Abstract
The nuclear internalization of biomolecules by Tat peptide provides a mechanism to deliver drugs to cells. However, translocation of molecular imaging probes to the nucleus may induce undesirable mutagenesis. To assess the feasibility of retaining its cell permeating effect without nuclear translocation, Tat-peptide was conjugated with a somatostatin receptor (STR)-avid ligand (Oct) and labeled with fluorescent dyes. The results show that Tat-Oct-5-FAM (fluorescein 5’-carboxylic acid) remained in the cytoplasm of STR-positive AR42J cells. Co-incubation of Tat-Oct-5-FAM with ATP induced nuclear translocation. These data suggest that both dye and Oct-STR endocytosis complex could modulate nuclear internalization of Tat peptides.
Keywords: Tat peptide, nuclear internalization, somatostatin receptor, bombesin peptide analogue, fluorescent probe
1. Introduction
Numerous studies have shown that the Tat protein basic domain residues 48–57 (GRKKRRQRRR) from human immunodeficiency virus (HIV-1) rapidly permeate plasma membranes and translocate into the nucleus [1–5]. This mechanism is currently used to deliver proteins and nucleic acids to cell nuclei through covalent linkages [2]. Similarly, conjugation of molecular probes such as 5-FAM [4] and green fluorescent protein (GFP) [3,5] with Tat peptides provides an opportunity to image intracellular processes by optical methods.
Recent advances in laser technology and image reconstruction algorithms have reignited interest in the use of optical imaging as a powerful method to diagnose pathologic conditions as well as complement existing imaging modalities [6–10]. The use of exogenous optical molecular probes can enhance the rapid visualization of pathologic tissues. In previous studies, we demonstrated that conjugating a somatostatin receptor-avid peptide (octreotate, Oct) with fluorescent dyes improves the specificity of imaging cancers in rodents by contrast agent-mediated optical imaging [11]. Targeting somatostatin receptor subtype 2 (STR2) is attractive because it is up-regulated in cancer cells and several clinical studies have successfully used STR-targeted radiopharmaceuticals to image various forms of tumors and pathologic conditions [12,13]. To increase the rate of tumor localization, we explored the effects of labeling a tandem Tat-Oct peptide conjugate with fluorescent dyes, which are useful for cellular and whole-body optical imaging studies. Unfortunately, Tat-peptides are known to translocate into cell nuclei, rendering this approach less attractive for imaging agents because of potential undesirable mutagenesis.
In this study, we prepared a pair of three groups of compounds and assessed their cellular uptake and localization in STR-positive and negative cell lines: (a) 5-FAM and Cypate2-Tat peptides, (b) Oct-5-FAM and Oct-Cypate2, and (c) Tat-Oct-5-FAM and Tat-Oct-Cypate2 conjugates. We also prepared bombesin (BN) receptor-avid peptide [14] to assess the effects of other secondary peptides on modulating the activity of Tat peptides. The results show that nearly all the Tat peptide conjugates internalized and localized rapidly in cellular nuclei but a careful choice of a dye and a secondary peptide can modulate the nuclear internalization of Tat peptides. Particularly, we found that Tat-Oct-Cypate2 promoted, but Tat-Oct-5-FAM inhibited, the nuclear internalization of the Tat peptide residue. Interestingly, addition of ATP to the culture medium can reestablish the internalization of the product in the cell nucleus. This finding is applicable to another receptor-avid bombesin peptide when conjugated to Tat peptide, suggesting the potential to use a variety of secondary peptides to alter the intracellular distribution of Tat peptides. Thus, the internalization of Tat peptides in the nucleus can be modulated by a combination of Oct and 5-FAM and the potential to reverse this process with ATP provides a strategy to selectively destroy target tissues by mutagenesis.
2. Materials and Methods
2.1. Synthesis of Probe Cypate2
A solution of triethyl orthoformate (0.6 g, 4.0 mmol) in acetonitrile (7.0 mL) was added dropwise into a stirred solution of 1,1,2-trimethyl[1H]-benz[e]indole-3-propanoic acid (0.72 g, 2.0 mmol) in pyridine (10.0 mL) under reflux for 2 h and concentrated. The resulting residue was triturated with dilute HCl (5%), filtered, washed with water, and dried to afford the desired product (0.61g, 93% yield). The observed molecular mass in electrospray ionization mass spectrum (ESI-MS) corresponded to the calculated [MH]+ value of 573.41.
2.2. Peptide Synthesis and conjugation with 5-FAM or Cypate2
All amino acids were purchased from Novabiochem (San Diego, CA, USA). The peptides were prepared on solid support by standard fluorenylmethyloxy (Fmoc) chemistry as described previously [15]. The tandem Tat-octreotate peptide was prepared by a one-pot synthetic strategy on an automated peptide synthesizer (ACT 396). Starting with threonine on Wang resin the tandem Tat-Oct peptide moieties were prepared from the C-terminus of Oct to the N-terminus of Tat 48–57 (Fig. 1). The linker lysine between the OCT and Tat peptide sequences was protected with the orthogonal N-α-1-(4,4-dimethyl-2,6-dixoxcyclohex-1-ylidene)ethyl-N-ε-Fmoc group (Dde-Lys(Fmoc)-OH) to allow the automated synthesis of the tandem peptide moieties using Fmoc strategy. After peptide synthesis, the Dde group was removed with 2% hydrazine in DMF before dye conjugation. The carboxyl groups of Cypate2 or 5-FAM (Molecular Probes, Inc., Eugene, OR, USA) were pre-activated with 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU)/N-hydroxybenzotriazole (HOBt) coupling reagent and coupled to the peptide on resin, as described previously [14]. A similar approach was used to prepare the analogous 5-FAM bombesin peptide analogues used in this study. Cleavage of the peptide-dye conjugate from the solid support and concomitant removal of the side-chain protecting groups with TFA afforded the desired products, which were purified by HPLC and characterized by ESI-MS and spectroscopic methods. Each of the molecular probes was characterized by absorption and fluorescence spectroscopy in 20% aqueous DMSO.
Fig. 1.
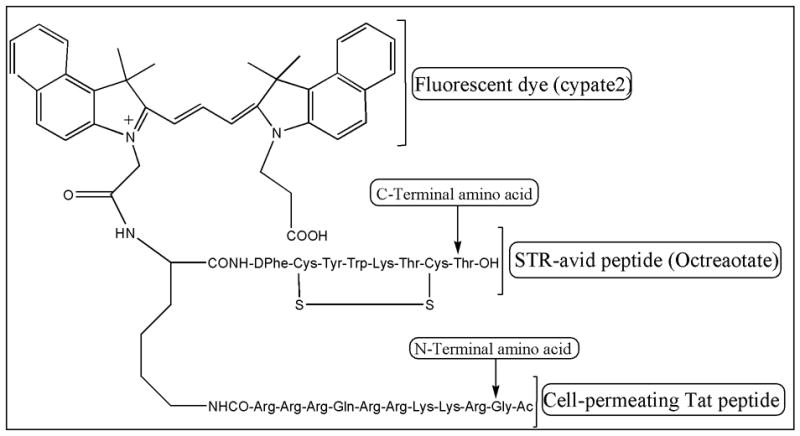
Structure of Tat-Oct-Cypate2. The fluorescent dye was replaced with 5-FAM in Tat-Oct-5-FAM and octreotate was replaced with bombesin peptide analogue (BN7-14) in Tat-BN-Cypate2. See text for details.
Cypate2-K(RRRQRRKKRG-Ac)-OH (Tat-Cypate2), ESI-MS: 693.8 [M+3H+]3+.
5-FAM-K(RRRQRRKKRG-Ac)-OH (Tat-5-FAM), ESI-MS: 942 [M+H+]2+.
Cypate2-K(Ac)-f-cyclo(C-Y-w-K-T-C)-T-OH (Oct-Cypate2), ESI-MS: 1604.5 [M+H+], 802.9 [M+H+]2+; f and w represent D-Phenylalanine and D-tyrosine, respectively.
5-FAM-K(Ac)-f-cyclo(C-Y-w-K-T-C)-T-OH (Oct-5-FAM), ESI-MS: 1407 [M+H+].
Cypate2-K(RRRQRRKKRG-Ac)-f-cyclo(C-Y-w-K-T-C)-T-OH (Tat-Oct-Cypate2), ESI-MS: 1577.3 [M+H+]2+; 1051.9 [M+3H+]3+.
5-FAM-K(RRRQRRKKRG-Ac)-f-cyclo(C-Y-w-K-T-C)-T-OH (Tat-Oct-5-FAM), ESI-MS: 2957 [M+H+]; 1478.4 [M+2H+]2+, 992.1 [M+3H+]3+
5-FAM-K(RRRQRRKKRG-Ac)-GSGGQWVAGHLM-NH2 (Tat-BN-5-FAM), ESI-MS: 2957 [M+H+]; 1478.4 [M+2H+]2+; 992.1 [M+3H+]3+.
5-Fam-Gly-Ser-Gly-Gly-Gln-Trp-Val-Ala-Gly-His-Leu-Met-NH2 (BN-5-FAM), ESI-MS: 1499 [M+H+]; 749 [M+2H+]2+.
2.3. Cell Culture and Internalization Studies
The STR-positive rat pancreatic carcinoma (AR42J) and the STR-negative human lung cancer (A549) cell lines were purchased from ATCC (Manassas, VA) and stored in 95% air/5% CO2 37 ºC. AR42J cells were cultured in Ham’s F-12K medium supplemented with 20% FBS, 1.5 mg/ml NaHCO3 and 2 mM L-glutamine. A549 cells were cultured in Ham’s F-12K medium supplemented with 10% FBS, 1.5 mg/ml NaHCO3 and 2 mM L-glutamine. Sub-culturing was performed in a solution of 0.25% trypsin/0.1% EDTA. STR-negative human embryonic kidney 293A (HEK 293) cells (Invitrogen, Carlsbad, CA) were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS). The cells (1 x 105 cells/well) were grown on LabTek 8-chamber slides (Nunc Inc. Rochester, NY) overnight prior to the experiment. All compounds were dissolved in 20% aqueous DMSO and diluted in 0.01 M PBS (pH 7.4) (Sigma, St. Louis, MO) to a concentration of 10 μM. Each compound (10 μM) was added to the cells in chambers to obtain final concentration of 1 μM and incubated at 37 ºC or 4 ºC. The samples were analyzed at different time points. For blocking studies, cells were pre-incubated in 1 μM of the corresponding unlabeled peptides for 3–4 h at 37 ºC before adding 1 μM of the molecular probes and incubated for another 15 h at 37 ºC. After washing (4X) with 0.01 M PBS (pH 7.4), cells were fixed with 4% paraformaldehyde for 30 min and treated with 0.1% Triton-X for 2 min at room temperature. This step was omitted for the live cell study. Subsequently, the cells were stained for 30 min with 1:2000 ToPro3 (Invitrogen, Carlsbad, CA) for fixed cells at room temperature, and 2 h for live cells at 37 ºC. Cells were mounted in 50% glycerol in 0.01 M PBS (pH 7.4), covered with coverslips and sealed with nail polish. Olympus confocal laser scanning microscope (FV1000) was used to analyze all samples.
2.4. Determination of molecular probe internalization by confocal microscopy
The internalization of Cypate2 labeled compounds was monitored by using 568 laser sources for excitation and monitored at 605–620 nm. The 5-FAM labeled compounds were excited at 488 nm and monitored at 510–550 nm. Internalization of the molecular probes in cells was observed at 0.5 h, 1 h, 2 h, 4 h, and 15 h incubation in fixed and live AR42J cells. The cells incubated with culture medium or Topro3 (nuclear dye) alone in 0.01M PBS buffer were used as control. The imaging parameters were kept constant between control and sample groups. A 60X water-merged objective lens was used for microscopy. For optical sectioning by confocal microscopy, a few cells were used to avoid color overlap of the different cells in X-Y and X-Z images. Z-axis dimension of each image was 5–6 μm and the X-Z images are presented as short stacks of three optical sections reconstructed with Olympus Fluoview software (v1.4a). Quantitation was performed by counting the number of cells with nuclear internalization of the molecular probes (Table 1) and measurement of fluorescence intensity in the nucleus and cytoplasm at different time points. For the kinetics experiment, three lines were drawn in the nucleus of different cells in each image and the mean fluorescence intensity of the lines was measured by the Fluoview software. The fluorescence intensity was normalized to control (5-FAM or Cypate2) at different time points.
Table 1.
The total cell number of the nuclear or/and cytoplasmic internalization of 1μM Tat or Oct peptides in AR42J cells.
| Total cell number (% cell number of nuclear internalization)
|
|||||
|---|---|---|---|---|---|
| Compounds | 0.5h | 1h | 2h | 4h | 15h |
| Tat-Cypate2 | 96(100) | 76(100) | 126(100) | 70(100) | 140(100) |
| Oct-Cypate2 | 105(69) | 80(89) | 77(100) | 100(92) | 92(97) |
| T-O-Cypate2 | 82(100) | 95(100) | 81(100) | 108(100) | 169(100) |
| T-5-FAM | 79(100) | 83(100) | 73(100) | 87(100) | 76(100) |
| O-5-FAM | 87(3) | 80(3) | 81(3) | 72(3) | 104(2) |
| T-O-5-FAM | 82(3) | 108(2) | 76(1) | 74(2) | 106(4) |
3. Results and discussion
3.1. Peptide design and synthesis
Tat peptide-mediated cell permeation is a viable method for intracellular delivery of drugs but the nonspecific uptake and translocation into cell nucleus may limit their use in molecular imaging. To overcome these problems, we assessed the feasibility of controlling the nuclear internalization of Tat peptides and their specific delivery to tumor cells by using a three-component molecular design. The new molecule consists of the decapeptide sequence of the HIV Tat protein for cell permeation, a STR-avid octapeptide sequence for specific cell uptake by STR-positive cells, and a fluorescent carbocyanine (Cypate2) or xanthene (5-FAM) dye to serve as the antenna for optical imaging. Analogues of these dyes (indocyanine green and fluorescein disodium salt) have been used in human patients, providing a strategy to incorporate findings with these molecular probes in human studies. A lysine residue was used to link the Tat and Oct peptide residues to obtain Tat-Oct-5-FAM and Tat-Oct-Cypate2. To evaluate the modulation of nuclear internalization of Tat-Oct tandem peptide and delineate the effects of the individual peptide components, we also prepared and labeled Tat peptide and Oct with 5-FAM and Cypate2. These compounds were used to assess the effects of each component. Similarly, bombesin receptor-avid octapeptide was conjugated to 5-FAM-Tat peptide to further evaluate the applicability of the nuclear internalization pattern to other molecular constructs. A representative structure (Tat-Oct-Cypate2) of the molecular probes is shown in Fig. 1. The absorption/emission maxima in 20% aqueous DMSO centered at 585/606 nm for Cypate2 and 498/522 nm for 5-FAM. The spectral profiles of the dyes were retained after conjugation to the peptides on solid support.
3.2. Endocytosis of molecular probes was not a fixation artifact
We studied the internalization profiles of the novel molecular probes in STR-positive tumor cells AR42J [16], STR-negative tumor cells A549 and STR-negative HEK 293 cells of non-tumor origin. The AR42J was used to assess STR–mediated internalization in tumor cells [17,18] and the A549 and HEK 293 cells served as negative controls for receptor-mediated endocytosis.
Some studies have suggested that translocation of Tat peptides across cell membrane occurred in fixed cells but to a lesser extent in live cells. These data were obtained by using either 4% formaldehyde or 100% methanol that induced a massive nuclear redistribution [5,19]. Consequently, we fixed the cells with 4% paraformaldehyde to avoid the fixation artifact and compared the effects of this fixation solution on live and the fixed cells. After incubating with 1 μM Tat-5-FAM or Oct-5-FAM, we observed that the internalization pattern in AR42J cells were the same between live and fixed cells. However, when the cells were fixed with 100% methanol, Oct-5-FAM internalized in the nucleus (not shown), which is attributable to a fixation artifact. This result is similar to the observed nuclear translocation of FITC conjugated PTD peptide after 100% methanol fixation [5]. For this reason, we fixed cells with 4% paraformaldehyde instead of 4% formaldehyde or 100% methanol.
3.3. 5-FAM and Oct cytoplasmic endocytosis complex modulate nuclear internalization of Tat peptides
Three-dimensional confocal microscopy images show the internalization of 5-FAM- and Cypate2- labeled Tat, Oct, and Tat-Oct in AR42J cells. At 30 minutes post-incubation the Tat-5-FAM distributed in both the nucleus and cytoplasm of the cells, with predominant localization in the nucleus after 4 h (Figure 2A–C). Expectedly, the STR-avid molecular probe, Oct-5-FAM, was retained in the cytoplasm of AR42J cells at all time points examined (Figure 2G–I). As a G-protein-coupled receptor, the Oct-5-FAM was likely endocytosized by STR into the lysosomal compartment before re-distribution in the cytosol or other cellular organelles [20]. Interestingly, Tat-Oct-5-FAM was also internalized in AR42J cells (Fig. 2M–O) but had intracellular distribution pattern similar to Oct-5-FAM instead of Tat-5-FAM. By contrast, all of the Cypate2-labeled peptides distributed in the cytoplasm and nucleus, irrespective of the internalization mechanism (Fig. 2). Compared with Tat-cypate2 and Tat-Oct-cypate2, the level of Oct-Cypate2 in the nucleus is low. These results suggest that the influence of Tat peptide moiety is not mitigated by either Cypate2 or Oct-Cypate2 conjugate and reflect the contribution of Oct-5-FAM endocytosis complex in modulating nuclear internalization of Tat peptide.
Fig. 2.
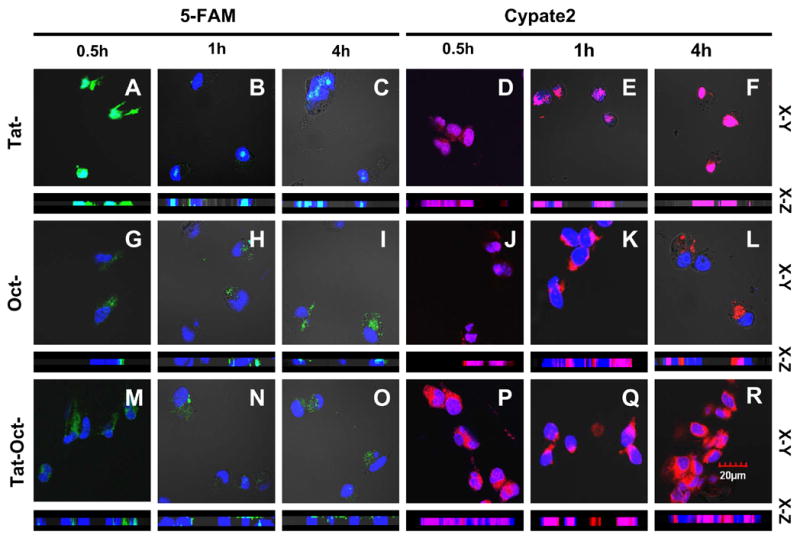
Intracellular distribution of 1μM 5-FAM and cypate 2 labeled Tat-(A–F), Oct-(G–L ) and Tat-Oct-(M-R) compounds in AR42J cells at 37 ºC for 0.5 h, 1 h, and 4 h. Color scheme: nuclear stain, blue; 5-FAM, green; Cypate2, red; Green/blue overlap, light green; red/blue overlap, purple.
Unlike receptor-mediated endocytosis, previous studies have shown that intracellular retention of Tat-peptide conjugates is not inhibited at low temperatures [5]. To evaluate how the cell trafficking of Tat peptide will be affected, we incubated AR42J cells with six molecular probes at 4°C (Fig. 3). Consistent with STR- and Tat peptide-mediated internalization mechanisms, the uptake of OCT-5-FAM and Oct-Cypate2 was inhibited at this low temperature while internalization of Tat-5-FAM and Tat-Cypate2 was not affected. By comparing the fluorescence intensity in Fig. 2A and 2D with Fig. 3A and 3D, the amount of these Tat peptide conjugates in the nucleus at 4°C decreased relative to incubation at 37 °C. Evaluation of the molecular probes possessing both Tat peptide and Oct in the same molecule showed that uptake of Tat-Oct-5-FAM but not Tat-Oct-Cypate2 was inhibited (Fig. 3C, 3F). However, comparison of Fig. 2P and Fig. 3F conducted at 37 °C and 4 °C, respectively shows that the fluorescence intensity of 3F decreased by about 50%, indicating the contribution of the Tat-peptide moiety to the observed cell uptake. These results demonstrate that the internalization of Tat peptide can be modulated by a combination of a receptor-avid peptide and a fluorescent dye. In addition, the ability to inhibit the internalization of the molecular probes in AR42J with equimolar amounts of analogous non-labeled peptides suggests that the endocytosis of each labeled and unlabeled compounds occurred through a common pathway.
Fig. 3.
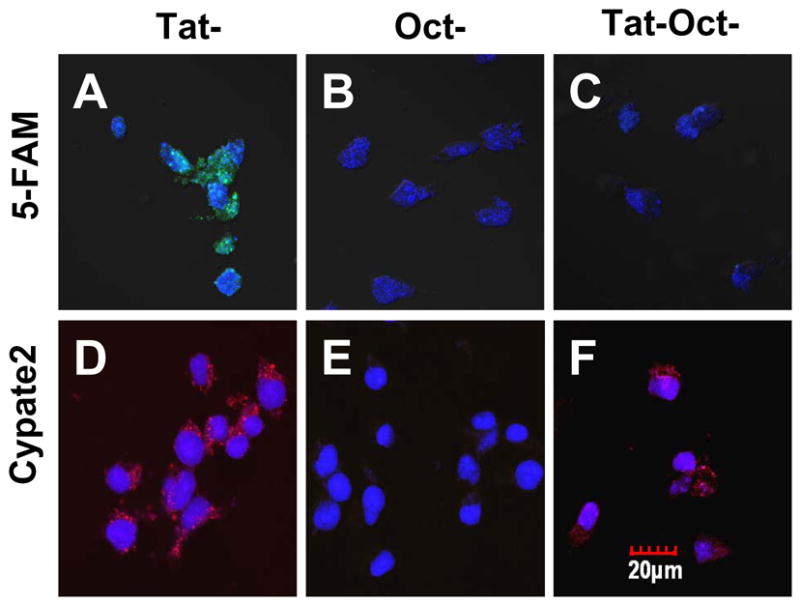
Incubation of AR42J cells with molecular probes at 4 ºC for 30 minutes: Tat-5-FAM (A), Oct-5-FAM (B) and Tat-Oct-5-FAM (C), Tat-cypate2 (D), Oct-cypate2 (E) and Tat-Oct-cypate2 (F). Except for (C), incubation at 4 °C did not abolished the nuclear translocation of 1 μM Tat compounds. Color scheme: nuclear stain, blue; 5-FAM, green; Cypate2, red; Green/blue overlap, light green; red/blue overlap, purple.
3.4. Conjugates of octreotate are selectively retained by AR42J tumor cells
In the light of the results above indicating the internalization of the peptide molecular probes in STR-positive AR42J cells, we further assessed the uptake of the molecular probes in STR-negative (A549) tumor and nontumor (HEK) cell lines. A similar internalization pattern was observed for the Tat-5-FAM and Tat-Cypate2 in all cell lines, with the molecular probes distributed between the cell nucleus and cytoplasm (Fig. 4A–D). Expectedly, the STR-avid molecular probes, Oct-Cypate2 and Oct-5-FAM, did not internalize in both HEK and A549 cells (Fig. 4E–H) lines, indicating the role of the receptor in mediating their intracellular trafficking in AR42J cells. However, we also noted that both Cypate2- and 5-FAM-labeled Tat-Oct peptides did not accumulate in the STR-negative cell lines (Figure 4I–L). While this finding may be expected for Tat-Oct-5-FAM, the absence of internalization of Tat-Oct-Cypate2 in these cells was surprising because of its enhanced uptake in the AR42J cells. The ensemble of the results suggests that the initial binding of Oct to STR on cell membrane facilitates intracellular transport by Tat peptide even at low temperature for Tat-Oct-Cypate2 in AR42J cells (Fig. 3F). Conversely, the absence or low expression of STR in HEK and A459 cells may allow Oct moiety to interfere with the internalization of Tat-Oct-Cypate2 at 30 minutes post-incubation. These findings could be useful in designing molecular probes for assessing cell trafficking pathways.
Fig. 4.
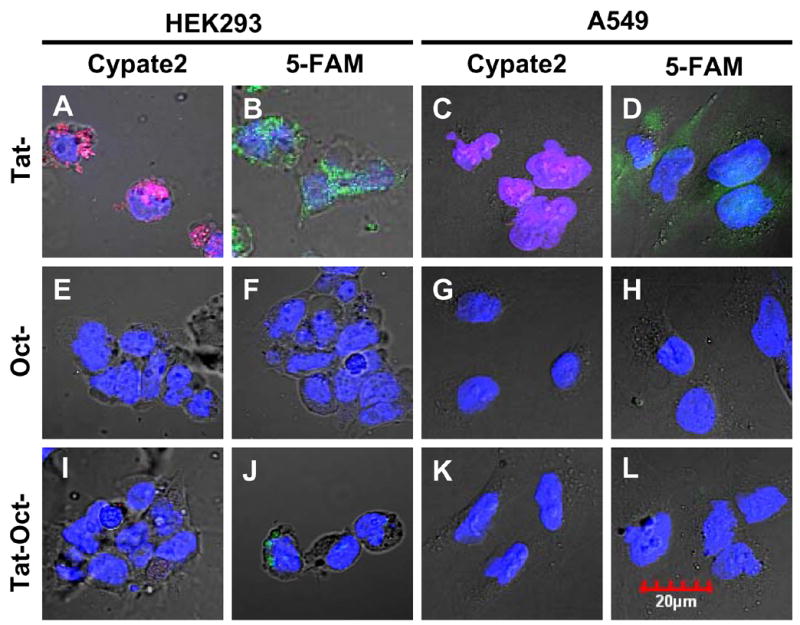
Intracellular distribution of molecular probes (1 μM) in HEK293 and A549 cells at 37 ºC for 30 min. Incubation of Tat-cypate2, Oct-cypate2, and Tat-Oct-cypate2 (A, E, I) or Tat-5-FAM, Oct-5-FAM, and Tat-Oct-5-FAM (B,F,J) with HEK cells and Tat-cypate2, Oct-cypate2, and Tat-Oct-cypate2 (C, G, K) or Tat-5-FAM, Oct-5-FAM, and Tat-Oct-5-FAM (D, H, L) with A549 cells. Color scheme: nuclear stain, blue; 5-FAM, green; Cypate2, red; Green/blue overlap, light green; red/blue overlap, purple.
3.5. Oct-Cypate2 promotes and Oct-5-FAM inhibits the nuclear internalization of Tat peptide
Regardless of the incubation time, Table 1 shows that the long-term distribution of the Tat peptides in AR42J cells is predictable within 30 minutes post-incubation. Nearly all the cells incubated with Cypate2-labeled compounds showed nuclear internalization spanning from 0.5 h to 15 h. By comparison, less than 5% of the cell population examined internalized the 5-FAM-labeled analogues in their nuclei, with the exception of Tat-5-FAM which had similar distribution pattern as Cypate2-labeled compounds. An interesting trend in the nuclear internalization is shown in Fig. 5, where either 5-FAM or Cypate2 was used as reference. When labeled with 5-FAM, the fluorescence intensity of the nuclear component peaked after 1 h incubation and progressively decreased with time, flattening out at about 4 h post-incubation. At maximum intensity, the fluorescence of Tat-5-FAM was nearly 3X those of Oct-5-FAM and Tat-Oct-5-FAM, which were close to background fluorescence. In contrast, the Tat-Oct-Cypate2 had similar nuclear internalization dynamics as Tat-Cypate2 than Oct-Cypate2 up to 4 h post-incubation. After 4 h, the nuclear intensity of Tat-Oct-Cypate2 continued to increase but that of Tat-Cypate2 decreased progressively towards the level of Oct-Cypate2.
Fig. 5.
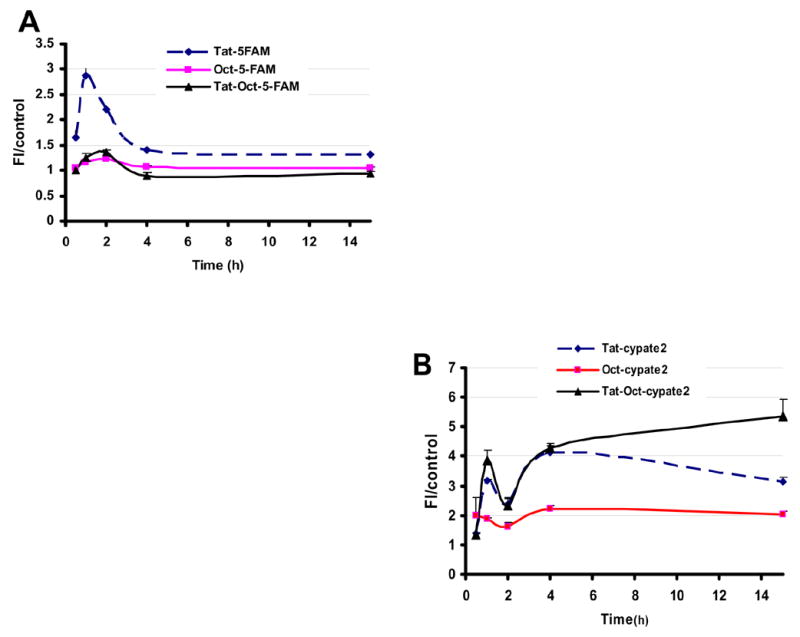
The dynamics of nuclear internalization of 5-FAM-labeled (A) and cypate2-labeled (B) Tat-, Oct-, and Tat-Oct- compounds in AR42J cells at 37 ºC. 5-FAM (A) or Cypate2 (B) was used as control for the internalization study.
Although the exact rationale for this reversal is not obvious at this time, it is likely that the internalization complex between STR and Tat-Oct-Cypate2 is resistant to the p-glycoprotein-mediated efflux of endocytosized compounds [21,22]. Such disposition would favor retention of the molecular probes in cells, thereby enhancing their uptake in the nucleus. It also allows the cells to take advantage of receptor-recycling time to increase the amount of internalized molecular probes at longer incubation times. This finding provides a strategy to deliver more materials to the nucleus and retain them for prolonged period if needed. Considering that the difference between Tat-Oct-Cypate2 and Tat-Oct-5-FAM resides in the fluorescent dye used, this observation indicates the potential to control nuclear internalization of Tat peptide derivatives by a combination of peptide modifiers and fluorescent dyes.
3.6. ATP facilitates nuclear internalization of all Tat peptide derivatives
The mechanism of nuclear internalization of Tat peptide remains poorly understood and controversial [3]. Recent studies showed that Tat peptide promote endocytosis by binding to negatively charged heparan sulfate proteoglycans (HSPGs) on the cell surface [23,24]. Once internalized, the Tat peptide sequence allowed active transport through the nuclear pore. Notably, glypican-1, an abundant cell-surface HSPG, contains nuclear localization signal (NLS) sequence and it is found in the nuclear compartment of neurons and glioma cells [25]. Binding of HIV-1 Tat protein to low-density lipoprotein receptor-related protein (LRP) promoted efficient uptake of Tat into neurons, and LRP-mediated uptake of Tat was followed by translocation to the neuronal nucleus [26]. Thus, it is conceivable that LRP and HSPG cooperate during internalization of Tat peptides, some of which may escape lysosomal degradation to exert biological activities in the nuclear compartment [27]. Moreover, cytoplasmic and nuclear delivery of Tat-derived peptides has been shown to be an ATP-dependent pathway after endocytic uptake into HeLa cells [4].
In this study, the observed inability of Tat-Oct-5-FAM to translocate into the nucleus of AR42J cells is in sharp contrast to a previous report that Tat-p27-5-FITC protein localized in cytoplasm and nucleus of cells [28]. It should be noted that FITC and 5-FAM are fluorescein derivatives. The Cdk inhibition protein, p27, have an NLS domain near its carboxy terminus that can mediate nuclear translocation of the labeled compound [28–31]. The lack of nuclear internalization by Tat-Oct-5-FAM, coupled with competing binding of the molecular construct to STR on the surface of AR42J cells, could modulate the subcellular distribution. It is also known that fluorescein is a selective inhibitor of (Na-K)-ATPase and this inhibition of ATPase activity can be reversed by ATP [32]. Apparently, in the presence of the octreotate peptide, 5-FAM, but not Cypate2 acted as an ATPase inhibitor. Consequently, addition of 4 mM ATP to the culture medium facilitated the translocation of Oct-5-FAM and Tat-Oct-5-FAM to nucleus (Fig. 6A–F), resulting in a similar subcellular distribution found in most Tat peptide-mediated intracellular transport of materials. Thus, nuclear internalization of Tat-Oct peptides can be enhanced by ATP, which is consistent with the finding of Potocky et al [4]. Apparently, in the presence of Oct, 5-FAM, but not Cypate2 acted as an ATPase inhibitor. Our data further suggest that neither Oct or 5-FAM alone is sufficient to modulate the nuclear internalization of Tat-Oct-5-FAM, establishing the importance of the endoplasmic complex between STR and Oct-5-FAM in nuclear translocation.
Fig. 6.
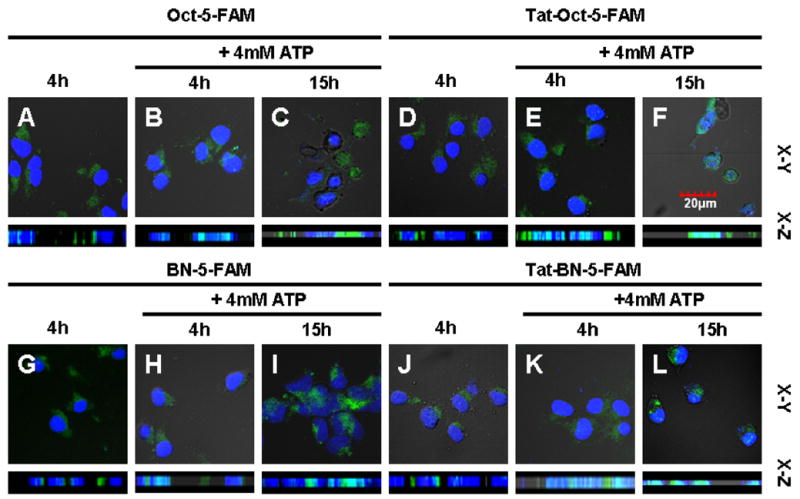
ATP facilitated nuclear localization of 1 μM Oct-5-FAM (B,C), Tat-Oct-5-FAM (E,F), BN-5-FAM (H,I), and Tat-BN-5-FAM (K,L) in AR42J cells at 37 ºC for 4 h. Panels A, D, G, and J are non-ATP treated components. Color scheme: nuclear stain, blue; 5-FAM, green; Cypate2, red; Green/blue overlap, light green; red/blue overlap, purple.
To explore the applicability of this finding to other receptor-mediated molecular constructs, we also prepared 5-FAM-labeled bombesin peptide (BN) analogues similar to Oct derivatives. BN and its truncated peptide analogue, BN7-14, bind gastrin releasing peptide receptor (GRPr) in epithelial cells [33] and AR42J cells [9,34] by a similar mechanism to STR. In sharp similarity to Oct-5-FAM and Tat-Oct-5-FAM, we found that 4 mM ATP also facilitated the nuclear uptake of BN-5-FAM and Tat-BN-5-FAM in AR42J cells (Fig. 6G–L). Together, our data demonstrate that a synergistic effect of the complex between Oct and STR or BN and GRPr with 5-FAM modulate the translocation of Tat-Oct-5-FAM to the nucleus. More importantly, the results show that incorporating secondary peptides and fluorescent dyes to Tat peptides could provide an important strategy to increase, retain, or inhibit nuclear internalization of Tat peptides.
In conclusion, incorporating a dye and a secondary peptide into Tat peptides can modulate its nuclear internalization over a long period. Conjugation of a hydrophobic dye such as Cypate2 to Tat peptide can enhance and retain nuclear internalization. Together with a secondary peptide, a hydrophilic dye such as 5-FAM inhibits nuclear internalization of Tat peptides. Addition of ATP overcomes this inhibition and provides a viable strategy to modulate the activities of Tat peptides for in vitro and in vivo applications. It is hoped that the rapid internalization of site-specific Tat peptides will enhance their localization and retention in target tissues, increase clearance from non-target tissues, and reduce the amount of the molecular probe administered in patients.
Acknowledgments
We thank Baogang Xu and Susan Adam for technical assistance with cell culture and Dr. David Piwnica-Worms for advice. This study was funded by the National Institutes of Health (P50 CA9405601, R01 EB1430, and R33 100972.
List of abbreviations
- STR2
somatostatin receptor subtype 2
- Oct
Octreotate
- BN
bombesin peptide analogue
- 5-FAM
fluorescein 5’-carboxylic acid
- NLS
nuclear localization signal
- LRP
lipoprotein receptor-related protein
- HSPGs
heparan sulfate proteoglycans
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272 (25):16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 2.Vives E, Charneau P, Vanrietschoten J, Rochat H, Bahraoui E. Effects of the Tat Basic Domain on Human-Immunodeficiency-Virus Type-1 Transactivation, Using Chemically Synthesized Tat Protein and Tat Peptides. J Virol. 1994;68 (5):3343–3353. doi: 10.1128/jvi.68.5.3343-3353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caron NJ, Quenneville SP, Tremblay JP. Endosome disruption enhances the functional nuclear delivery of Tat-fusion proteins. Biochem Biophys Res Commun. 2004;319 (1):12–20. doi: 10.1016/j.bbrc.2004.04.180. [DOI] [PubMed] [Google Scholar]
- 4.Potocky TB, Menon AK, Gellman SH. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J Biol Chem. 2003;278 (50):50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg M, Wikstrom S, Johansson M. Cell surface adherence and endocytosis of protein transduction domains. Mol Ther. 2003;8 (1):143–150. doi: 10.1016/s1525-0016(03)00135-7. [DOI] [PubMed] [Google Scholar]
- 6.Sevick-Muraca EM, Houston JP, Gurfinkel M. Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents. Curr Opin Chem Biol. 2002;6 (5):642–650. doi: 10.1016/s1367-5931(02)00356-3. [DOI] [PubMed] [Google Scholar]
- 7.Weissleder R. Scaling down imaging: Molecular mapping of cancer in mice. Nat Rev Cancer. 2002;2 (1):11–18. doi: 10.1038/nrc701. [DOI] [PubMed] [Google Scholar]
- 8.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7 (5):626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Achilefu S. Lighting up tumors with receptor-specific optical molecular probes. Technol Cancer Res Treat. 2004;3 (4):393–409. doi: 10.1177/153303460400300410. [DOI] [PubMed] [Google Scholar]
- 10.Benaron DA. The future of cancer imaging. Cancer Metastasis Rev. 2002;21 (1):45–78. doi: 10.1023/a:1020131208786. [DOI] [PubMed] [Google Scholar]
- 11.Achilefu S, Dorshow RB, Bugaj JE, Rajagopalan R. Novel receptor-targeted fluorescent contrast agents for in vivo tumor imaging. Invest Radiol. 2000;35 (8):479–485. doi: 10.1097/00004424-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bakker WH, et al. In-111-Dtpa-D-Phe1 -Octreotide, a Potential Radiopharmaceutical for Imaging of Somatostatin Receptor-Positive Tumors - Synthesis, Radiolabeling and Invitro Validation. Life Sci. 1991;49 (22):1583–1591. doi: 10.1016/0024-3205(91)90052-d. [DOI] [PubMed] [Google Scholar]
- 13.Stolz B, Weckbecker G, Smith-Jones PM, Albert R, Raulf F, Bruns C. The somatostatin receptor-targeted radiotherapeutic Y-90-DOTA-DPhe(1),Tyr(3) octreotide (Y-90-SMT 487) eradicates experimental rat pancreatic CA 20948 tumours. Eur J Nucl Med. 1998;25 (7):668–674. doi: 10.1007/s002590050268. [DOI] [PubMed] [Google Scholar]
- 14.Achilefu S, et al. Synthesis, in vitro receptor binding, and in vivo evaluation of fluorescein and carbocyanine peptide-based optical contrast agents. J Med Chem. 2002;45 (10):2003–2015. doi: 10.1021/jm010519l. [DOI] [PubMed] [Google Scholar]
- 15.Achilefu S, Wilhelm RR, Jimenez HN, Schmidt MA, Srinivasan A. A new method for the synthesis of tri-tert-butyl diethylenetriaminepentaacetic acid and its derivatives. J Org Chem. 2000;65 (5):1562–1565. doi: 10.1021/jo991453t. [DOI] [PubMed] [Google Scholar]
- 16.Coy DH, Taylor JE. Receptor-specific somatostatin analogs: Correlations with biological activity. Metab-Clin Exp. 1996;45 (8):21–23. doi: 10.1016/s0026-0495(96)90073-6. [DOI] [PubMed] [Google Scholar]
- 17.Whetstone PA, Akizawa H, Meares CF. Evaluation of cleavable (Tyr(3))-octreotate derivatives for longer intracellular probe residence. Bioconjugate Chem. 2004;15 (3):647–657. doi: 10.1021/bc049972c. [DOI] [PubMed] [Google Scholar]
- 18.Taylor JE. Somatostatin (Sstr2) Receptors Mediate Phospholipase C-Independent Ca2+ Mobilization in Rat Ar42j Pancreas Cells. Biochem Biophys Res Commun. 1995;214 (1):81–85. doi: 10.1006/bbrc.1995.2259. [DOI] [PubMed] [Google Scholar]
- 19.Fretz MM, Koning GA, Mastrobattista A, Jiskoot W, Storm G. OVCAR-3 cells internalize TAT-peptide modified liposomes by endocytosis. Biochim Biophys Acta-Biomembr. 2004;1665 (1–2):48–56. doi: 10.1016/j.bbamem.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Krisch B, Feindt J, Mentlein R. Immunoelectronmicroscopic analysis of the ligand-induced internalization of the somatostatin receptor subtype 2 in cultured human glioma cells. J Histochem Cytochem. 1998;46 (11):1233–1242. doi: 10.1177/002215549804601103. [DOI] [PubMed] [Google Scholar]
- 21.Dyszlewski M, Blake HM, Dahlheimer JL, Pica CM, Piwnica-Worms D. Characterization of a novel 99mTc-carbonyl complex as a functional probe of MDR1 P-glycoprotein transport activity. Mol Imaging. 2002;1 (1):24–35. doi: 10.1162/15353500200200002. [DOI] [PubMed] [Google Scholar]
- 22.Egudina SV, Stromskaya TP, Frolova EA, Stavrovskaya AA. Early steps of the P-glycoprotein expression in cell cultures studied with vital fluorochrome. FEBS Lett. 1993;329 (1–2):63–66. doi: 10.1016/0014-5793(93)80194-y. [DOI] [PubMed] [Google Scholar]
- 23.Vives E. Cellular uptake of the Tat peptide: an endocytosis mechanism following ionic interactions. J Mol Recognit. 2003;16 (5):265–271. doi: 10.1002/jmr.636. [DOI] [PubMed] [Google Scholar]
- 24.Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276 (5):3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 25.Liang Y, Haring M, Roughley PJ, Margolis RK, Margolis RU. Glypican and biglycan in the nuclei of neurons and glioma cells: Presence of functional nuclear localization signals and dynamic changes in glypican during the cell cycle. J Cell Biol. 1997;139 (4):851–864. doi: 10.1083/jcb.139.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, et al. Uptake of HIV-1 Tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6 (12):1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- 27.Belting M, Sandgren S, Wittrup A. Nuclear delivery of macromolecules: barriers and carriers. Adv Drug Deliv Rev. 2005;57 (4):505–527. doi: 10.1016/j.addr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Toyoshima H, Hunter T. P27, a Novel Inhibitor of G1 Cyclin-Cdk Protein-Kinase Activity, Is Related to P21. Cell. 1994;78 (1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Hurwitz J, Massague J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995;375(6527):159–161. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 30.Nagahara H, et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4 (12):1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 31.Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79 (3):487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 32.Farley RA, Tran CM, Carilli CT, Hawke D, Shively JE. The amino acid sequence of a fluorescein-labeled peptide from the active site of (Na,K)-ATPase. J Biol Chem. 1984;259 (15):9532–9535. [PubMed] [Google Scholar]
- 33.Slice LW, Yee HF, Jr, Walsh JH. Visualization of internalization and recycling of the gastrin releasing peptide receptor-green fluorescent protein chimera expressed in epithelial cells. Receptors Channels. 1998;6 (3):201–212. [PubMed] [Google Scholar]
- 34.Bugaj JE, Achilefu S, Dorshow RB, Rajagopalan R. Novel fluorescent contrast agents for optical imaging of in vivo tumors based on a receptor-targeted dye-peptide conjugate platform. J Biomed Opt. 2001;6 (2):122–133. doi: 10.1117/1.1352748. [DOI] [PubMed] [Google Scholar]


