Abstract
Objectives
To define the natural history and outcomes of children infected with hepatitis C virus (HCV) at birth or in early childhood.
Study design
This retrospective- prospective study identified of 60 HCV infected children through a transfusion look-back program (group 1) and by referrals (group 2). Perinatal/transfusion history, clinical course and laboratory studies were correlated with findings from 42 liver biopsies.
Results
Mean age at infection was 7.1 months and duration of infection 13.4 years. The sources of infection were blood transfusion (68%), perinatal transmission (13%) and both (7%). Most patients were asymptomatic; 3 referral patients had advanced liver disease at presentation. Mean ALT was normal in 25%, 1 to 3 times normal in 62 % and over 3 times normal in 13%. Liver biopsies showed minimal to mild inflammation in 71 %, absent or minimal fibrosis in 88 %, bridging fibrosis in 12 %. Age at infection and serum GGT correlated with fibrosis; serum ALT correlated with inflammation unless complicated by co-morbidity. Repeat biopsies within 1–4 years in 4 patients showed no significant progression in 3 and cirrhosis in one. Two patients died following liver transplantation.
Conclusions
Children with chronic HCV infection are generally asymptomatic. By 13 years post-infection, 12 % of patients developed significant fibrosis. Patients enrolled by referral had more severe liver disease than those identified through the look-back program, demonstrating the importance of selection bias in assessing the long term outcome of HCV infection.
Keywords: Transfusion look-back program, alanine aminotransferase (ALT), hepatitis C Polymerase Chain Reaction (PCR, grading and staging of liver biopsy, enzyme immune assay (EIA), recombinant immuno-blot assay (RIBA), hepatitis C viral load
Hepatitis C virus (HCV) progresses insidiously and incrementally, and results in potentially serious complications such as cirrhosis and hepatocellular carcinoma in approximately 20% and 4 % of adult patients respectively (1–4). However, data on the natural history and histopathology of HCV-related liver disease in children are conflicting (5–13). Studies from Japan and Europe point to relatively benign clinical and histopathologic liver disease (14–20) whereas studies from the USA suggest a more aggressive course with development of early fibrosis (21–23). Geographic variation in genotypes and diversity of the infected population studied may account for some of these differences, in addition to, as yet unknown viral/host factors. The lack of uniformity in the descriptions of the natural history, clinical presentation and histologic features of HCV infection in children prompted us to evaluate a cohort of 60 HCV infected children followed in our institute over a 5-year period.
The broad objectives of this study were to identify children with HCV who were infected perinatally or during early childhood, follow their natural history through adolescence, correlate clinical and laboratory data with liver histology, identify children who would benefit from medical therapy, and follow treatment or natural outcomes.
Methods
Study subjects
2,100 children who received blood/blood product transfusions between 1982 and 1992 were tested for HCV through a look-back program established at Children’s National Medical Center, Washington D.C. The primary objective was to study the natural history of HCV infection in children who had no other risk factors for liver disease than HCV acquired through transfusions in the newborn period or early in life. Patients with sickle cell disease, hemophilia, renal disease and malignancy within 5 years of treatment were excluded from the study. Study subjects included 30 HCV-positive children who were transfused prior to the availability of anti HCV testing, identified and recalled by the look-back program (group1). In addition, we studied 30 children (group 2), identified through referrals for evaluation of elevated liver enzymes and/or a known history of hepatitis C or jaundice. Included in the referral group were 6 adoptees identified as HCV positive during evaluation by their primary care physician. Potential sources of HCV infection were investigated through a questionnaire administered to parents and to children above the age of 14. Parental blood samples were obtained for anti-HCV testing for all patients from group 1 and those patients from group 2, when there were no obvious risk factors for HCV infection. Patients were recruited and followed from 1996 through 2001; the study was approved by the Institutional Review Board of our institute.
Laboratory data
HCV status was evaluated using an HCV enzyme immune assay (EIA, 2nd and 3rd generation, Abbott Laboratories, Chicago, IL) and confirmed with a second-generation recombinant immuno-blot assay, (RIBA 2, Chiron Strip Immunoblot assay, Chiron, Emmeryville, CA). Anti-HCV positive or indeterminate patients were further tested for qualitative and quantitative HCV RNA by polymerase chain reaction (RT-PCR and Cobas Amplicor HCV Monitor v2.0 assay, Roche Diagnostics, Branchburg, NJ). Viral genotype was identified by line-probe assay (InnoLiPa, Innogenetics, Belgium). Alanine aminotransferase (ALT), gamma glutamyltranspeptidase (GGTP), creatinine phosphokinase (CPK), albumin and serum iron were measured in serum by Ektachem 500 (Ortho Diagnostics, Raritan, NJ). Alpha-fetoprotein was measured by EIA (Immuno I, Bayer, Tarreytown, New York). At the initial visit, other causes of liver diseases including alpha-1-antitrypsin deficiency, Wilson’s disease, autoimmune hepatitis and co-infections with HIV, hepatitis A and B were investigated. An abdominal sonogram with Doppler study of portal venous flow was performed to identify mass lesions in the liver, splenomegaly and portal hypertension. Patients had follow-up visits at 4–6 month intervals. Anti-HCV EIA/RIBA, HCV PCR and quantitative HCV-RNA were done once or twice a year unless signs and symptoms prompted more frequent analyses. A negative test for HCV RNA on at least two samples tested within one year was considered as evidence for viral clearance.
Liver biopsy
Following informed consent, liver biopsy was performed if the ALT was two times the age related normal value on two or more visits or if autoimmune markers were present. If the ALT was less than 1 ½ times normal, a biopsy was deferred during the initial 6 –12 months, but was recommended subsequently, within the five year study period. Most of the liver biopsies were obtained by a closed, ultrasound-guided procedure using 16–18 gauge Bard Monopty needles. Transjugular biopsies were performed if the patients had thrombocytopenia. All specimens were stained by hematoxylin and eosin, Masson trichrome, Periodic Acid Schiff (PAS), Perl stain for iron and Rhodamine stain for copper. Two independent pathologists, blinded to the clinical information, evaluated the biopsies, using the scoring systems of Batts and Ludwig (24) and the Knodell Histologic Activity Index (HAI) (25). The ALT levels at the time of biopsy were recorded. The onset of infection was considered to be the date of a transfusion or the date of any surgery prior to 1992 or the date of birth when the mother was anti-HCV positive and in the case of adopted children, the age at infection was presumed to be the date of birth.
Statistical Analysis
The continuous data were summarized as mean ± standard deviation (SD), range, minimum and maximum observations. The nominal, categorical or ordinal data were summarized by count and/or proportion (%). Using the Wilcoxon and Kruskall-Wallis statistics, the two groups were compared with respect to the averages of variables such as age an infection, age at biopsy, duration of infection, laboratory values and biopsy grade and stage (26). To assess the effects of these covariates on the dependent variables, namely the histologic grade and stage, cumulative logistic regression models with proportional odds ratios were fitted to each group and to the two groups of patients pooled together. The HAI grade was compressed into three ordinal values to reduce the number of proportional odds ratio assumptions needed in the model i.e., HAI grade 0 for the original grades 0–4, 1 for 5–8 and 2 for 9 and above. A p value of < 0.05 was considered significant.
Results
Demography and clinical features
Demographics, source of infection and follow-up data for the 60 patients is given in Table I. The majority of subjects were infected in infancy at a mean age of 7.1 months. The mean age at diagnosis was 11.9 years and duration of infection to the end of the study was 13.4 years. Transfusion was the primary source of infection, occurring in 68%. The reasons for HCV testing included a history of transfusion in 48%, elevated aminotransferase levels in 28%, HCV positive parent in 8%, screening of adoptees in 8%, blood donor screening and acute jaundice in 2 % each. Patients were mostly asymptomatic. Symptoms, when reported, were mild and transient and were similar in both groups. They included fatigue in 17 patients (28%), diffuse abdominal pain in 10 (17%), nose bleeds in 7 (12%), poor weight gain in 7 (12%), muscle aches in 5 (8%), and headaches in 2 (3%). Two patients from group 2 presented with acute, self-limited jaundice and two others with edema of the legs, shortness of breath and laboratory evidence of chronic liver failure
Table I.
Population characteristics of 60 HCV-infected children.
| Demographics (mean SD, * p < 0.05) | Total | Group 1 (30) | Group 2 (30) |
|---|---|---|---|
| Sex (Male/female) | 30/30 | 16/14 | 16/14 |
| Age at infection (months) | 7.11 ±16.9 | 6.92 ± 17.8* | 7.3 ± 16.1 |
| Age at diagnosis (years) | 11.9 ±14.1 | 11.53 ± 4.1* | 8.74 ± 5.0 |
| Duration of follow-up (years) (n=60) | 3.3 ± 1.7 | 2.99 ± 1.5 | 3.45 ± 2.1 |
| Duration of infection | 13.35 ± 4.12 | 14.9 ± 2.9 | 11.8 ± 4.6 |
| Ethnicity (White/Black/Hispanic/Russian/others/mixed) | 15/13/1/1/0 | 10/8/1/6/3/2 | |
|
| |||
| Source of infection | |||
| Transfusion alone | 41 (68 %) | 28 | 13 |
| Perinatal | 8 (13 %) | - | 8 |
| Transfusions + perinatal | 4 (7 %) | 2 | 2 |
| Unknown | 7 (12 %) | - | 7 |
|
| |||
| Co-existing conditions | |||
| Sequele of prematurity | 29 | 23 | 6 |
| Cardiac/congenital anomalies | 9 | 7 | 2 |
| Hematologic/past malignancy | 4 | 0 | 4 |
| Viral hepatiis/HIV | 3 | 0 | 3 |
|
| |||
| Patients lost to follow-up | 10 (17 %) | 7 (12%) | 3 (5%) |
| Age at infection (mean in mo.) | 1.62 | 0.82 | 3.02 |
| Age at diagnosis (mean in yrs.) | 12.0 | 11.25 | 13.5 |
| Duration of infection (mean in yrs.) | 15.7 | 14.71 | 17.50 |
| Duration of follow up (mean in yrs.) | 2.0 | 1.83 | 2.25 |
Biochemical and Virologic Indices
The ALT varied widely among the patients and in the same patient at different times during the study period (mean ± SD = 98.0± 115.7 U/L). The mean values from each patient were expressed as normal, 1– 1½ times age adjusted upper limit of normal (UNL), 1½ to 3 times ULN and above 3 times the ULN (Table II; available at www.jpeds.com). 28/60 (47 %) patients had mean ALT levels within the normal range or below 1.5 times ULN, 24 (40 %) had mean ALT levels 1½-3 times of ULN and 8 (13 %) had mean values above 3 times ULN. A few patients skewed the data with inordinately high ALT at presentation although ALT levels generally normalized during the follow up period. They included an infant with vertically acquired HCV (1786 U/L), a patient with myopathy (27) presumed to be secondary to HCV infection (1005 U/L), and a transfused infant co-infected with HIV and cytomegalovirus (801 U/L). Serum bilirubin, albumin and iron levels were normal in all patients except in two patients who presented initially with acute obstructive jaundice and early liver failure. The mean ALT during the follow-up period (group 1= 86.3 ± 113.6 U/L vs. group 2 = 109.6 ± 95.4 U/L) and at the time of liver biopsy (Table IV) was significantly higher in group 2 vs. group 1 patients.
Table 2.
Comparison of ALT values among study cohorts
| ALT (U/L) (Mean ± SD) | Total | Group 1 | Group 2 |
|---|---|---|---|
| ALT during study period (n=60) | 98.0 ± 115.7 | 86.3 ± 133.6 | 109.6 ± 95.4* |
| ALT at biopsy (n = 42) | 131.5 ± 22 | 61.0 ± 59.5 | 154.7± 255.5* |
| Patients with normal ALT (%) | 15 (25%) | 11 | 4 |
| ALT > 1–1½ times normal | 13 (22%) | 5 | 8 |
| ALT > 1½ –3 times normal | 24 (40%) | 12 | 12 |
| ALT > 3 times normal | 8 (13%) | 4 | 4 |
p = < 0.05
Table 4.
Comparison of Cohort and Referral groups at the time of liver biopsy
| Variable | Group 1 (Cohort) n =19 | Group 2 (Referral) n=23 | Kruskal-Wallis test: * p <0.05 |
|---|---|---|---|
| Age at infection (mo) | 4.6 ± 11.8 | 7.3 ± 16.5 | 0.157 |
| Age at biopsy (yrs) | 15 ± 1.8 * | 8.8 ± 5.0 | 0.0001 |
| Duration of infection(yrs) | 14.1 ± 2.8 * | 8.3 ± 4.5 | 0.0002 |
| ALT at biopsy | 61 ± 59.5 | 154.7 ± 255.5* | 0.001 |
| GGT | 34.1 ± 21.4 | 114.1 ± 197.7 | 0.49 |
| AFP | 3.7 ± 3.7 | 26.2 ± 78.2 | 0.17 |
| Viral load (million) | 1.7 ± 2.8 | 2.0 ± 3.5 | 0.09 |
| HAI grade | 5.1 ± 2.1 | 7.4 ± 3.4* | 0.012 |
| HAI stage | 0.42 ± 0.5 | 1.2 ± 1.1 * | 0.012 |
The majority of patients (n=50, 83 %) tested positive for antibodies to HCV and HCV RNA throughout the 5 year study period. One patient was HCV RNA positive but EIA negative on two consecutive tests six months apart, but subsequently converted to seropositivity after 24 months (28). Seven anti-HCV and HCV RNA positive patients were lost to follow up after the initial testing. One patient each from the two groups was HCV RNA positive at enrollment but thereafter remained negative for the duration of the study. Viral genotype was performed on 45 patients (group 1 = 26, group 2 = 19); 80 % of group 1 and 78 % of group 2 were of genotype 1 and the rest were of genotype 2 and 3. The mean viral load at the time of liver biopsy did not differ significantly between groups 1 and 2 and only 15% had viral loads >2 million copies/ml.
Radiologic findings
Abdominal sonogram with Doppler study of the portal venous system was performed in 43/60 (72 %) patients. The study was normal in 27 children (63 %). Abnormal findings included mild splenomegaly in 5 (12%), gallstones/gall bladder sludge in 3 patients (7 %) and abnormal echogenicity in 2 (5 %). Splenomegaly was found only in the referral group (group 2). Abdominal CT scans in 2 of 5 patients with splenomegaly showed nodularity of the liver and esophageal varices.
Histology
42 initial 1iver biopsies were scored by HAI (25); the results are summarized in Table III. These 42 biopsies and two additional biopsies were also scored using the classification of Batts and Ludwig (24). Correlation between the two systems of classification was excellent both for the grade and stage of histology (Spearman correlation for grade: r = 0.75, p =0.0001; for stage: r = 0.62, p = 0.0001. Pearson correlation linear regression analysis for grade: r = 0.78; and stage r = 0.79). Overall, the inflammatory changes (the sum of portal, periportal and lobular inflammation) were mild (grade 0–8) in 71 %, moderate (grade 9–12) in 24% and severe (grade13–18) in 5 %. Fibrosis was absent or mild with only periportal expansion in 88 % and bridging fibrosis was seen in 12 %. All of the patients with severe inflammation (5 %) and bridging fibrosis (12%) belonged to the referral group. None of the initial biopsies showed cirrhosis. Lymphoid aggregates were present in 18/41 (44%). Mild steatosis was identified in four biopsies (10%) and bile duct involvement (“Poulson lesions”) in three biopsies (7 %). Stainable iron was detected in the biopsies from two patients who had received multiple transfusions for acute leukemia and thalassemia major. Four patients had repeat liver biopsies over a span of 1–4 years. Three biopsies showed no histological progression; one showed progression to early cirrhosis over the course of four years. Bridging fibrosis was seen in 5 of group 2 patients. Combining the initial and follow-up biopsies, bridging fibrosis or early cirrhosis was found in 6 (14%) of 42 patients undergoing percutaneous biopsy. In addition, two patients were found to have cirrhosis at the time of liver transplantation.
Table 3.
Histopathologic findings on liver biopsy by HAI scoring system (Group 1: n = 19, group 2: n = 23)
| Periportal Inflammation | Score | Group 1 | Group 2 | Total (%) |
|---|---|---|---|---|
| None | 0 | 1 | 2 | (7) |
| Mild | 1 | 12 | 7 | (45) |
| Moderate | 3 | 6 | 10 | (39) |
| Marked | 4–6 | 0 | 4 | (10) |
| Lobular inflammation | ||||
| None | 0 | 0 | 0 | (0) |
| Mild | 1 | 7 | 3 | (24) |
| Moderate | 3 | 5 | 6 | (24) |
| Marked | 4 | 7 | 14 | (50) |
| Portal inflammation | ||||
| None | 0 | 5 | 3 | (19) |
| Mild | 1 | 13 | 12 | (60) |
| Moderate | 3 | 1 | 8 | (21) |
| Marked | 4 | 0 | 0 | (0) |
| Total Grade (0 – 18)** | ||||
| Minimal | 0–4 | 6 | 4 | (24) |
| Mild | 5–8 | 10 | 10 | (47) |
| Moderate | 9–12 | 3 | 7 | (24) |
| Marked | 13–18 | 0 | 2 | (5) |
| Stage- Fibrosis (0 – 4) | ||||
| None | 0 | 11 | 6 | (40) |
| Periportal/Expansion | 1 | 8 | 12 | (48) |
| Bridging fibrosis | 3 | 0 | 5 | (12) |
| Cirrhosis | 4 | 0 | 0 | (0) |
Sum of portal, periportal and lobular inflammation
Correlation between histology, demography and laboratory parameters
When the two groups were pooled together, age at infection and serum GGT levels were significantly and positively associated with HAI stage (p = 0.002 and p = 0.032 respectively). When the groups were examined separately, there were no statistically significant predictors of stage in group 1, but age at infection was significantly and positively associated with the stage of liver disease in group 2 (p = 0.041). ALT at the time of biopsy correlated positively with histologic grade in group 1 (p = 0.021). No covariate was found to affect the grade significantly in group 2. Serum ALT was positively associated with histologic grade in group 1 and age at infection was positively associated with stage in group 2. Table IV compares the laboratory and histological findings between the two groups of patients.
Clinical Outcome
The clinical outcome of the 60 patients is summarized in the Figure. Of the 50 patients who had continuous follow up, 47 (94 %) had clinical and laboratory evidence of mild liver disease. Two patients underwent liver transplantation for portal hypertension and chronic liver failure; both of them succumbed to complications of the transplantation. The native liver of one of them showed evidence of hepatocellular carcinoma. A third patient whose repeat biopsy showed evidence of cirrhosis continues to exhibit deterioration in liver function. The only patients with cirrhosis were from the referral group and had acquired HCV infection via perinatal transmission.
Figure.
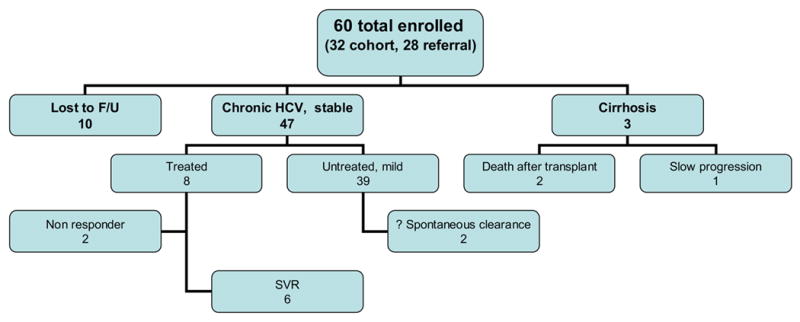
The clinical outcome of 60 patients over the 5 year study period.
Discussion
We describe the demographic, virologic and histopathologic data on 60 children and adolescents with chronic HCV infection, evaluated during a 5 year period. The onset of infection was reasonably established in 88% based on transfusion history and/or perinatal transmission from HCV-infected mothers. The mean duration of infection based upon the history of exposure was 13 years. Histological data was available for 60% of the children and only 13 % had treatment that would alter the natural history of the infection. We evaluated patients in two clinical settings. The primary focus of our study was HCV-infected children (group 1) who were transfused at our institution in the years prior to the availability of testing for HCV. Group 2 included patients referred to us for evaluation of established liver disease. Although inclusion of these patients introduced a referral bias, a prospective study of sufficient length to analyze the full spectrum of HCV outcomes in children is not feasible and hence we chose to combine a retrospective-prospective cohort analysis with that of an equal number of referral cases in whom more severe outcomes were expected, and indeed found. This dual approach aimed to achieve a balanced approximation of the full and varied spectrum of HCV outcomes as demonstrated in studies of HCV infection in adults (1–4). Surprisingly, only 1.5% of the 2,100 transfusion recipients investigated in the look-back study were found to be HCV-infected despite the absence of donor screening at the time of their transfusions. Other look-back studies in children (29–31) have reported HCV infection in 0.3 to 6 % of blood transfusion recipients. The lower than expected prevalence of HCV positivity in our look-back study may have been due in part to our entry criteria that excluded children with concurrent diseases. Only two of the 30 patients in group 1 appeared to have cleared HCV infection spontaneously.
Clinical and laboratory evaluation of the identified cases in our study showed an excellent correlation between ALT and the histologic grade on liver biopsy in group 1. Patients in group 2 had concurrent pathology such as hepatitis B and decompensated cirrhosis that may have affected the ALT. 26/28 children (93%) with normal ALT’s or levels persistently less than 1 ½ times ULN had only mild liver disease by biopsy. Patients with ALT greater than 3 times ULN generally had more severe pathology; however two patients with persistently normal ALT’s also had advanced liver disease. Although ALT was an excellent correlate of liver histology, discrepancy between ALT and histology in 7 % of our patients attests to the continued value of liver biopsy, as has been supported by other pediatric and adult studies (14,16,32–34).
The characteristic features of chronic HCV-associated hepatitis found in adults, namely, lymphoid aggregates and follicles, macrovesicular steatosis and bile duct damage were relatively rare in our series (35). Early cirrhosis was documented in only one patient on a repeat biopsy and frank cirrhosis was found in two other patients at the time of liver transplantation. Among four patients with repeat biopsy, only one showed fibrosis progression; this patient already had bridging fibrosis in the initial biopsy and then advanced to early cirrhosis over the course of 4 years. When the biopsies from the two groups were examined separately, we found that the ALT level at the time of liver biopsy, and the grade and the stage of histology were significantly higher in group 2 than in group 1 even though the duration of infection was longer in the latter (Table IV). 60 % of those with no (stage 0) fibrosis belonged to group 1 and all of the patients with stage 3–4 were from Group 2. The increased severity of liver disease in group 2 reflects, at least in part, a referral bias wherein these cases represented patients with established liver disease referred for further evaluation. In these patients, the degree of fibrosis correlated with the age at which they were infected, the older the age at infection the worse the fibrosis. This age relationship was not seen in group 1 patients, possibly because the vast majority was infected before age 2. In contrast, Guido et al (36) studied the progression of fibrosis in untreated patients who had acquired HCV infection in infancy and found that age at biopsy and duration of infection positively correlated with stage of fibrosis. In their study, 13 out of 112 patients were re-biopsied at a mean interval of 7.9 years; 54% showed a 1–2 stage increase in fibrosis, the progression was slower than reported in adult patients (37).
Long-term cohort studies in children have been limited. One of the earliest longitudinal studies involved 77 children from Europe who were HCV-positive following transfusion-related and community-acquired infection (13). When followed over a 6-year period, 27 % had active but mild histologic liver disease, 10 % achieved biochemical remission and only 3 % developed cirrhosis (13). The same group recently reported that 7–10% of these children subsequently developed anti-LKM antibodies that were associated with a more severe outcome of their liver disease (38). Vogt et al emphasized the relatively benign course of liver disease in persons who acquire HCV infection early in life (18). They evaluated 458 children who had been transfused for cardiac surgery approximately 20 years earlier at a mean age of 2.8 years (18). 15 % were found to be anti-HCV positive; but only 55% were HCV RNA positive, suggesting a spontaneous viral clearance in 45%. Liver biopsy in 17 chronically infected patients showed mild histological disease in (82%); only one patient had cirrhosis attributable to HCV infection (18). Casiraghi et al reported mild liver disease with slow progression in persons who acquired HCV infection via mini-transfusions in the newborn period; 35 years post-exposure, 9 of 11 (82%) had minimal or no inflammatory activity or fibrosis and 2 had stage 3–4 fibrosis. Repeat biopsies in 5 patients after 5 years showed progression in only one patient (5). Studies in young adults also demonstrate a high spontaneous recovery rate and predominantly mild histological changes. Two studies involving young women, inadvertently exposed to HCV contaminated Rh immune globulin revealed cirrhosis in less than 4% and bridging fibrosis in only 10–15 % approximately 25 years after the onset of HCV infection (4, 39). Minola et al (6) studied 392 patients with post-transfusion HCV infection and calculated that the median time to develop end stage liver disease was 33 years for those infected between ages 21–30 years, and 16 years for those infected after age 40. In 77 patients infected below the age of 20 years, only 4% developed cirrhosis (5). These and other studies demonstrate that the younger the individual at the time of infection, the less the severity of HCV related liver disease (4,5,6,18,37). The reasons for this observation are not known, but may relate to more vigorous immune responses to an infection acquired early in life, reduced fibrogenic mechanisms in children and/or the confounding effects of alcohol, obesity and co-infections in adult patients (8, 53). Although children tend to have more indolent HCV-infection than adults, the development of severe liver diseases can be accelerated in the presence of co-morbid conditions such as thalassemia (9), iron overload (9, 21), childhood cancer (7,8,22,23), and HIV co-infection (40).
Retrospective studies involving patients referred to tertiary care centers for identified liver diseases demonstrate yet another population of patients with more severe outcomes (21,22). Illustrative of these retrospective studies, Badizadegan et al examined 50 liver biopsies from 40 HCV-infected children aged 2 to 18 years, and found significant fibrosis in 78%, including bridging fibrosis and architectural distortion in 58%, and cirrhosis in 8% (21). The degree of fibrosis correlated positively with age and duration of infection. These study subjects are comparable to our group 2 (referral) patients.
We conclude that children who acquire HCV infection early in life generally manifest only mild liver disease over the first two decades of their infection. However, it is apparent that severe liver disease may develop in select groups of children infected with HCV. Despite the inherent bias of patient selection, referral studies prove that cirrhosis and rarely hepatocellular carcinoma are potential sequelae of childhood HCV infection and emphasize the need for identification and careful follow-up of those with potential risk factors. However, only a very long-term prospective study on unselected cohort groups can provide an accurate accounting of the relative proportion of children who manifest severe outcomes. It is uncertain at present whether the slow progression of hepatitis C in immuno-competent children will accelerate as they grow older or will remain stable. This question has considerable implications for therapy. A slowly progressive course of HCV infection in children would favor deferred treatment, given that the multiple side effects of the currently available therapy observed in adults might be compounded by growth retardation in children. On the other hand, if pediatric trials show that children tolerate anti-viral therapy well with high sustained response rates, early treatment would be preferred over the potential for serious liver disease decades later. Our natural history study and other pediatric studies (10–20) suggest that treatment can be deferred to adolescence or later as long as patients are followed closely. However, it is important that this premise be tested by longer-term natural history studies and by carefully conducted pediatric drug treatment trials to assess toxicity and sustained response rates.
Acknowledgments
We wish to thank Dr. Albert Hoang and Dr. Robert McCarter for their help with the statistical analyses of our data during the revision of this paper, following the demise of Dr Kantilal Patel.
List of Abbreviations
- ALT
Alanine aminotransferase
- ANA
Anti Nuclear Antibody
- CPK
Creatinine Phosphokinase
- HCV PCR
Hepatitis C Polymerase Chain Reaction
- EIA
Enzyme Immune Assay (EIA)
- RIBA
Recombinant Immuno-Blot Assay
- Anti LKM antibodies
Anti Liver Kidney Microsomal antibodies
Footnotes
The study was funded in part by a grant (RO1 HL 56060) from the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alter HJ, Seeff LB. Recovery, persistence and sequelae in hepatitis C virus infection: A perspective on long-term outcome. Semin Liv Dis. 2000;20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 2.Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CMB, Buskell ZL, et al. Long term morbidity and mortality of transfusion-associated non-A, non-B, and type C hepatitis: A National heart, Lung and Blood Institute collaborative study. Hepatology. 2001;33:455–463. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 3.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 4.Kinney-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–33. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 5.Casiraghi MA, Paschale MD, Romano L, Biffi R, Assi A, Binelli G, et al. Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology. 2004;39:90–96. doi: 10.1002/hep.20030. [DOI] [PubMed] [Google Scholar]
- 6.Minola E, Prati D, Suter F, Maggiolo F, Caprioli F, Zogni A, et al. Age at infection affects the long term outcome of transfusion-associated chronic hepatitis C. Blood. 2002;99:4588–4591. doi: 10.1182/blood-2001-12-0192. [DOI] [PubMed] [Google Scholar]
- 7.Cesaro S, Petris MG, Rossetti F, Cusinato R, Pipan C, Guido M, et al. Chronic hepatitis C virus infection after treatment for pediatric malignancy. Blood. 1997;90:1315–1320. [PubMed] [Google Scholar]
- 8.Locasciulli A, Testa M, Pontisso P, Benvegnu L, Fraschini D, Corbetta A, et al. Prevalence and natural history of hepatitis C infection in patients cured of childhood leukemia. Blood. 1997;90:4628–33. [PubMed] [Google Scholar]
- 9.Lai ME, DeVirgilis S, Argiolu F, Farci P, Mazzoleni AP, Lisci V, et al. Evaluation of antibodies to hepatitis C virus in a long-term prospective study of post transfusion hepatitis among thalassemic children: comparison between first and second generation assay. J Ped Gastroenterol Nutr. 1993;16:458–464. doi: 10.1097/00005176-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Mohan P, Glymph C, Baxter C, Chandra RR, Kleiner DE, Luban NLC, et al. Histopathology and epidemiology of hepatitis C in children: a single institution study. J Pediatr Gastroenterol Nutr. 2000;31:S115. [Google Scholar]
- 11.Schwimmer JB, Balistreri WF. Transmission, natural history and treatment of hepatitis C virus infection in the pediatric population. Semin Liv Dis. 2000;20:37–46. doi: 10.1055/s-2000-9257. [DOI] [PubMed] [Google Scholar]
- 12.Aach RD, Yomtovian RA, Hack M. Neonatal and pediatric post transfusion hepatitis C- A look back and a look forward. Pediatrics. 2000;105:836–842. doi: 10.1542/peds.105.4.836. [DOI] [PubMed] [Google Scholar]
- 13.Bortolloti F, Jara P, Diaz C, Vajro P, Hierro L, Giacchino R, et al. Posttransfusion and community-acquired hepatitis C in childhood. J Pediatr Gastroenterol Nutr. 1994;18:279–283. doi: 10.1097/00005176-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka S, Tatara K, Hayabuchi Y, Taguchi Y, Mori K, Honda H, et al. Serologic, virologic, and histologic characteristics of chronic phase hepatitis C virus disease in children infected by transfusion. Pediartics. 1994;94:919–922. [PubMed] [Google Scholar]
- 15.Kage M, Fujisawa T, Shiraki K, Tanaka T, Fugisawa T, Kimura A, et al. Pathology of chronic hepatitis C in children. Hepatology. 1997;26:771–75. doi: 10.1002/hep.510260333. [DOI] [PubMed] [Google Scholar]
- 16.Guido M, Rugge M, Jara P, Hierro L, Giacchino R, Larrauri J, et al. Chronic hepatitis C in children: the pathological and clinical spectrum. Gastroenterol. 1998;115:1525–1529. doi: 10.1016/s0016-5085(98)70032-0. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Monzone C, Jara P, Fernandez-Bermejo M, Hierro L, Frauca E, Camarena C, et al. Chronic hepatitis C in children: A clinical and immunohistochemical comparative study with adult patients. Hepatology. 1998;28:1696–1701. doi: 10.1002/hep.510280633. [DOI] [PubMed] [Google Scholar]
- 18.Vogt M, Lang T, Frosner G, Klingler C, Sendl AF, Zeller A, et al. Prevalence and clinical outcome of HCV infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999;341:866–70. doi: 10.1056/NEJM199909163411202. [DOI] [PubMed] [Google Scholar]
- 19.Hoshiyama A, Kimura A, Fujisawa T, Kage M, Kato H. Clinical and histologic features of chronic hepatitis C virus infection after blood transfusion in Japanese children. Pediatrics. 2000;105:62–65. doi: 10.1542/peds.105.1.62. [DOI] [PubMed] [Google Scholar]
- 20.Jara P, Resti M, Hierro L, Giacchino R, Barbera C, Zancan L, et al. Chronic hepatitis C virus infection in childhood: clinical patterns and evolution in 224 white children. Clin Infect Dis. 2003;36:275–280. doi: 10.1086/345908. [DOI] [PubMed] [Google Scholar]
- 21.Badizagen K, Jonas MM, Ott MJ, Nelson SP, Perez-Atayde AR. Histopathology of the liver in children with chronic hepatitis C viral infection. Hepatology. 1998;28:1416–1423. doi: 10.1002/hep.510280534. [DOI] [PubMed] [Google Scholar]
- 22.Strickland DK, Reily CA, Patrick CC, Jones-Wallace D, Boyett JM, Waters B, et al. Hepatitis C infection among survivors of childhood cancer. Blood. 2000;95:3065–70. [PubMed] [Google Scholar]
- 23.Castellino S, Lensing S, Reily C, Rai SN, Davila R, Hayden RT, et al. The epidemiology of chronic hepatitis C infection in survivors of childhood cancer: an update of the St Jude Children’s Research Hospital hepatitis C seropositive cohort. Blood. 2004;103:2460–66. doi: 10.1182/blood-2003-07-2565. [DOI] [PubMed] [Google Scholar]
- 24.Batts KP, Ludwig J. Chronic hepatitis: an update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis C. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 26.SAS Institute Inc. SAS Procedures Guide, Release 6.03 Ed. Cary, NC: SAS Institute Inc.; 1988. The CORR Procedure; pp. 125–150. [Google Scholar]
- 27.Mohan P, Chandra RS, Escolar DM, Luban NLC. Inflammatory myopathy and hepatitis C in a pediatric patient: Role of liver biopsy in evaluating the severity of liver disease. Hepatology. 2001;34:851–2. doi: 10.1002/hep.510340439. [DOI] [PubMed] [Google Scholar]
- 28.Mohan P, Chandra RS, Kleiner DE, Luban NLC. An unusual presentation perinatally transmitted hepatitis C. Arch Dis Child. 2003;88:160–61. doi: 10.1136/adc.88.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts EA, King SM, Fearon M, McGee N. Hepatitis C in children after transfusion: assessment by look-back studies. Acta gastroenterol Belg. 1998;61:195–97. [PubMed] [Google Scholar]
- 30.Davoren A, Dillon AD, Power JP, Donnellan J, Quinn JM, Willis JW, et al. Outcome of an optional HCV screening program for blood transfusion recipiens in Ireland. Transfusion. 2002;42:1501–06. doi: 10.1046/j.1537-2995.2002.00224.x. [DOI] [PubMed] [Google Scholar]
- 31.Goldman M, Long A. Hepatitis C lookback in Canada. Vox Sang. 2000;78 (suppl 2):249–252. [PubMed] [Google Scholar]
- 32.Hoofnagle J. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26(suppl 1):15S–20S.5. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 33.McCormick SE, Goodman ZD, Maydonovitch CL, Sjogren MH. Evaluation of liver histology, ALT elevation, and HCV RNA titer in patients with chronic hepatitis C. Amer J Gastroenterol. 1996;91:1516–1522. [PubMed] [Google Scholar]
- 34.Pradat P, Alberti A, Poynard T, Esteban JI, Weiland O, Marcellin P, et al. Predictive value of ALT levels for histologic findings in chronic hepatitis C; A European collaborative study. Hepatology. 2002;36:973–977. doi: 10.1053/jhep.2002.35530. [DOI] [PubMed] [Google Scholar]
- 35.Ishak KG. Pathologic features of chronic hepatitis. Am J Clin Pathol. 2000;113:405. doi: 10.1309/42D6-W7PL-FX0A-LBXF. [DOI] [PubMed] [Google Scholar]
- 36.Guido M, Bortolotti F, Leandro G, Jara P, Hierro L, et al. Fibrosis in chronic hepatitis C acquired in infancy: is it only a matter of time? Am J Gastroenterol. 2003;98:660–663. doi: 10.1111/j.1572-0241.2003.07293.x. [DOI] [PubMed] [Google Scholar]
- 37.Poynard T, Badossa P, Opolon P, OBSVIRC, METAVIR, CLINIVIR & DOSVIRC groups Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 38.Bortolliti F, Muratori I, Jara P, Hierro L,Verucchi G, Giacchino R, et al. Hepatitis C virus infection associated with liver-kidney microsomal antibody type 1 (LKM 1) autoantibodies in children. J Pediatr. 2003;142:185–90. doi: 10.1067/mpd.2003.45. [DOI] [PubMed] [Google Scholar]
- 39.Weise M, Gr ngreiff K, G thoff W, Lafrenz M, Oesen U, Porst H. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany- a 25- year multicenter study. J Hepatol. 2005;43:590–598. doi: 10.1016/j.jhep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Benhamou Y, Bochet M, DiMartino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]


