Abstract
Granule cells of the mammalian dentate gyrus normally form a discrete layer, and virtually all granule cells migrate to this location. Exceptional granule cells that are positioned incorrectly, in ‘ectopic’ locations, are rare. Although the characteristics of such ectopic granule cells appear similar in many respects to granule cells located in the granule cell layer, their rare occurrence has limited a full evaluation of their structure and function. More information about ectopic granule cells has been obtained by studying those that develop after experimental manipulations that increase their number. For example, after severe seizures, the number of ectopic granule cells located in the hilus increases dramatically. These experimentally induced ectopic granule cells may not be equivalent to normal ectopic granule cells necessarily, but the vastly increased numbers have allowed much more information to be obtained. Remarkably, the granule cells that are positioned ectopically develop intrinsic properties and an axonal projection that are similar to granule cells that are located normally, i.e., in the granule cell layer. However, dendritic structure and synaptic structure/function appear to differ. These studies have provided new insight into a rare type of granule cell in the dentate gyrus, and the plastic characteristics of dentate granule cells that appear to depend on the location of the cell body.
Keywords: Neurogenesis, Hilus, Seizure, Epilepsy, Hippocampus, Neuronal migration, Calbindin, PROX1
Introduction
The dentate gyrus is known for its compact layer of granule cells. In virtually all mammals studied to date, this layer is the place where the vast majority of mature granule cells reside after normal development and migration. Indeed, it is often assumed that this is the only place where granule cells of the dentate gyrus are located. It is relatively unappreciated that some granule cells are located outside the granule cell layer, in ‘ectopic’ locations. However, under some conditions, this number can be substantial. In light of recent interest in the factors that control granule cell migration, as well as the function of abnormally situated neurons in developmental disorders, it is timely to review the current understanding of ectopic granule cells, not only those that occur normally, but those that develop after experimental intervention.
Historical Overview
Almost all considerations of the granule cells of the dentate gyrus discuss them in the context of their location in the dense, compact, granule cell layer. Very few studies mention the possibility that they may occur elsewhere, and when they do, it appears to be the exception rather than the rule. Nevertheless, even some of the earliest analyses of hippocampus included reference to the existence of neurons that might be ectopic granule cells. For example, Ramón y Cajal [1911] suggested that ‘displaced’ granule cells occurred in the molecular layer. However, the nature of the material used, namely the Golgi preparation, made it difficult to be sure these cells were definitely granule neurons, and it was unclear whether they were present in substantial numbers. The fact that these displaced granule cells were rare may have contributed to the fact that there were few subsequent studies about ectopic granule cells after Ramón y Cajal [1911]. Even to this day, the number of ectopic granule cells has not been established. Preliminary studies using stereological methods in the adult male Sprague-Dawley rat suggest approximately 1,000–2,000 ectopic granule cells develop in the hilus per hippocampus [McCloskey et al., 2005], which means that they represent approximately 0.1–0.2% of the total granule cell population [West et al., 1991; Boss et al., 1995]. More quantitative studies and more information in general will provide a much better foundation to evaluate the potential role of ectopic granule cells in the dentate gyrus network.
Previous Studies of Ectopic Granule Cells
As mentioned above, there have been few studies of ectopic granule cells since the time of Ramón y Cajal. All of them have examined the laboratory rat, and agree that these cells are rare. Seress and Pokorny [1981] discussed neurons that resembled granule cells in the hilus, as did Gaarskjaer and Laurberg [1983]. The study of Gaarskjaer and Laurberg [1983] provided anatomical evidence that the hilar cells were granule-like because of a morphological similarity to granule cells of the granule cell layer. In addition, they found that the ectopically situated neurons projected to area CA3, like normal granule cells located in the granule cell layer, and unlike other hilar cell types.
There are also other studies that describe neurons in the hilus with morphological features of granule cells, but these reports do not discuss them as if they are granule cells, presumably because they were not found in the granule cell layer, and all morphological features of granule cells were not demonstrated. For example, in Amaral’s [1978] classic study of the hilar region, he described a granule-like hilar cell, but did not define it as such. Amaral and Woodward [1977] reported an ‘interneuron of regio inferior’ that they identified in area CA3, close to the border with the hilus, and this cell had many morphological similarities to granule cells located in the granule cell layer. However, it was not discussed as if it were a granule cell, because it failed to show all characteristics of granule cells. For example, the dendrites were more elaborate than granule cells. In addition, and perhaps most convincing, was the absence of mossy fiber expansions. In comparison, ectopic granule cells that have subsequently been identified by electrophysiology and intracellular dye injection have remarkably complex and lengthy dendrites, and they do have mossy fiber axons [Scharfman et al., 2000, 2003].
In 1986, a detailed Golgi study was provided to elucidate more of the anatomical characteristics of ectopic granule cells [Marti-Subirana et al., 1986]. In this study, 50 adult (70-day-old) male Sprague-Dawley rats were examined. Neurons with the morphology of granule cells were detected in many locations, not only in the hilus and molecular layer, but also within CA3.
Defining Ectopic Granule Cells
General Approaches
The studies described above raise the question of how a granule cell can be defined if it does not exist within the granule cell layer. The soma size and shape, asymmetric or ‘polarized’ dendritic tree, and mossy fiber axon are useful attributes, as are dendritic spines, and these were used by Marti-Subirana et al. [1986] to define granule cells. In addition, the granule cell has characteristic ultrastructural features [Lübbers and Frotscher, 1987; Shapiro and Ribak, 2005]. However, there is some variability in these features even in the granule cells located in the granule cell layer. Thus, some granule cells have variability in the apical dendritic tree as a function of their location in the granule cell layer [Green and Juraska, 1985; Claiborne et al., 1990]. Granule cells may have basal dendrites, which is rare in the rat but common in primates [Seress and Mrzjlak, 1987; Shapiro and Ribak, 2005]. Some granule cells may have recurrent basal dendrites, or apical axons [Yan et al., 2001].
Immunohistochemical Methods to Differentiate Granule Cells
Markers of granule cells are also useful to distinguish them from other cell types, and two markers appear to preferentially label granule cells relative to other dentate gyrus neurons: calbindin D28K, a calcium-binding protein, and PROX1, a homeobox protein. However, even these markers are not able to define granule cells perfectly. Calbindin does not label granule cells that are immature, for example [Goodman et al., 1993], and calbindin does not label all granule cells after seizures, because the expression of calbindin appears to decrease in granule cells due to the seizure activity [Magloczky et al., 1997; Nagerl et al., 2000] (fig. 1, 2). Although calbindin immunoreactivity is present in many GABAergic neurons of the hippocampal pyramidal cell subfields, few are evident in the dentate gyrus of the normal adult rat [Toth and Freund, 1992; Scharfman et al., 2000] (fig. 1).
Fig. 1.
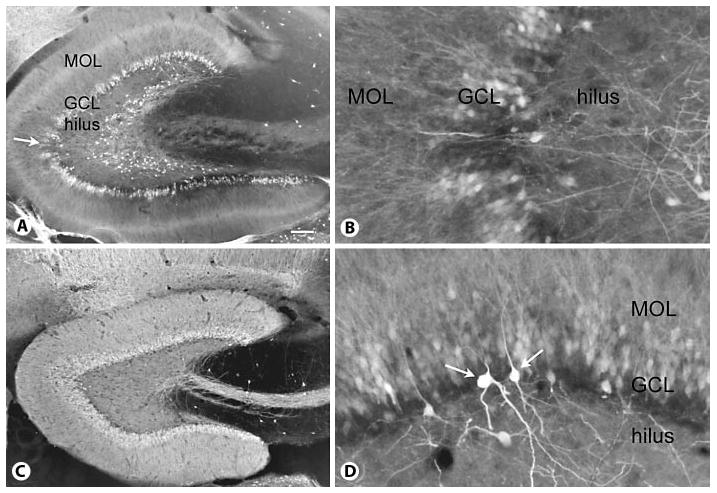
Calbindin expression in a control and epileptic rat. A Calbindin expression in a rat that had status epilepticus and recurrent seizures shows irregular loss of calbindin expression in the granule cell layer (GCL) and numerous ectopically located calbindin-immunoreactive cells throughout the deep hilus. MOL = Molecular layer. Calibration = 100 μm. For methods used for calbindin immunocytochemistry, see Scharfman et al. [2000, 2002]. B Higher magnification of the area in A marked by the arrow. Same orientation as in A. Calibration (in A) = 50 μm. C Calbindin expression in a saline-treated rat that was of a similar age at the time of euthanasia as the rat in A. Note the even, homogeneous expression of calbindin throughout the granule cell layer and the lamina containing granule cell dendrites and the mossy fiber pathway. However, calbindin-immunoreactive cells are not present in the hilus. Same calibration as in A. D Example of unusual calbindin immunoreactivity in a pilocarpine-treated rat with chronic seizures. Dense reactivity is apparent in nongranule cells (arrows) relative to granule cells, suggesting seizure-induced up-regulation of calbindin expression in nongranule cells at the same time as seizure-induced downregulation of calbindin in granule cells. Same calibration as in B. MOL = Molecular layer; GCL = granule cell layer.
Fig. 2.
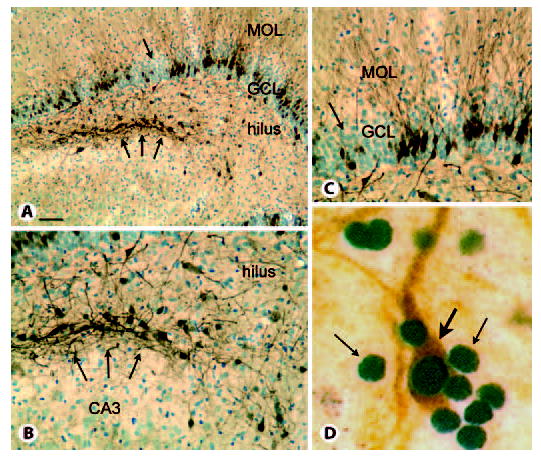
Calbindin and PROX1 expression in the epileptic rat. MOL = Molecular layer; GCL = granule cell layer. A Calbindin and cresyl violet staining of a section through the septal pole of a pilocarpine-treated rat with recurrent seizures illustrates irregular loss of calbindin expression despite persistence of apparently normal granule cell cresyl violet staining (arrow) and clusters of calbindin-immunoreactive cells at the border of the hilus and CA3 region (triple arrows). Calibration = 100 μm. B Higher magnification of the clusters of hilar calbindin cells shown in A. Calibration (in A) = 50 μm. C Higher magnification of the irregular loss of calbindin in the granule cell layer (arrow in A). Calibration as for B. D Double-labeling of sections from the hilus of a pilocarpine-treated rat with recurrent seizures demonstrates that numerous PROX1-immunoreactive nuclei are present (small arrows), but only one is double-labeled with calbindin (orange, large arrow), suggesting that PROX1 is the more reliable marker of mature granule cells than calbindin in rats with chronic seizures. Calibration (in A) = 5 μm.
After seizures, the reliance on calbindin as a marker of granule cells is difficult not only for the reasons discussed above, that its presence in granule cells appears to decline, but it also appears to decline inconsistently, so that some granule cells retain calbindin expression levels, but others lose it entirely (fig. 1). Moreover, in other cell types that do not normally express calbindin, it may increase. The increase in calbindin immunoreactivity appears to occur primarily in a subpopulation of hilar neurons after pilocarpine-induced status epilepticus (specifically when status is curtailed after 1 h by the anticonvulsant diazepam; for more detailed methods, see Scharfman et al. [2000, 2001]). Figure 1 shows a representative section from a rat that had pilocarpine-induced status epilepticus, developed recurrent spontaneous seizures in the following weeks, and was perfusion fixed 2 months after status. Calbindin expression was distinct from a saline-treated, age-matched control (fig. 1). First, there were numerous ‘patches’ of decreased calbindin immunoreactivity in the granule cell layer (fig. 1). Second, large hilar neurons were intensely reactive, which was not apparent in control tissue (fig. 1). Third, numerous small, granule-like neurons that express calbindin were present in the hilus (fig. 1). The latter have been described: they are ectopic hilar granule cells that were born after seizures and migrated incorrectly [Parent et al., 1997, 2006; Scharfman et al., 2000; Scharfman, 2004].
The second marker of granule cells, PROX1, appears to be a superior marker relative to calbindin, because no other dentate gyrus neuronal cell types have been reported to stain positively using antibodies to PROX1. However, it can be argued that PROX1 is imperfect, because under some conditions, namely status epilepticus, PROX1 expression of granule cells may decline [Elliott et al., 2001].
Physiological Differentiation of Granule Cells
Physiological recordings are another way to identify granule cells from other neuronal subtypes in the dentate gyrus. In the hilus, these differences are important, because many cell types are present: the mossy cells, various GABAergic neurons, as well as ectopic granule cells. They can be easily confused because, for example, the shape and size of the granule cell soma are similar to some other types of hilar neurons (GABAergic neurons). In addition to common hilar neuronal cell types, there can also be stray CA3 pyramidal cells that are located in the hilus, close to the tip of the CA3 pyramidal cell layer.
Using ‘sharp’ microelectrodes, the problem can be resolved because each type of neuronal cells is distinguishable. Thus, there are several characteristics of granule cells that distinguish them from the other cell types in the hilus [Scharfman, 1992, 1999]. Interestingly, patch electrodes are less useful, because some distinguishing characteristics detected with sharp electrodes are homogeneous when patch electrodes are used [Lübke et al., 1998]. Patch electrodes probably create a more homogeneous view of cellular physiology because the solution used to fill the patch pipette is more influential on the characteristics of the cell than it is when sharp electrodes are used. Indeed, spike frequency adaptation is not as distinct when comparing hilar cells if patch electrodes are used relative to sharp electrodes [Lübke et al., 1998; Scharfman, 1992, 1995, 1999; Scharfman et al., 2000]. Therefore, sharp microelectrodes are more useful in detecting whether an impaled cell is a granule cell on the basis of electrical recordings. However, it is important to point out that differentiation of cell types using sharp electrodes requires optimal recordings: all dentate gyrus neurons, including granule cells, become more homogeneous if not impaled well (i.e., input resistance is below 50 MΩ). Therefore, strict criteria must be used to sample cells, such as resting potentials over −65 mV for granule cells, overshooting action potentials, input resistance over 50 MΩ at resting potential.
Characteristics that are useful in distinguishing granule cells include the resting potential, because granule cells maintain a high (very negative) resting membrane potential [Staley et al., 1992]. Although the concentration of extracellular K+ alters the resting potential, and many investigators use slightly different concentrations in their extracellular buffer, the range that is commonly used (3–5 mM K+) leads to a very hyperpolarized granule cell resting potential relative to the other neurons in the dentate gyrus. If [K+]o is 5 mM, granule cell resting potential is approximately −70 mV, whereas other hilar cells exhibit resting potentials that are closer to −60 mV [Scharfman, 1992, 1999]. Granule cells also have a relatively short time constant, like many of the GABAergic interneurons, but can be distinguished from the interneurons by spike frequency adaptation, action potential slope/width, and different phases and kinetics of afterhyperpolarizations following their action potentials [Scharfman, 1995]. In contrast, most mossy cells and pyramidal cells, the other cell types one might encounter with microelectrodes in the hilar region, have extremely long time constants, discharge with irregular patterns (mossy cells) [Scharfman and Schwartzkroin, 1988] or bursts upon a triangular depolarizing envelope (CA3 pyramidal cells) [Scharfman, 1993], have distinct afterhyperpolarizations, and many other characteristics unlike granule cells [Scharfman, 1992, 1999].
The Mossy Fiber as the Defining Feature of a Granule Cell
Unfortunately, as mentioned above, physiological methods are not always possible to use in evaluating granule cells, or simply inconvenient, making another approach imperative for many studies of ectopic granule cells. In addition, high-quality recordings are usually difficult unless young animals are used, i.e., at postnatal days 15–30. For these reasons, another option besides recordings is important to consider.
Fortuitously, there is one feature of granule cells that appears to be universal to them and one that does not require electrophysiological characterization. This is the presence of a unique axon, the mossy fiber axon. The unique features of this axon include not only the large terminal size, as mentioned above, but also its projection and other characteristics. The projection is to the stratum lucidum of area CA3, and there are also collaterals throughout the hilus [Claiborne et al., 1986]. Within the stratum lucidum, the axon makes a straight course that parallels the cell layer of area CA3 pyramidal cells, until it ends at the border of area CA3 and area CA2. Along this route, periodic large and complex expansions are present, which have intricate structural relationships with the thorny excrescences of the proximal dendrites of CA3 pyramidal cells [Blackstad and Kjaerheim, 1961; Hamlyn, 1962; Claiborne et al., 1986]. These boutons are extremely large in size, packed with vesicles, and possess filamentous extensions with varicosities [Amaral, 1979; Acsady, 2000]. The size, extensions, and ultrastructure of these boutons are unlike any other cell type in the hippocampus. In summary, one can argue that the best characteristic to use to define a granule cell is its mossy fiber axon.
On the Development and Function of Ectopic Hilar Granule Cells
Development of Normal Ectopic Granule Cells
There could be many reasons why ectopic granule cells develop in a normal brain. It may be that they are simply outliers in a normal process, i.e., some animals have slightly more or less of the molecular cues that coordinate migration and termination, such as reelin [Forster et al., 2006]. One important question that seems fundamental to our understanding of the development of ectopic hilar granule cells is whether they develop in the subgranular zone and migrate into the hilus [Parent et al., 2006]. This view has been supported by studies of ectopic hilar granule cells after status epilepticus, which appear to migrate into the hilus along glial processes [Parent et al., 2006]. A second hypothesis is that ectopic granule cells develop in the tertiary matrix of the hilus and fail to migrate to the granule cell layer. Thus, it has been suggested that hilar ectopic granule cells may develop abnormally late, and be ‘trapped’ in the hilus due to the constraints that develop in the rapidly changing hilar environment [Bayer, 1980; Gaarskjaer and Laurberg, 1983]. A third hypothesis for the development of hilar ectopic granule cells, specifically those that occur after severe seizures, is that the ectopic cells are derived from blood-borne precursors that pass through breaks in the blood-brain barrier occurring during seizures. The fact that the hilus is richly vascularized may be the reason this leads to ectopic hilar cells, rather than cells located in another region. The hilus may also provide ‘a vascular niche’ [Palmer et al., 2000].
Function of Normal Ectopic Granule Cells
The functional implications of ectopic granule cells that develop in the normal brain have not been elucidated in detail as yet. However, studies of hilar ectopic granule cells in hippocampal slices of normal adult rats have provided some initial insights. In these experiments, ectopic cells were sampled that were close to the border of the granule cell layer and the hilus, as well as deep within the hilus (i.e., near the border of the hilus with area CA3) [Scharfman et al., 2000, 2003]. These neurons were similar to granule cells in the granule cell layer, at least in terms of their intrinsic properties (fig. 3). In addition, their response to electrical stimulation of the outer molecular layer elicited a composite synaptic potential that resembled that of granule cells located in the granule cell layer (fig. 3). However, the results of intracellular dye injection showed that they were morphologically unlike granule cells in the granule cell layer in at least two ways: (1) they possessed basal dendrites (fig. 3), and (2) they often possessed an unusually thick primary dendrite (fig. 3).
Fig. 3.
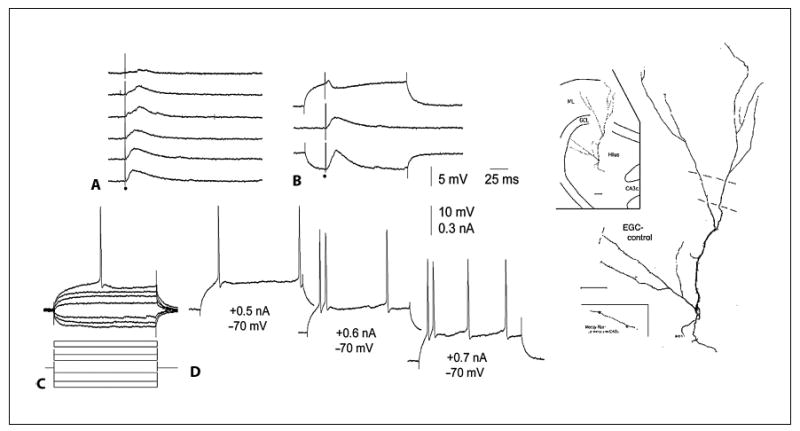
Characteristics of ectopic hilar granule cells in the normal rat. A Synaptic responses to outer molecular layer stimulation of the cell shown on the far right, an ectopic granule cell located in the deep hilus of a normal adult rat. B Synaptic responses at different holding potentials illustrate the common synaptic response of a granule cell to molecular layer stimulation in vitro. C, D Intracellular current injection evoked responses in the ectopic cell that were similar to granule cells in the granule cell layer. For further details, see Scharfman et al. [2003]. Reprinted from Neuroscience. EGC = Ectopic granule cell.
Taken together, the data accumulated to date suggest that granule cells located in the hilus in the normal dentate gyrus are able to function very much like a normal adult granule cell that is located in the correct place, i.e., the granule cell layer. Therefore, ectopic granule cells may simply add to the population of granule cells that normally resides in the layer. Yet several questions remain to be addressed, and suggest caution before concluding that ectopic hilar granule cells simply function as additional granule cells. One important question is whether ectopic hilar granule cells in the normal dentate gyrus develop inputs or synaptic connections that are atypical of a granule cell, and more like a normal hilar cell (i.e., GABAergic interneurons, mossy cells). In addition, very little is understood about ectopic granule cell plasticity, expression of substances specific to the normal granule cell (e.g. opiates, zinc), and responses to experimental interventions. Thus, more information will be necessary before clarifying the degree of similarity of ectopic hilar granule cells to granule cells of the granule cell layer in the normal adult rat, and the functional implications of ectopic hilar granule cells in the normal dentate gyrus network.
Increased Ectopic Granule Cells after Experimental Manipulations that Increase Neurogenesis
Numerous studies to date have documented the wide range of manipulations that can increase neurogenesis. These include exercise, altered diet, growth factors, modulation of serotoninergic systems, reproductive or adrenal steroids, as well as pathological conditions such as traumatic injury, hypoxia/ischemia, and seizure induction [for a review, see Cameron et al., 1998; Kempermann, 2005]. Given this long list, it is surprising that there are few reports of ectopic granule cells. One would expect that with increased adult neurogenesis, some neurons would not obtain the correct cues for migration, and migrate ectopically. The lack of reports could be due to the focus of most investigators on the granule cell layer rather than other locations, or it could be that, remarkably, most granule cells born in the adult brain are provided all the cues necessary and sufficient to support correct migration.
What experimental approaches have reported an increase in ectopic granule cells? Most of these involve growth factor delivery or increased growth factor expression. For example, infusion of insulin-like growth factor led to increased neurogenesis and evidence of numerous granule-like hilar profiles [Åberg et al., 2000; Lichtenwalner et al., 2001]. VEGF infusion also led to increased neurogenesis and evidence of hilar granule cells [Jin et al., 2002], although experiments did not address directly whether the hilar cells were granule cells. BDNF infusion, which increased neurogenesis, also led to ectopic hilar granule cells [Scharfman et al., 2005]. Developmental disorders also appear to foster ectopic granule cells. Thus, in the reeler mouse [Stanfield and Cowan, 1979], the p35 knockout mouse [Patel et al., 2004] and the Pcmt1 knockout mouse [Farrar et al., 2005], there are increased ectopic granule cells.
Perhaps the most robust means to generate ectopic hilar granule cells is seizures. In particular, status epilepticus (defined here as severe, continuous seizures) is very effective [Parent et al., 1997; Scharfman et al., 2000]. Status can be induced by many methods (explained in more detail below), and lead to numerous ectopic granule cells [Parent et al., 1997; Scharfman et al., 2000; Jung et al., 2004; Mohapel et al., 2004].
Interestingly, seizures that are not severe, or seizures that are not continuous, could influence ectopic granule cell development, even if they are not as powerful a stimulus to produce ectopic granule cells as status epilepticus. The reason to suggest this is because the p35 knockout mouse and Pcmt1 knockout mouse have intermittent seizures, and the reeler mouse has increased excitability [Patrylo et al., 2006]. These are the knockouts that develop ectopic granule cells, so the phenotype may depend on the developmental defect and intermittent seizures (presumably due to altered circuitry resulting from the defect). However, the role of the intermittent seizures and the role of the developmental defect are difficult to tease apart. In the adult, intermittent seizures do not appear to initiate ectopic granule cell formation, because animals that have had numerous individual kindled seizures do not develop substantial numbers of ectopic granule cells [Scharfman and Goodman, unpublished].
It is notable that the robust ability of severe seizures to initiate ectopic granule cell formation is not as simple as one might think. There may not be a linear relationship between the severity of seizures and ectopic granule cell number. Thus, status epilepticus that is prolonged beyond 1 h appears to promote death of new neurons [Mohapel et al., 2004]. This result suggests a balance between the ability of severe seizures to increase proliferation, and the fact that severe seizures increase death. Death of neurons appears to escalate as status epilepticus increases in duration. This may reflect a permissive influence of robust neuronal activity, balanced by the negative impact of seizure-induced energy depletion, and hypoxia.
The permissive effect of increased neuronal activity may be due to the increase in growth factor expression in the dentate gyrus, a robust effect of seizures [for a review, see Scharfman, 2006]. Status may also change the expression of the molecules thought to control migration of granule cells. One of these is reelin, normally a ‘stop’ signal for granule cells, keeping them from migrating beyond the granule cell layer [Frotscher, 1997]. In animal models of epilepsy, as well as humans with intractable temporal lobe epilepsy, reelin expression diminishes [Haas et al., 2002]. Thus, status may facilitate neuroproliferation by increasing growth factors that normally stimulate proliferation. In addition, status may change chemotactic factors, and these changes might lead to abnormal migration of newly born cells [Bagri et al., 2002; Lu et al., 2002; Minami et al., 2002; Scharfman, 2006].
Other seizure-induced changes in the dentate gyrus may also be involved in ectopic granule cell formation. Seizures have been reported to delay maturation [Overstreet-Wadiche et al., 2006] and delay proliferation of newly born granule cells, and these delays may influence their migration. Thus, it was found that induction of seizures after treatment with the convulsant kainic acid did not alter the normal turnover of nestin-immunoreactive precursors of granule cells, but did delay the maturation of type 3 precursors, which normally express doublecortin [Jessberger et al., 2005]. Doublecortin expression could be key, because it ordinarily is a critical element in the normal migration of cortical neurons during development. By perturbing the stage in development when doublecortin is expressed, migration may become perturbed itself. Other studies have defined alternate potential factors that could contribute to aberrant migration, such as changes in the proliferaton of radial glia [Huttmann et al., 2003]. These could stimulate an unusual relationship with newly born granule cells, as hypothesized elsewhere [Shapiro et al., 2005]. Proinflammatory cytokines may play a role in ectopic granule cell formation, because after seizures there is an increase in cytokines in response to seizure-induced damage, and some members of the proinflammatory cytokine family influence dentate gyrus neurogenesis [Monje et al., 2003].
The Characteristics of Ectopic Granule Cells after Experimental Status Epilepticus in the Rat
Comparison of Results from Different Animal Models of Status Epilepticus
The first study of seizure-induced ectopic granule cells was conducted by Parent et al. [1997], who used the muscarinic agonist pilocarpine to initiate status epilepticus. Status was then diminished in its severity and length by administration of the anticonvulsant. It was reported that the majority of neurons were born within the first weeks after status, and a number were present in the hilus or molecular layer, i.e., in ectopic locations.
Subsequent studies of ectopic granule cells used pilocarpine to induce status, or kainic acid [Scharfman et al., 2000]. In these experiments, status was decreased by diazepam administration after 1 h. This led to the appearance of robust numbers of hilar ectopic granule cells, but there were few ectopic granule cells in the molecular layer. Why were fewer ectopic cells in the molecular layer than in previous studies? It is possible that the shorter delay before diazepam was administered (1 vs. several hours after the onset of status) could have influenced the location of ectopic granule cells. This might occur if there was less seizure-induced damage, leading to fewer changes in reelin, for example.
Lithium-pilocarpine has also been used to induce status and examine hilar ectopic granule cells [Jung et al., 2004]. Finally, electrical stimulation has been used to elicit status epilepticus, and the results have indicated that this method can also produce robust numbers of ectopic hilar granule cells [Mohapel et al., 2004].
Distribution
In our laboratory, ectopic hilar granule cells that develop after pilocarpine-induced status have been studied after a few weeks, when spontaneous seizures recur regularly. Studies of animals that were examined from 1 month to 18 months after status show little change over time using manual counts [Scharfman et al., 2000] or stereological methods [McCloskey et al., 2005]. Therefore, ectopic granule cells which are born after status appear to survive for a long period of time, and they appear to be a stable population. The caveat to this conclusion is that there may be effects of intermittent recurrent seizures that have not been detected. For example, after a single spontaneous seizure, there may be transient increases in neurogenesis and ectopic granule cells may form. They may not survive for long periods of time, which would lead to the result that the population appears to remain stable over months. The problem with this hypothesis is that individual kindled seizures do not appear to initiate ectopic granule cell formation in the adult rat (discussed above). In addition, neurogenesis has been shown to decline during the period of chronic recurrent seizures that follows status epilepticus [Hattiangady et al., 2004]. However, proliferation remains high in those animals with very frequent spontaneous seizures [Cha et al., 2004]. More experiments will be required to determine whether such increases in proliferation would be followed by ectopic granule cell formation. If so, there could be dynamic fluctuations in ectopic granule cells over time, but no substantial net change in the population, at least in animals with frequent spontaneous seizures.
The distribution of the ectopic hilar granule cells is very interesting because it can be homogeneous, with evenly scattered cells throughout the hilus (usually temporally), and it can occur in clusters (usually septally). However, few septotemporal differences are consistent across all animals [McCloskey et al., 2005]. One of the most robust characteristics is that few cells develop in the CA3 cell layer, although ectopic granule cells often cluster at the border of the hilus and CA3 (fig. 2), suggesting a structural or chemical barrier that prevents their migration into the CA3 subfield.
Morphology
Ectopic hilar granule cells are widely varied in the orientation of their dendrites [Scharfman et al., 2003]. Remarkably, there are abnormalities in dendritic organization whether the hilar ectopic granule cell is present in a normal animal or one that had a history of status epilepticus and recurrent seizures. Thus, one of the proximal dendrites is often relatively thick compared to normal granule cell dendrites [Dashtipour et al., 2001]. Basal dendrites are almost always present [Spigelman et al., 1998; Scharfman et al., 2000, 2003]. The mossy fibers innervate dendrites more densely than normal granule cells [Dashtipour et al., 2001; Pierce et al., 2005].
In contrast to the dendrites, the axons of ectopic granule cells are remarkably similar to normal granule cells located in the granule cell layer, i.e., they demonstrate a classic mossy fiber axon. Thus, the axon has numerous collaterals in the hilus, and a major projection that follows the stratum lucidum until it ends, as well as mossy fiber boutons that are similar to those described in classic studies of granule cells from the granule cell layer [Blackstad and Kjaerheim, 1961; Hamlyn, 1962; Claiborne et al., 1986]. The regular periodicity of mossy fiber boutons in the stratum lucidum, the variation within the hilus of both small and large boutons, and numerous other details of the normal mossy fiber pathway are also characteristic of the hilar ectopic granule cell axons [Scharfman et al., 2000]. Furthermore, all ectopic granule cells that have been injected intracellularly, so that their axon is visible, demonstrate collaterals in the inner molecular layer [Scharfman et al., 2000], similar to other granule cells in epileptic tissue.
Thus, ectopic hilar granule cells appear to make errors in maturing normally with respect to their dendritic tree, but this does not appear to be the case with respect to their axon. This remarkable contrast is potentially informative: it suggests that the factors controlling dendrites are distinct from axons, at least for granule cells. Possibly the axon simply is able to find its correct path because it is thin and can meander through the mature neuropil better than the larger dendritic processes. Another factor may be that dendrites require normal signals or afferent input from fibers in the molecular layer. Consistent with this hypothesis, the ectopic hilar granule cells closest to the molecular layer (proximal to the granule cell layer in the location of their soma) develop dendrites that are much more like normal granule cells than ectopic hilar granule cells with somata that are far from the molecular layer [Scharfman et al., 2003].
Physiology
Intrinsic Characteristics and Firing Behavior
Ectopic granule cells have been studied electrophysiologically from 1 month to 8 months after status epilepticus, a time when initial responses to status epilepticus have waned, such as seizure-induced damage. It is thought to be a period when ongoing changes in response to status have peaked and are relatively stable. The majority of seizure-induced neurogenesis is thought to occur much earlier, in the initial week or two after status epilepticus [Parent et al., 1997]. Indeed, ectopic granule cells sampled 1 month or more after status appear to be mature neurons. The impression of maturity is based on their intrinsic properties, which are difficult to distinguish from a normal adult granule cell [Scharfman et al., 2000]. Morphology also appears mature because the dendrites intensely spine, axons are elaborate, and no growth cones are evident at the tips of any of the processes [Scharfman et al., 2000, 2003].
These ectopic granule cells have only been sampled from the hilus, because this is where the majority develop. Notably, the ectopic hilar granule cells seem similar in their membrane properties and firing behavior to cells that are located ectopically in the hilus of the normal adult rat [Scharfman et al., 2003]. Thus, membrane properties such as resting potential, input resistance, time constant, and characteristics of the action potential (duration, slope, amplitude) are not statistically distinct [Scharfman et al., 2000]. Firing behavior, defined by the pattern of action potential discharge in response to direct current injection, is also difficult to distinguish [Scharfman et al., 2000, 2003]. These data suggest that spontaneous seizures seem to do little to influence this aspect of physiology, which is surprising given the fact that status epilepticus changes many aspects of the dentate gyrus structure and function. It is also remarkable because it suggests that such characteristics are ‘hard-wired’, i.e., granule cells that are located in abnormal positions might develop according to an inherent program, rather than an influence from their immediate environment. However, all biophysical analyses of ectopic granule cells have not been conducted, and further studies may reveal distinctions.
Synaptic and Network Properties
In contrast to intrinsic properties, differences between ectopic granule cells and granule cells of the granule cell layer have been apparent when synaptic or network activity has been examined. When there are differences, ectopic hilar granule cells behave more like their neighbors in the hilus than cells located in the granule cell layer. For example, after a spontaneous seizure, which normally leads to c-fos expression in neurons that were active during the seizure, c-fos expression in ectopic granule cells and hilar cells developed when c-fos expression was low in the granule cell layer [Scharfman et al., 2002].
Other distinctions have been elucidated by intracellular recordings that have compared ectopic and normally located granule cells in slices from rats with chronic seizures. Some ectopic granule cells had unusually high levels of spontaneous synaptic activity (fig. 4), presumably reflecting a higher degree of mossy fiber input than occurs in the normally situated granule cell [Dashtipour et al., 2001; Pierce et al., 2005]. This high degree of spontaneous activity is similar to the high level of spontaneous depolarizations previous studies have shown in hilar cells, such as the mossy cell [Scharfman and Schwartzkroin, 1988; Scharfman, 1992].
Fig. 4.
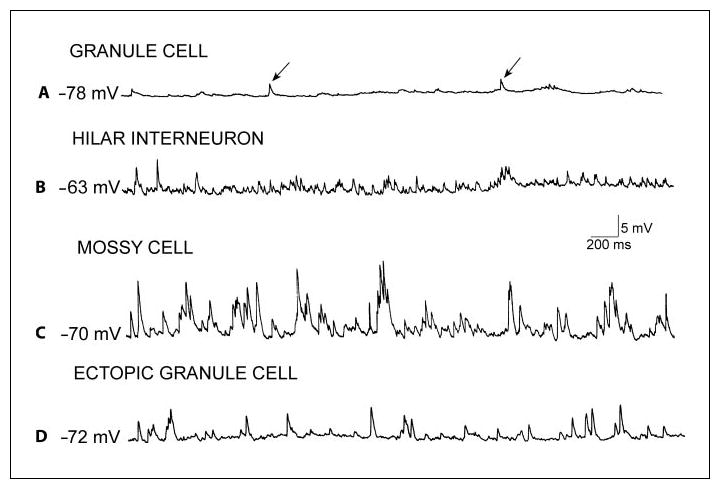
Comparison of spontaneous synaptic activity in neurons recorded intracellularly in hippocampal slices of rats with spontaneous recurrent seizures. A Representative recording from a granule cell located in the granule cell layer at a hyperpolarized potential shows spontaneous depolarizations (arrows), which are likely to be IPSPs, because they reversed polarity at approximately −70 mV (not shown), typical of GABAergic IPSPs under these recording conditions. B Example of spontaneous activity in a GABAergic interneuron located in the hilar region. C Spontaneous activity in a mossy cell demonstrates the typical high frequency and large amplitude of spontaneous depolarizations recorded in these cells. D A recording from an ectopic granule cell shows a higher frequency and amplitude of spontaneous events relative to granule cells located in the normal position (compare to A). The spontaneous potentials are likely to be a mixture of IPSPs and EPSPs, because of their reversal potentials (not shown). Thus, some events reverse at −70 mV, like IPSPs mediated by Cl−, and the others reverse at much more depolarized potentials, like EPSPs.
Interestingly, the similarity to hilar neurons seems to increase with the distance of the ectopic cell body from the granule cell layer. Thus, when the ectopic granule cell is close to the cell layer, it has a well-developed dendritic arborization in the molecular layer, like granule cells located in the granule cell layer [Scharfman et al., 2003]. In contrast, ectopic granule cells located closer to area CA3 have a poorly developed dendritic tree in the molecular layer. The ectopic granule cells located close to the cell layer have a similar response to perforant path input as granule cells sampled from the cell layer [Scharfman et al., 2003], but ectopic granule cells that are located deep in the hilus have delayed synaptic responses [Scharfman et al., 2003]. The delay is consistent with a lack of perforant path input and instead, disynaptic activation, via normally located granule cells [Scharfman et al., 2003]. The delay makes the ectopic granule cells near CA3 seem more like hilar neurons, or even CA3 neurons, than a granule cell.
Perhaps the most dramatic distinction between ectopic and normal granule cell physiology is the regular burst discharges that have been observed in ectopic granule cells, but not normally situated granule cells (fig. 5). These epileptiform bursts occur only in slices from animals with recurrent seizures, presumably reflecting the epileptic condition. Remarkably, all ectopic granule cells recorded to date have been found to discharge synchronously with these epileptiform bursts of CA3 neurons. Mossy cells and hilar interneurons also do [Scharfman et al., 2001], suggesting that the ectopic granule cell behaves like its neighbors in the hilus. However, granule cells in the granule cell layer do not exhibit these burst discharges, so the ectopic hilar cells are distinct from granule cells located in the granule cell layer, at least in this respect (fig. 5). Notably, many of the granule cells of the granule cell layer exhibit depolarizations long after burst discharges of other cell types [Scharfman et al., 2003], although this was not the case for those granule cells that were sampled in the slice from which the recordings in figure 5 were made. This is important because it suggests that the burst discharges may relay excitation to the granule cell layer in some conditions.
Fig. 5.
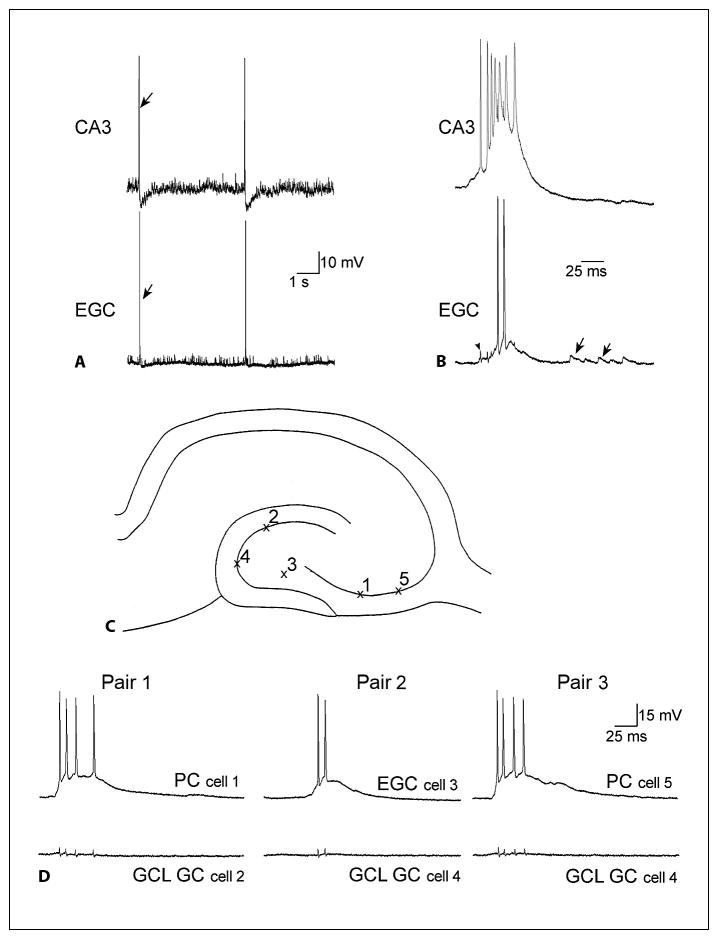
Abnormal synchronized bursts of ectopic hilar granule cells with CA3 neurons, recorded in hippocampal slices from rats with spontaneous recurrent seizures. A A continuous recording from a CA3 pyramidal cell (top, CA3) and an ectopic granule cell (bottom, EGC) in a slice from a rat that had spontaneous recurrent seizures. There are two spontaneous bursts of action potentials that are synchronized. The arrows point to one of the synchronized events. B One of the synchronous events in A is shown with higher temporal resolution. The arrowhead marks the capacitative artifact of the CA3 pyramidal cell’s first action potential in the recording of the ectopic granule cell. It shows that the first action potential of CA3 occurs at the onset of the depolarization in the ectopic granule cell. The arrows denote spontaneous synaptic depolarizations. C The schematic illustrates recording positions for D. D Three consecutively recorded pairs of simultaneous intracellular recordings are shown. Recordings were from the same slice, from a rat that had status epilepticus and spontaneous, recurrent seizures. Left: the first pair of cells to be recorded simultaneously was a CA3 pyramidal cell (top, PC) and a granule cell (GC) of the granule cell layer (bottom, GCL). The CA3 recording shows the intracellular correlate of the population burst discharge: a large depolarization with several action potentials at its peak. There was no activity in the simultaneously recorded granule cell when the CA3 pyramidal cell had the spontaneous depolarization and discharge of action potentials. This was true at the potential of the recording, −70 mV, and other membrane potentials between −50 and −80 mV (data not shown). There are four small deflections in the granule cell recording that do not reflect activity, but are the capacitative artifacts of the action potentials in the simultaneous CA3 pyramidal cell recording. Center: the second pair of cells included an ectopic hilar granule cell (EGC) and a second granule cell (GC) from the granule cell layer (GCL), which were both impaled after the first two cells shown at left. The neuron from the granule cell layer was silent when there was a burst discharge that occurred in the ectopic granule cell. Right: another CA3 pyramidal cell (PC) was impaled subsequent to the ectopic granule cell shown in the center, while maintaining the impalement of the granule cell (GC) in the granule cell layer (GCL) shown in the center. During the spontaneous burst discharge of the CA3 neuron, the granule cell demonstrated little activity. These recordings demonstrate that during the spontaneous discharges of CA3 and hilar ectopic neurons, there is no evidence of synchronized burst discharges in the granule cells that are located in the normal position, the granule cell layer. Note, however, that there can be subthreshold depolarizations that follow the burst after 5–25 ms, suggesting that recurrent excitatory circuits may activate granule cells after the CA3-ectopic bursts occur. For further discussion, see Scharfman et al. [2000, 2003], and Scharfman [2004].
The burst discharges of CA3 neurons are presumably driven by EPSPs, because AMPA receptor antagonists block them (n = 3, 10 μM CNQX; n = 1, 10 μM NBQX) [Scharfman et al., unpublished], the GABAA antagonist bicuculline prolongs them (n = 10; 10μM) [Scharfman and McCloskey, unpublished], and because they trigger action potentials at their peak. The depolarizations are rhythmic, although the frequencies are not all the same (from 0.05 to 0.3 Hz), and there are highly variable numbers of action potentials (typically 3–5 in ectopic hilar granule cells, more in CA3 neurons; fig. 5C). Because simultaneous intracellular recordings showed that CA3 burst discharges preceded the bursts of ectopic hilar cells, as well as bursts of other hilar cells [Scharfman et al., 2000, 2001], CA3 neurons are likely to initiate the epileptiform activity. In support of this hypothesis, ectopic granule cells do not exhibit burst discharges when a slice from a rat with chronic seizures fails to demonstrate regular burst discharges in CA3 neurons [Scharfman et al., 2000].
What is the reason for the close synchrony between CA3 neuron and ectopic granule cell burst discharges? Clues have come from simultaneous intracellular recordings, which showed that there can be just 1–2 ms between the onsets of CA3 and ectopic burst discharges. This delay is similar to a single synaptic delay, and suggests that CA3 pyramidal cells may develop synaptic connections with ectopic hilar granule cells. Indeed, CA3 pyramidal cells normally extend axon collaterals deep into the hilus [Ishizuka et al., 1990; Li et al., 1994], and make monosynaptic connections with hilar cells [Scharfman, 1994a]. When a slice from a normal adult rat is disinhibited with an antagonist of GABAA receptors, all hilar cells develop burst discharges that follow CA3 bursts by a few milliseconds [Scharfman, 1994b, c]. Taken together, these data suggest that CA3 can normally excite hilar neurons, and burst discharges can occur after disinhibition. In the epileptic animal, new cells in the hilus may join this burst activity, and this might depend on the ability of CA3 neurons to develop new synaptic connections with the ectopic granule cells. Such new synapses may occur in response to the loss of some hilar neurons after status epilepticus, because the loss of the normal targets of CA3 pyramidal cell axons might stimulate the axons to find new targets, and the closest neurons available would include new ectopic granule cells. Indeed, newly born ectopic granule cells may be a likely target if they have not yet been innervated densely by other afferents, as one would expect of a new cell. In summary, a new, monosynaptic, glutamatergic pathway from CA3 neurons to ectopic hilar cells could explain the almost synchronous burst discharges in CA3 pyramidal cells and ectopic granule cells. Proof of this explanation will require more analyses of the structural and functional characteristics of the burst discharges.
In summary, studies about ectopic granule cells in the rat dentate gyrus have been rare, just as the ectopic neurons appear to be. There are still many questions to be addressed with respect to their characteristics and function under normal conditions. New interest in this area has been spawned by the demonstrations that there are many more ectopic granule cells after severe seizures, and recent studies have been able to determine much more about them under these conditions. The studies of normal and epileptic tissue have both provided important information about how ectopic granule cells may differ from normal granule cells because of their ectopic position. Future studies will be necessary to elucidate why they develop, what regulates their similarities and differences from normal granule cells, and their functional influence on the dentate gyrus and hippocampus.
Acknowledgments
This study was supported by NIH NS 41490, New York State Department of Health, the Epilepsy Foundation, and the Helen Hayes Foundation. We thank Karen L. Smith and Joseph P. Pierce for their past contributions.
References
- Åberg M, Åberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acsady L, Katona I, Martinez-Guijarro FJ, Buzsáki G, Freund TF. Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus. J Neurosci. 2000;20:6907–6919. doi: 10.1523/JNEUROSCI.20-18-06907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Amaral DG. Synaptic extensions from the mossy fibers of the fascia dentata. Anat Embryol. 1979;155:241–251. doi: 10.1007/BF00317638. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Woodward DJ. A hippocampal interneuron observed in the inferior region. Brain Res. 1977;124:225–236. doi: 10.1016/0006-8993(77)90881-2. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. 1. Neurogenesis examined with [3H]thymidine autoradiography. J Comp Neurol. 1980;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Blackstad TW, Kjaerheim A. Special axodendritic synapses in the hippocampal cortex: electron and light microscopic studies on the layer of mossy fibers. J Comp Neurol. 1961;117:133–159. doi: 10.1002/cne.901170202. [DOI] [PubMed] [Google Scholar]
- Boss ▪▪▪.
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- Cha ▪▪▪ 2004.
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Claiborne BM, Amaral DG, Cowan WM. Quantitative, three-dimensional analysis of granule cell dendrites in the rat dentate gyrus. J Comp Neurol. 1990;302:206–219. doi: 10.1002/cne.903020203. [DOI] [PubMed] [Google Scholar]
- Dashtipour K, Tran PH, Okazaki MM, Nadler JV, Ribak CE. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Res. 2001;890:261–271. doi: 10.1016/s0006-8993(00)03119-x. [DOI] [PubMed] [Google Scholar]
- Elliott RC, Khademi S, Pleasure SJ, Parent JM, Lowenstein DH. Differential regulation of basic helix-loop-helix mRNAs in the dentate gyrus following status epilepticus. Neuroscience. 2001;106:79–88. doi: 10.1016/s0306-4522(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Farrar CE, Huang CS, Clarke SG, Houser CR. Increased cell proliferation and granule cell number in the dentate gyrus of protein repair-deficient mice. J Comp Neurol. 2005;493:524–537. doi: 10.1002/cne.20780. [DOI] [PubMed] [Google Scholar]
- Forster E, Zhao S, Frotscher M. Laminating the hippocampus. Nat Rev Neurosci. 2006;7:259–268. doi: 10.1038/nrn1882. [DOI] [PubMed] [Google Scholar]
- Frotscher M. Dual role of Cajal-Retzius cells and reelin in cortical development. Cell Tissue Res. 1997;290:315–322. doi: 10.1007/s004410050936. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Seress L, Schwerdtfeger WK, Buhl E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J Comp Neurol. 1991;312:145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB, Laurberg S. Ectopic granule cells of hilus fasciae dentatae projecting to the ipsilateral region inferior of the rat hippocampus. Brain Res. 1983;274:11–16. doi: 10.1016/0006-8993(83)90516-4. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Wasterlain CG, Massarweh WF, Dean E, Sollas AL, Sloviter RS. Calbindin-D28k immunoreactivity and selective vulnerability to ischemia in the dentate gyrus of the developing rat. Brain Res. 1993;606:309–314. doi: 10.1016/0006-8993(93)90999-4. [DOI] [PubMed] [Google Scholar]
- Green EJ, Juraska JM. The dendritic morphology of hippocampal dentate granule cells varies with their position in the granule cell layer: a quantitative Golgi study. Exp Brain Res. 1985;59:582–586. doi: 10.1007/BF00261350. [DOI] [PubMed] [Google Scholar]
- Haas ▪▪▪ 2004.
- Hamlyn LH. The fine structure of the mossy fiber endings in the hippocampus of the rabbit. J Anat. 1962;6:112–120. [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhauser C, Gray WP. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci. 2003;18:2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Romer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol. 2005;196:342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Lin X, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ▪▪▪ 2004.
- Kempermann G. Adult Neurogenesis: Stem Cells and Neuronal Development in the Adult Brain. New York: Oxford University Press; 2005. [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intra-cerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbers K, Frotscher M. Fine structure and synaptic connections of identified neurons in the rat fascia dentata. Anat Embryol. 1987;177:1–14. doi: 10.1007/BF00325285. [DOI] [PubMed] [Google Scholar]
- Lübke J, Frotscher M, Spruston N. Specialized electrophysiological properties of anatomically identified neurons in the hilar region of the rat fascia dentata. J Neurophysiol. 1998;79:1518–1534. doi: 10.1152/jn.1998.79.3.1518. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Halasz P, Vajda J, Czirjak S, Freund TF. Loss of Calbindin-D28K immunoreactivity from dentate granule cells in human temporal lobe epilepsy. Neuroscience. 1997;76:377–385. doi: 10.1016/s0306-4522(96)00440-x. [DOI] [PubMed] [Google Scholar]
- Marti-Subirana A, Soriano E, Garcia-Verdugo JM. Morphological aspects of the ectopic granule-like cellular population in the albino rat hippocampal formation: a Golgi study. J Anat. 1986;144:31–47. [PMC free article] [PubMed] [Google Scholar]
- McCloskey DP, Hintz T, Pierce JP, Scharfman HE. Stereological estimation of the number of ectopic granule cells (EGCs) in the hilus following pilocarpine-induced status epilepticus (PILO) Epilepsia. 2005;46:295–296. [Google Scholar]
- Minami M, Maekawa K, Yamakuni H, Katayama T, Nakamura J, Satoh M. Kainic acid induces leukemia inhibitory factor mRNA expression in the rat brain: differences in the time course of mRNA expression between the dentate gyrus and pal CA1/CA3 subfields. Brain Res Mol Brain Res. 2002;107:39–46. doi: 10.1016/s0169-328x(02)00443-6. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Ekdahl CT, Lindvall O. Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiol Dis. 2004;15:196–205. doi: 10.1016/j.nbd.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Nagerl UV, Mody I, Jeub M, Lie AA, Elger CE, Beck H. Surviving granule cells of the sclerotic human hippocampus have reduced Ca2+ influx because of a loss of calbindin-D(28k) in temporal lobe epilepsy. J Neurosci. 2000;20:1831–1836. doi: 10.1523/JNEUROSCI.20-05-01831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nek N, Schwegler H, Crusio WE, Frotscher M. Are the fine-structural characteristics of mouse hippocampal mossy fiber synapses determined by the density of mossy fiber axons? Neurosci Lett. 1993;158:756–778. doi: 10.1016/0304-3940(93)90616-s. [DOI] [PubMed] [Google Scholar]
- Nitsch R, Leranth C. GABAergic neurons in the rat dentate gyrus are innervated by subcortical calretinin-containing afferents. J Comp Neurol. 1996;364:425–438. doi: 10.1002/(SICI)1096-9861(19960115)364:3<425::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliot RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel LS, Wenzel HJ, Schwartzkroin PA. Physiological and morphological characterization of dentate granule cells in the p35 knock-out mouse hippocampus: evidence for an epileptic circuit. J Neurosci. 2004;24:9005–9014. doi: 10.1523/JNEUROSCI.2943-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Browning RA, Cranick S. Reeler homozygous mice exhibit enhanced susceptibility to epileptiform activity. Epilepsia. 2006;47:257–266. doi: 10.1111/j.1528-1167.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Melton J, Punsoni M, McCloskey DP, Scharfman HE. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005;196:316–331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du système nerveux de l’homme et des vertébrés. Vol. 2 Paris: Maloine; 1911. Fourth-order olfactory areas: Ammons horn and the dentate gyrus; . [Google Scholar]
- Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Differentiation of rat dentate neurons by morphology and electrophysiology in hippocampal slices: granule cells, spiny hilar cells and aspiny ‘fast-spiking’ cells. Epilepsy Res Suppl. 1992;7:93–109. [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Spiny neurons of area CA3c in rat hippocampal slices have similar electrophysiological characteristics and synaptic responses despite morphological variation. Hippocampus. 1993;3:9–28. doi: 10.1002/hipo.450030103. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. EPSPs of dentate gyrus granule cells during epileptiform bursts of dentate hilar ‘mossy’ cells and area CA3 pyramidal cells in disinhibited rat hippocampal slices. J Neurosci. 1994a;14:6041–6057. doi: 10.1523/JNEUROSCI.14-10-06041.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Evidence from simultaneous intracellular recordings in rat hippocampal slices that area CA3 pyramidal cells innervate dentate hilar mossy cells. J Neurophysiol. 1994b;72:2167–2180. doi: 10.1152/jn.1994.72.5.2167. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Synchronization of area CA3 hippocampal pyramidal cells and non-granule cells of the dentate gyrus in bicuculline-treated rat hippocampal slices. Neuroscience. 1994c;59:245–257. doi: 10.1016/0306-4522(94)90593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Electrophysiological diversity of pyramidal-shaped neurons at the granule cell layer/hilus border of the rat dentate gyrus recorded in vitro. Hippocampus. 1995;5:287–305. doi: 10.1002/hipo.450050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The role of nonprincipal cells in dentate gyrus excitability and its relevance to animal models of epilepsy and temporal lobe epilepsy. In: Delgado-Esqueta AV, Wilson W, Olsen RW, Porter RJ, editors. Basic Mechanisms of the Epilepsies: Molecular and Cellular Approaches. New York: Lip-pincott-Raven; 1999. pp. 805–820. [PubMed] [Google Scholar]
- Scharfman HE. Functional implications of seizure-induced neurogenesis. Adv Exp Med Biol. 2004;548:192–212. doi: 10.1007/978-1-4757-6376-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Seizure-induced neurogenesis and its dependence on growth factors and cytokines. In: Binder DK, Scharfman HE, editors. Growth Factors and Epilepsy. New York: Nova Sciences; 2005. [Google Scholar]
- Scharfman HE, Goodman JH, MacLeod A, Phani S, Antonelli C, Croll SD. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Schwartzkroin PA. Electrophysiology of morphologically identified mossy cells of the dentate hilus recorded in guinea pig hippocampal slices. J Neurosci. 1988;8:3812–3821. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Smith KL, Goodman JH, Sollas AL. Survival of dentate hilar mossy cells after pilocarpine-induced seizures and their synchronized burst discharges with area CA3 pyramidal cells. Neuroscience. 2001;104:741–759. doi: 10.1016/s0306-4522(01)00132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AE, Berger RE, Goodman JH, Pierce JP. Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience. 2003;121:1017–1029. doi: 10.1016/s0306-4522(03)00481-0. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2002;111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Seress L, Mrzljak L. Basal dendrites of granule cells are normal features of the fetal and adult dentate gyrus of both monkey and human hippocampal formations. Brain Res. 1987;405:169–174. doi: 10.1016/0006-8993(87)91003-1. [DOI] [PubMed] [Google Scholar]
- Seress L, Pokorny J. Structure of the granular layer of the rat dentate gyrus. A light microscopic and Golgi study. J Anat. 1981;133:181–195. [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, Korn MJ, Shan Z, Ribak CE. GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res. 2005;1040:81–91. doi: 10.1016/j.brainres.2005.01.098. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE. Integration of newly born dentate granule cells into adult brains: hypotheses based on normal and epileptic rodents. Brain Res Rev. 2005;48:43–56. doi: 10.1016/j.brainresrev.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Yan XX, Obenaus A, Lee EY, Wasterlain CG, Ribak CE. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998;86:109–120. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, Cowan WM. The morphology of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979;185:393–422. doi: 10.1002/cne.901850302. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Otis TS, Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol. 1992;67:1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF. Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity of GABA and projection to the medial septum. Neuroscience. 1992;49:793–805. doi: 10.1016/0306-4522(92)90357-8. [DOI] [PubMed] [Google Scholar]
- Yan XX, Spigelman I, Tran PH, Ribak CE. Atypical features of rat dentate granule cells: recurrent basal dendrites and apical axons. Anat Embryol. 2001;203:203–209. doi: 10.1007/s004290000150. [DOI] [PubMed] [Google Scholar]


