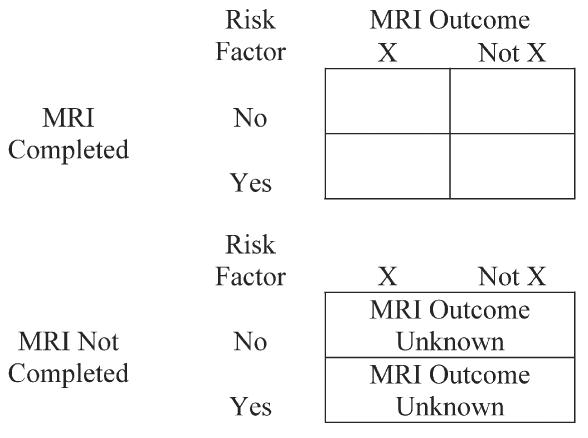
Observed and unobserved data.
If MRI data are missing completely at random (ie, the probability of missing data is unrelated to MRI outcomes), the lower panel of Fig 2 may be ignored in analyses. WHIMS-MRI data suggest that this may not be the case because “missingness” is related to many factors that have associations with neuropathology. If missingness is indeed related to the risk factor or MRI outcome, ignoring the lower panel may result in bias. In such cases, it may be possible to develop models that statistically account for these relationships. If successful, this approach yields data that are “missing at random,” for which many analytical approaches are appropriate (29). If data are missing at random and if data collection rates are fairly high, estimation bias can be controlled to be relatively modest through the use of statistical methods (30). If models cannot be found to account for relationships, missing data are not ignorable. In such cases, expected bias is related to the rate of consent and the strength of associations causing the “non-ignorability” (31).
Sensitivity analyses may be used to examine the potential breadth of biases. As an example of these, we consider a situation in which rates of missing data are conditionally independent of whether an MRI scan is performed, but vary depending on an intermediate or confounding variable and a risk factor that are both linked to MRI outcomes. An example may be cognitive decline, which may be associated both with MRI risk factors and MRI outcomes. We simulated data (n = 100,000) in which the odds ratio relating a risk factor to an MRI outcome was 4.0 both among individuals with and without the confounding factors. We examined situations in which the confounding factor had relationships with the risk factor and MRI outcome described by odds ratios of 2.0 and 3.0, respectively, and in which the overall prevalence of the risk factor, MRI outcome, and confounding factor were all 50%. (The range of associations associated with odds ratios of 2.0 to 4.0 may be described as moderate to large) (32). We then randomly withdrew individuals from the cohort at rates that varied depending on the confounding factor (as if their MRI scans were not completed). We considered the impact of analyses on the remaining cohort (relative to an analysis based on the entire cohort), when rates of missing MRI scans ranged from 20% to 40% for individuals with or without the confounding factor and according to the status of the risk factor, as displayed in Table 6. The range of assumptions we explored produced non-ignorable missing data in some cases and was sufficient to introduce biases ranging from −17% to 19% in estimates of odds ratios relating risk factors to outcomes. Thus, analyses limited to women providing MRI scans may both under- and overestimate the strength of relationships between risk factors and outcomes in the target population. In our example, the magnitude of this bias may be as large as 19%.
Adverse Events
The low frequency of adverse events may be associated with the WHIMS-MRI screening procedures and the use of special guidelines for participants with a history of claustrophobia. Even when rigorous exclusion criteria are used, however, some adverse events must be expected. Therefore, in addition to careful documentation and categorization of adverse study events, safety protocols should be in place for managing these as they occur.
Limitations
Our analyses are limited to women who have met the criteria for participation in the WHI and WHIMS clinical trials and who had previously agreed to participate in these trials. As documented, these women may not be representative of the general US population, tending to be healthier, better educated, and having already committed to participating in rigorous clinical trials (12,33). Enrollment in WHIMS-MRI was from an existing cohort with a history of volunteerism for medical research. The rates we report likely exceed those associated with de novo recruitment, and other selection biases may occur (5,22).
CONCLUSIONS
Enrollment of older individuals from established cohorts into brain MRI studies appears to be very feasible. Selection factors differ from those for other biomedical studies and may, if not addressed, introduce moderate levels of bias in findings.
APPENDIX A
Roster of WHIMS-MRI Sites and Staff
Clinical Centers:—Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller, Mimi Goodwin, Richard DeNise, Michael Lipton, James Hannigan; Medical College of Wisconsin, Milwaukee: Jane Morley Kotchen, Diana Kerwin, John Ulmer, Steve Censky; Stanford Center for Research in Disease Prevention, Stanford University, Stanford, CA: Marcia L. Stefanick, Sue Swope, Anne Marie Sawyer-Glover; The Ohio State University, Columbus: Rebecca Jackson, Rose Hallarn, Bonnie Kennedy; University of California at Davis, Sacramento: John Robbins, Sophia Zaragoza, Cameron Carter, John Ryan; University of California at Los Angeles: Lauren Nathan, Barbara Voigt, Pablo Villablanca, Glen Nyborg; University of Florida, Gainesville/Jacksonville: Marian Limacher, Sheila Anderson, Mary Ellen Toombs, Jeffrey Bennett, Kevin Jones, Sandy Brum, Shane Chatfield; University of Iowa, Davenport: Jennifer Robinson, Candy Wilson, Kevin Koch, Suzette Hart; University of Massachusetts, Worcester: Judith K.Ockene, Linda Churchill, Sharon Jackson, Douglas Fellows, Anthony Serio; University of Minnesota, Minneapolis: Karen Margolis, Cindy Bjerk, Chip Truwitt, Margaret Peitso; University of Nevada, Reno: Robert Brunner, Ross Golding, Leslie Pansky; University of North Carolina, Chapel Hill: Carol Murphy, Maggie Morgan, Mauricio Castillo, Thomas Beckman; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller, Pat McHugh, Carolyn Meltzer, Denise Davis.
Clinical Coordinating Center:—Wake Forest University Health Sciences, Winston-Salem, NC: Sally Shu-maker, Mark Espeland, Steve Rapp, Claudine Legault, Laura Coker, Maggie Dailey, Josh Tan, Debbie Felton, LeeAnn Andrews, Julia Robertson, Patricia Hogan, Sarah Jaramillo, Jeff Williamson.
WHIMS-MRI Quality Control Center:—University of Pennsylvania, Philadelphia, PA: Nick Bryan, Christos Davatzikos, Lisa Desiderio.
U.S. National Institutes of Health:—National Institute on Aging, Baltimore, MD: Susan Resnick.
U.S. National Institutes of Health: National Heart, Lung, and Blood Institute, Bethesda, MD:—Jacques Rossouw, Linda Pottern; National Institute on Aging: Neil Buckholtz, Susan Molchan.
Footnotes
The Women's Health Initiative program is funded by the National Heart, Lung, and Blood Institute, US Department of Health and Human Services. The Women's Health Initiative Magnetic Resonance Imaging Study was funded by contract N01-WH-44221, from the National Heart, Lung, and Blood Institute. Also included in the manuscript are data collected by the Women's Health Initiative Study of Cognitive Aging (supported by the Department of Health and Human Services and the National Institute on Aging, NO1-AG-1-2106, National Institutes of Health), and the Women's Health Initiative Memory Study (supported by Wyeth Pharmaceuticals Inc. and Wake Forest University Health Sciences).
REFERENCES
- 1.Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain—The Cardiovascular Health Study. Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 2.Manolio TA, Kronmal RA, Burke GL, et al. Magnetic-resonance abnormalities and cardiovascular disease in older adults: The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 3.Bryan RN, Cai J, Burke G, et al. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: the Atherosclerosis Risk in Communities Study. Am J Neuroradiol. 1999;20:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 4.Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions and hypertension, its treatment, and its control. The ARIC study. Stroke. 1996;27:2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 5.Illes J, Kirschen MP, Edwards E, et al. Incidental findings in brain research. Science. 2006;311:783–784. doi: 10.1126/science.1124665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 7.De Groot JC, de Leeuw F-E, Oudkerk M, et al. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57:1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 8.Havlik RJ, Foley DJ, Sayer B, et al. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging Study. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
- 9.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Shumaker SA, Reboussin BA, Espeland MA, et al. The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled Clin Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 11.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Controlled Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 12.Hays J, Hunt JR, Hubbell FA, et al. the Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 13.Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 14.The Women's Health Initiative Steering Committee Effects of conjugated equine estrogens in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 15.Shumaker S, Legault C, Rapp S, et al. The effects of estrogen plus progestin on the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study. JAMA. 2003;289:2651–2562. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 16.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;20:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 17.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogen alone, pooled hormone therapy, and incidence of probable dementia and mild cognitive impairment in postmenopausal women: results from the Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 18.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 19.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 20.Resnick SM, Coker LH, Maki PM, et al. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials. 2004;1:440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 21.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4:173–178. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- 22.SAS Institute Inc . SAS/STAT 9.1 user's guide. SAS Institute Inc; Cary, NC: 2004. [Google Scholar]
- 23.Katz RC, Wilson L, Frazer N. Anxiety and its determinants in patients undergoing magnetic resonance imaging. J Behav Ther Exp Psychiatry. 1994;25:131–134. doi: 10.1016/0005-7916(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 24.Lukins R, Davan IG, Drummond PD. A cognitive behavioural approach to preventing anxiety during magnetic resonance imaging. J Behav Ther Exp Psychiatry. 1997;28:97–104. doi: 10.1016/s0005-7916(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 25.Lovato LC, Hill K, Hertert S, et al. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Controlled Clin Trials. 1997;18:328–357. doi: 10.1016/s0197-2456(96)00236-x. [DOI] [PubMed] [Google Scholar]
- 26.Stoddart ML, Jarvis B, Blake B, et al. Recruitment of American Indians in epidemiologic research: the Strong Heart Study. Am Indian Alask Native Ment Health Res. 2000;9:20–37. doi: 10.5820/aian.0903.2000.20. [DOI] [PubMed] [Google Scholar]
- 27.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248–256. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- 28.Wyatt SB, Diekelmann N, Henderson F, et al. A community-driven model of research participation: the Jackson Heart Study Participant Recruitment and Retention Study. Ethn Dis. 2003;13:438–455. [PubMed] [Google Scholar]
- 29.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. John Wiley and Sons; New York: 2002. [Google Scholar]
- 30.Fitzmaurice GM, Lipsitz SR, Molenberghs G, et al. Bias in estimating association parameters for longitudinal binary responses with drop-outs. Biometrics. 2001;57:15–2131. doi: 10.1111/j.0006-341x.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhao LP, Lipsitz S, Lew D. Regression analysis with missing covariate data using estimating equations. Biometrics. 1996;52:1165–1182. [PubMed] [Google Scholar]
- 32.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 33.Stefanick ML, Cochrane BB, Hsia J, et al. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:1647–1657. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]


