Abstract
Background
The long-term use of benzodiazepines is highly prevalent in developed societies and is not devoid of risks. Withdrawing patients from these drugs is often difficult. Tapering off benzodiazepines has been shown to be a good strategy for discontinuing their long-term use.
Aim
To establish the efficacy of an intervention programme for reducing the chronic use of benzodiazepines.
Design of study
Randomised, two-arm, parallel, non-blinded controlled trial.
Setting
Three urban healthcare centres covering a population of 50 000 inhabitants (Mallorca, Spain).
Method
Adult patients (n = 139) taking benzodiazepines daily for more than a year and visited by their family physician were randomised into an intervention group (n = 73) that received standardised advice and a tapering off schedule with biweekly follow-up visits, or into a control group (n = 66), that was managed following routine clinical practice. Both were followed for a year.
Results
Patients achieved withdrawal or reduced their dose by at least 50% after 6 and 12 months. Abstinence and withdrawal symptoms were also measured. Both groups were homogeneous for personal, clinical and psychological characteristics and for benzodiazepine use. Only two patients from each group were lost to follow-up. After 12 months, 33 (45.2%) patients in the intervention group and six (9.1%) in the control group had discontinued benzodiazepine use; relative risk = 4.97 (95% confidence interval [CI] = 2.2 to 11.1), absolute risk reduction = 0.36 (95% CI = 0.22 to 0.50). For every three interventions, one patient achieved withdrawal. Sixteen (21.9%) subjects from the intervention group and 11 (16.7%) controls reduced their initial dose by more than 50%.
Conclusion
Standardised advice given by the family physician, together with a tapering off schedule, is effective for withdrawing patients from long-term benzodiazepine use and is feasible in primary care.
Keywords: benzodiazepines, primary health care, randomised trial, withdrawal symptoms
INTRODUCTION
Benzodiazepines are one of the most commonly prescribed drugs in Spain and other Western Europe countries.1 They are used for anxiety disorders, insomnia, alcohol withdrawal, as adjuvant therapy in schizophrenia and depression, and as muscle relaxants. Their short-term benefits are not in doubt, but their long-term use may cause daytime somnolence, blunted reflexes, memory impairment and an increased risk of falls and hip fractures in the elderly,2 as well as dependence. Most guidelines recommend restricting their use to no more than 3–4 weeks,3,4 but many patients take them for years,5,6 as addiction hinders their withdrawal, even when used at low doses. Attempts of withdrawing benzodiazepines may cause anticipatory anxiety, rebound insomnia, irritability and other symptoms that perpetuate a spiral of dependency and abuse.7 Spontaneous abandonment has been estimated to be only 6%.8
Since many benzodiazepine treatments are started by family physicians,6 they should also play a leading role in limiting their duration and in their withdrawal. Interventions for promoting benzodiazepine withdrawal have been assessed in several studies.9–14 After a gradual reduction of dose, rates of successful withdrawal, maintained at 12 months, ranged from 24 to 51%.11,12 The value of adding a cognitive-behavioural therapy is uncertain: it improved outcomes in two studies,12,13 but not in the third.11
Our goal was to appraise the efficacy of a structured intervention in primary care, consisting of a brief standardised advice and a stepwise dose reduction, for discontinuing benzodiazepine use.
METHOD
A randomised, non-blinded clinical trial was performed in the island of Mallorca (702 122 inhabitants) from 2001 to 2004. The trial was carried out by family physicians working in three public primary care centres set in urban areas.
Participants
Among all patients visiting the collaborating physicians, those aged 14–75 years and who were taking benzodiazepines at least five times a week for over a year, were asked for their consent. Recruitment was facilitated by the fact that, in Spain, pharmacies cannot dispense benzodiazepines without a prescription, so that patients taking these drugs must visit their doctor regularly.
We excluded patients diagnosed with severe depression or anxiety, psychotic or markedly symptomatic personality disorders, illegal drug or severe alcohol intake, as well as those being followed by a psychiatrist. We also excluded patients with an increase in anxiety or insomnia at the time of recruitment, inpatients and those with cognitive impairment or advanced disease.
Recruitment and randomisation
Physicians identified long-term benzodiazepine users in their practices and asked for their written consent. Thirteen family physicians from three primary care centres took part in the trial. The average population covered by each physician was 2000 subjects. Since chronic benzodiazepine use is estimated at about 2% of the population,1 approximately 40 cases were expected per physician. It was calculated that a sample size of 142 subjects would be enough to test the hypothesis, with 80% power and an αof 0.05. About 6% of chronic benzodiazepine users, spontaneously stop taking these drugs without any specific intervention.8 The prediction of withdrawals among the control group was increased to 10% (p1 = 10%), to allow for the fact that the patients were volunteers, and therefore more likely to be motivated to give up. The recruitment phase lasted 3 months. Patients were block randomised into two groups, with one block per physician. Each of them was given a set of envelopes, half of which enclosed a paper marked ‘C’ for the control group, and the other half ‘I’ for the intervention group. When a patient granted his or her consent, an envelope was opened. The patient was then followed for a year. An outline of the study is given in Figure 1.
Figure 1.
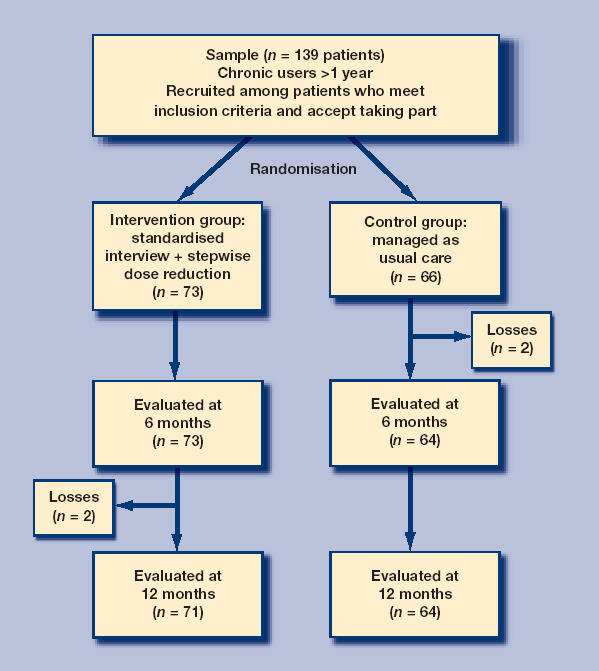
Outline of the study.
How this fits in
The long-term use of benzodiazepines is highly prevalent in developed societies and is not devoid of risks. Withdrawing patients from these drugs is often difficult. Tapering off benzodiazepines has been shown to be a good strategy for discontinuing their long-term use. Adding a standardised interview to the tapering-off schedule is five times more likely to achieve withdrawal than usual practice. One out of every three interventions is successful, with the benefit persisting at least 12 months. This approach is feasible in primary care.
Measurements and intervention
The type of benzodiazepine, daily dose and duration of use were recorded. The main efficacy variable was benzodiazepine use at 12 months, although data was also taken at 6 months. End-points were: success, no use or no more than once every 15 days; reduced, at least a 50% reduction in initial dose; failure, no change or a decrease smaller than 50%.
On visit 1, personal, clinical and drug use variables were taken. Level of anxiety and depression were measured through the Spanish versions of the STAI-E and STAI-R questionnaires15,16 and the Hamilton Depression Scale.17 The intervention consisted of an interview with a standardised message that had been developed previously through a qualitative study on four focal groups, each one with eight to 12 chronic consumers of benzodiazepines (one group of men and three of women, grouped per age). The content of the message is given in Box 1. Patients in the intervention group underwent a gradual reduction of benzodiazepine dose, with control visits every 15 days. The dose was reduced between 10 and 25% of the initial dose fortnightly.
Content of the standardised message given on the first and the follow up visits.
First visit:
-
▸
What are benzodiazepines and what are they used for?
-
▸
Treatment of symptoms versus treatment of their cause.
-
▸
Untoward effects of benzodiazepines (memory impairment, decreased reflexes, risk of falls and of hip fracture in the elderly, and so on).
-
▸
Problems of long-term use of benzodiazepines: dependence, tolerance and abstinence syndrome.
-
▸
Information on how to withdraw benzodiazepines through a stepwise reduction in dose.
Follow up surgery based consultations:
-
▸
Stressing the issues discussed on the first visit.
-
▸
Evaluate possible abstinence or withdrawal symptoms.
-
▸
Positive reinforcement of achievements.
For patients in the control group, the same information was taken on personal, clinical, benzodiazepine use and psychological tests. They did not receive the structured intervention, being managed according to usual practice and informed of the convenience of reducing the use of benzodiazepines.
Throughout the study, the patient's own statement on their use of benzodiazepines was accepted. Since prescriptions were adapted to a lower dose in each control visit, and as benzodiazepines cannot be purchased without a prescription, it was decided that there was no need for an analytical control.
The interview took place on a visit lasting about 15–20 minutes. Subsequent follow up surgery-based consultations lasted about 10 minutes. Abstinence symptoms were monitored throughout the study in the intervention group.
The adequacy of data recording was piloted on 20 patients. Participating physicians received training on the administration of questionnaires, the structured interview and the guidelines for tapering off doses. This training was given in a 2–hour workshop.18
Data analysis
A descriptive analysis of patient's characteristics was performed. The results from both groups were compared to see if there were relevant differences between them for any of the independent variables related to personal or clinical data, comorbidity, benzodiazepine use and reason for prescription. Once the homogeneity of both groups had been confirmed, results were analysed for the populations per protocol and by intention to treat. Losses — always counted as treatment failures — were limited to two subjects per group, so that results per protocol and by intention to treat were similar.
Relative risk (RR) was calculated at 6 and 12 months of follow up. The absolute risk reduction (ARR) and the number of subjects that need to be treated to obtain one withdrawal (NNT) were estimated at 12 months. All parameters are given with their 95% confidence intervals (CI). Although subgroup analysis had not been considered when the study was designed, it was explored post-hoc whether the results differed among subgroups according to relevant variables such as sex, half-life of the benzodiazepine, initial dose and Hamilton Depression Scale. Withdrawal symptoms were also analysed. Statistical analysis was performed using SPSS (version 11) and Epidat (version 3). RR, ARR, NNT and 95% CI were calculated using the program at: http://www.healthcare.ubc.ca/calc/clinsig.html.
RESULTS
A total of 139 patients were included (73 in the intervention group, 66 in the control group). Four were lost to follow up, two per group, because they had moved or could not be contacted. Average age was 59 years (standard deviation = 11.4) and 82% were women.
In the general population, the median score for STAI A-E and A-R among adult men is 19 points for both scales; in this sample, it was 22 and 21.5 points, respectively. For women, results are 21 and 24 in the population, versus 28.5 and 29 in this study. Criteria for depression on the Hamilton Depression Scale were met by 27.3% of subjects.
No relevant differences were found between both groups in personal or clinical data, comorbidity, type of benzodiazepine nor anxiety or depression scales (Table 1). The main reasons for starting the prescription of benzodiazepines were, in order of frequency, anxiety, insomnia and depressive symptoms. The treatment was started by a family physician in 67.5% of cases (95% CI = 58.7 to 76.3), with psychiatrists and other specialists accounting for the remainder. The drugs more frequently prescribed were those with a short-to-intermediate half-life (81.2%, 95% CI = 74.3 to 88.0). Most patients (79.6%, 95% CI = 72.4 to 86.7) received doses equivalent to 10 mg or less of diazepam.
Table 1.
Base line characteristics of the intervention and control groups.
| Variable | Intervention subjects (%) | Control subjects (%) |
|---|---|---|
| Age mean (SD) | 60.0 (12.3) | 58.0 (10.4) |
| Sex | n = 73 | n = 66 |
| Male | 13 (17.8) | 12 (18.2) |
| Female | 60 (82.2) | 54 (81.8) |
| Education | n = 71 | n = 64 |
| No studies | 20 (28.2) | 16 (25.0) |
| Primary | 37 (52.1) | 36 (56.3) |
| Secondary | 11 (15.5) | 9 (14.1) |
| College | 3 (4.2) | 3 (4.7) |
| Residence | n = 69 | n = 61 |
| Rural | 9 (13.0) | 9 (14.8) |
| Single-family house | 20 (29.0) | 14 (23.0) |
| Block | 40 (58.0) | 38 (62.3) |
| Marital status | n = 72 | n = 63 |
| Married/partner | 47 (65.3) | 46 (73) |
| Single | 5 (6.9) | 3 (4.8) |
| Divorced/widowed | 20 (27.8) | 14 (22.2) |
| Number of people in the home | n = 73 | n = 62 |
| Alone | 14 (19.2) | 8 (12.9) |
| 1–2 | 39 (53.5) | 31 (50) |
| ≥3 | 20 (27.3) | 23 (37.1) |
| Smoking | n = 73 | n = 64 |
| Never/ex-smoker | 62 (84.9) | 50 (78.1) |
| Yes | 11 (15.1) | 14 (21.9) |
| Alcohol (>30 g/day) | n = 72 | n = 64 |
| yes | 2 (2.8) | 2 (3.1) |
| no | 70 (97.8) | 62 (96.9) |
| Chronic conditions | n = 73 | n = 66 |
| Hypertension | 24 (32.9) | 18 (27.3) |
| COPD | 6 (8.2) | 2 (3.0) |
| Diabetes | 8 (11.0) | 5 (7.6) |
| Obesity | 11 (15.1) | 8 (12.1) |
| Osteoarthritic pain | 26 (35.6) | 26 (39.4) |
| Main reason for prescription | n = 68 | n = 51 |
| Anxiety | 41 (59.4) | 37 (72.5) |
| Depression | 9 (13) | 4 (7.8) |
| Insomnia | 18 (26.1) | 10 (19.6) |
| Prescriptor | n = 69 | n = 51 |
| Family physician | 43 (62.3) | 38 (74.5) |
| Psychiatrist | 8 (11.6) | 3 (5.9) |
| Other | 18 (26.1) | 10 (19.6) |
| Withdrawal attempts | n = 64 | n = 36 |
| Yes | 28 (43.8) | 3 (4.7) |
| No | 33 (51.6) | 15 (41.7) |
| Unknown | 19 (52.8) | 2 (5.6) |
| Type of benzodiazepine | n = 73 | n = 65 |
| Short-intermediate half-life | 62 (84.9) | 50 (76.9) |
| Long half-life | 11 (15.1) | 15 (23.1) |
| Dose | n = 72 | n = 65 |
| >10 mg diazepam or equivalent | 15 (20.8) | 13 (20.0) |
| ≤10 mg diazepam or equivalent | 57 (79.2) | 52 (80.0) |
| Anxiety | n = 65 | n = 59 |
| STAI A-R, mean (SD) | 28.6 (12.7) | 29.1 (11.2) |
| STAI A-E, mean (SD) | 25.2 (14.3) | 25.73 (13.0) |
| Depression | n = 67 | n = 61 |
| Hamilton Depression Scale, mean (SD) | 13.3 (12.9) | 14.1 (7.1) |
COPD = chronic obstructive pulmonary disease. SD = standard deviation.
The intervention lasted an average of 2.6 months (range = 1 to 7). After 6 months of follow up, 29 patients from the intervention group (39.7%; 95% CI = 27.8 to 51.6) and two from the control group (3.1%; 95% CI = 0.5 to 10.8) had stopped benzodiazepine treatment.
At 12 months, 33 patients (45.2%; 95% CI = 31.1 to 57.3) of the intervention group and six (9.1%; 95% CI 1.4 to 16.8) of the control group had discontinued their use. The RR for complete withdrawal at 12 months was 4.97 (95% CI = 2.22 to 11.10). The ARR was 0.361 (95% CI = 0.22 to 0.50) and NNT = 3 (95% CI = 2 to 4). A further 16 subjects of the intervention group (21.9%) and 11 of the control group (16.7%) lowered their consumption at least by 50% after 1 year (Table 2).
Table 2.
Main outcomes at 12 months.
| Intervention group (n = 73) | Control group (n = 66) | Relative risk (95% CI) | |
|---|---|---|---|
| Complete withdrawal (%) | 33 (45.2) | 6 (9.1) | 4.97 (2.2 to 11.1) |
| Reduce dose>50% (%) | 16 (21.9) | 11 (16.7) | |
| Persist (%) | 22 (30.1) | 47 (71.3) | |
| Dropouts (%) | 2 (2.7) | 2 (3) | |
Results for different subgroups are given in Table 3. Abstinence symptoms in the intervention group included insomnia (15 cases, 20.5%), anxiety (11, 15.0%) and irritability (10, 13.6%).
Table 3.
Results in subsets of patients.
| Intervention | Control | |||||
|---|---|---|---|---|---|---|
| Complete withdrawal | Reduce dose >50% or persist | Complete withdrawal | Reduce dose >50% or persist | RR | 95% CI | |
| Total | 33 | 38 | 6 | 58 | 4.97 | 2.2 to 11.1 |
| Men | 6 | 7 | 2 | 10 | 2.8 | 0.7 to 11.2 |
| Women | 27 | 33 | 4 | 50 | 6.1 | 2.3 to 16.2 |
| Without depressiona | 20 | 27 | 3 | 36 | 5.5 | 1.8 to 17.2 |
| Depressed patientsa | 7 | 9 | 3 | 19 | 3.2 | 0.9 to 10.5 |
| Low doseb | 30 | 27 | 5 | 47 | 5.5 | 2.3 to 13.0 |
| High doseb | 2 | 13 | 1 | 13 | 1.9 | 0.2 to 18.4 |
| Short half-lifec | 28 | 34 | 4 | 46 | 5.6 | 2.1 to 15.0 |
| Long half-lifec | 5 | 6 | 2 | 13 | 3.4 | 0.8 to 14.4 |
Depression was defined as score of 17 or more patients on the Hamilton depression scale.
Higher or lower than 10mg of diazepam or an equipotent dose of another benzodiazepine.
Referred to the benzodiazepine taken by patients.
RR = relative risk.
DISCUSSION
Summary of main findings
The results of the present study suggest that a structured intervention consisting of a standardised interview together with a tapering-off process is five times more effective for discontinuing long-term benzodiazepine use than usual practice. There is one success for every three interventions, and the benefit persists for at least 12 months.
No serious adverse effect was detected, neither during the tapering-off process nor in the 12 months of follow up. The more common symptoms of withdrawal were insomnia, anxiety and irritability, but their overall prevalence was not high.
Differences in outcome between subsets of patients were found. However, the number of individuals in each of these subsets was too small for the differences to reach statistical significance, especially when considering the case of multiple comparisons (eight subgroups) where the significance level would be established with Bonferroni's correction.
Strengths and limitations of the study
This study proves the effectiveness of our intervention with a fair degree of accuracy, although the size of the study limited the capacity to estimate relative risks or contrast hypothesis among subgroups of patients. Another possible weakness is that in a study aiming to measure a physician's capacity to influence patient's habits the number of physicians taking part was relatively low.
On the other hand, the patient drop-out rate was low, probably because the intervention was performed personally by the family physician, a fact that may have improved both adherence and follow up.
The group of family physicians involved in the trial were interested in benzodiazepine dependence but had no previous specific training in this area.
The study intervention has the value of being feasible in primary care. The training associated with the intervention is simple and it can be readily incorporated into the everyday general practice.
Comparisons with existing literature
Several approaches for discontinuing benzodiazepine use have been tested. In some studies, patients were advised about the need to discontinue the use of benzodiazepines with written information about how to achieve this.8,19 The success rate was 18%, while only 5% of controls stopped taking the drug,8 a similar result to that expected for spontaneous withdrawal.11 On the other hand, studies with a precise tapering off schedule led by the physician had response rates between 24 and 51%.11–13 These studies also evaluated the benefit of adding a cognitive-behavioural therapy to the stepwise reduction of dose.11–13 This showed no benefit in one study,11 while in the other two there was a significant increase in the rate of withdrawals.12,13 Similar to the current study, Oude Voshaar and colleagues included a control group that followed usual practice.11
Implications for future research or clinical practice
A larger clinical trial, involving a larger sample of family physicians from different settings would increase the external validity of results and allow for exploring efficacy in different subsets of patients (for example, according to length of benzodiazepine use, initial dose or type of drug).
In conclusion, these results have implications for daily clinical practice, since they show that this intervention is both effective for achieving the withdrawal of long-term benzodiazepine use, and feasible in primary care practice. Its simplicity and the limited time needed for its application mean that it is a good strategy to discontinue benzodiazepine use and that it can be easily fitted into the daily routine of most physicians.
Acknowledgments
With thanks to Alberto Eek, Alfonso Ramón, Irene Sempere, Francisca Bestard, Ana Badosa, Micaela Llull, Isabel Borras, María Garau, Isabel Flórez.
Funding body
This study has received a grant from the Spanish Society of Family and Community Medicine (2000/08) and has been supported by the Gerència d’Atenció Primària de Mallorca and by the Primary Care Preventive and Health Promotion Activities Network (redIAPP)
Ethics committee
The ethical research committee of Primary Care of Mallorca Island approved the study on 2 June 1999 (exp: 99–103)
Competing interests
The authors have stated that there are none
REFERENCES
- 1.Rayon P, Montero D, Santamaría B. Benzodiazepine consumption in Spain. Eur J Clin Pharmacol. 1997;52(4):321–323. doi: 10.1007/s00228-997-4015-9. [DOI] [PubMed] [Google Scholar]
- 2.Herings RM, Stricker BH, De Boer A. Benzodiazepines and the risk of falling leading to femur fractures. Dosage more important than elimination half-life. Arch Intern Med. 1995;155(16):1801–1807. [PubMed] [Google Scholar]
- 3.National Health Committee. Guidelines for assessing and treating anxiety disorders. Wellington (New Zealand): National Health Committee; 1998. [Google Scholar]
- 4.Estivill E, Bové A, García-Borreguero D. Consensus on drug treatment, definition and diagnosis for insomnia. Clin Drug Invest. 2003;23:351–385. doi: 10.2165/00044011-200323060-00001. [DOI] [PubMed] [Google Scholar]
- 5.Cañellas F, Ochogavía J, Llobera J, et al. Trastornos del sueño y consumo de hipnóticos en la Isla de Mallorca [Sleep disorders and the consumption of hypnotics on the island of Mallorca] Rev Clín Esp. 1998;198:719–725. [PubMed] [Google Scholar]
- 6.Vicens C, Piquer MJ, Carro M, et al. Psychotropic drugs prescription in a primary health care centre. Aten Prim. 1997;20(Suppl 1):331–332. [Google Scholar]
- 7.Schweizer E, Rickels K. Benzodiazepine dependence and withdrawal: a review of the syndrome and its clinical management. Acta Psychiatr Scand. 1998;393(Suppl):95–101. doi: 10.1111/j.1600-0447.1998.tb05973.x. [DOI] [PubMed] [Google Scholar]
- 8.Bashir K, King M, Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br J Gen Pract. 1994;44(386):408–412. [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel DA. Psychological strategies for discontinuing benzodiazepine treatment. J Clin Psychopharmacol. 1999;19(6 Suppl 2):17S–22S. doi: 10.1097/00004714-199912002-00004. [DOI] [PubMed] [Google Scholar]
- 10.Zitman FG, Couvee JE. Chronic benzodiazepine use in general practice patients with depression: an evaluation of controlled treatment and taper-off: report on behalf of the Dutch Chronic benzodiazepine working group. Br J Psychiatry. 2001;178:317–324. doi: 10.1192/bjp.178.4.317. [DOI] [PubMed] [Google Scholar]
- 11.Voshaar RC, Gorgels W, Mol AJ, et al. Tapering off long-term benzodiazepine use with or without group cognitive-behavioural therapy: three condition, randomised controlled trial. Br J Psychiatry. 2003;182:498–504. doi: 10.1192/bjp.182.6.498. [DOI] [PubMed] [Google Scholar]
- 12.Baillargeon L, Landreville P, Verreault R, et al. Discontinuation of benzodiazepines among older insomniac adults treated with cognitive-behavioural therapy combined with gradual tapering: a randomised trial. CMAJ. 2003;169:1015–1020. [PMC free article] [PubMed] [Google Scholar]
- 13.Morin CM, Bastien C, Guay B, et al. Randomised clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation on older adults with chronic insomnia. Am J Psychiatry. 2004;161:332–342. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- 14.Rickels K, DeMartinis N, Rynn M, Mandos L. Pharmacologic strategies for discontinuing benzodiazepine treatment [review] J Clin Psychopharmacol. 1999;19(6 Suppl 2):12S–16S. doi: 10.1097/00004714-199912002-00003. [DOI] [PubMed] [Google Scholar]
- 15.Spielberger CD, Gorsuch RL, Lushene RE. STAI: The state-trait Anxiety Inventory. Palo Alto, California: Consulting Psychologist Press; 1970. [Google Scholar]
- 16.Speilberger CD, Gorsuch RL, Lushene RE, Seisdedos N. Cuestionario de ansiedad Estado-Rasgo. 6th edn. Madrid: TEA; 2002. [Google Scholar]
- 17.Ramos-Brieva JA, Cordero A. Spanish validation of the Hamilton Rating Scale for depression. Actas Luso Esp Neurol Psiquiatr. 1986;14:324–334. [PubMed] [Google Scholar]
- 18.Vicens C, Fiol F, Socias I. Use and withdrawal from benzodiazepines. FMC. 2003;10(suppl 4):39–45. [Google Scholar]
- 19.Cormack MA, Sweeney KG, Hughes-Jones H, Foot GA. Evaluation of an easy, cost-effective strategy for cutting benzodiazepine use in general practice. Br J Gen Pract. 1994;44:5–8. [PMC free article] [PubMed] [Google Scholar]


