Abstract
Objective
The aim of this study was to confer an antigen presenting cell (APC) ability on multiple myeloma cell lines (HMCLs) using B7-1 and/or 4-1BBL gene transfer.
Materials and methods
HMCLs were retrovirally transduced with B7-1 and/or 4-1 BBL cDNAs. Allogeneic or autologous T cells were stimulated by coculture with B7-1- and/or 4-1 BBL-transduced HMCLs in the presence of IL-2. T cell clones were obtained by limiting dilution. T cell activation was assessed by IFNγ Elispot assays and cytotoxicity by 51Cr release assays.
Results
Neither primary multiple myeloma cells (MMC) nor HMCLs expressed B7-1 nor 4-1BBL and these molecules could not be induced by CD40 triggering. HMCLs failed to stimulate allogeneic or autologous T cells. Transduction of HMCLs with B7-1 and/or 4-1BBL retroviruses induced a high expression of B7-1 and 4-1 BBL molecules and a strong T cell activation ability. Long-term cultured CD8+ T cell lines could be obtained by stimulation with the autologous B7-1/4-1BBL XG-19 HMCL. These cytotoxic T lymphocytes (CTL) efficiently killed the autologous parental XG-19 HMCL as well as autologous primary MMC and allogeneic HMCLs. They did not kill autologous CD34 cells and autologous EBV cell line or NK target K562 cells. Cloned CTL could recognize allogeneic HMCLs, demonstrating that a shared anti-MMC repertoire was expanded.
Conclusion
Transduction with B7-1 and 4-1 BBL retroviruses turned HMCLs into efficient APC. It permits the long-term expansion of autologous anti-tumor CTL with a shared anti-MMC repertoire, for one HMCL. These data suggest developing an immunotherapy using modified tumor cells in patients with multiple myeloma.
Introduction
A major goal of tumor immunotherapy is the induction of tumor-specific T lymphocytes that will be effective in eradicating disseminated tumors. The full activation of antigen-specific T cells requires a dialog between T cells and antigen presenting cells (APC) resulting in T cell receptor (TCR) activation together with costimulatory molecules. First, the APC takes up antigen and processes it into antigenic peptides that are presented through major histocompatibility complex (MHC) class I or class II molecules. Engagement of TCR by peptide/MHC complexes yields a transient expression of CD40 ligand on T cells that further activates CD40 molecules on the APC. CD40 activation induces or increases costimulatory molecules on the APC. The prototypical costimulatory molecules are B7-1 and B7-2 which activate the CD28 T cell activation molecule [1,2]. Engagement of TCR by peptide-MHC complexes without costimulatory molecules results in profoundly anergic T cells which are unable to respond to further activation [3,4]. B7/CD28 T cell activation is downregulated by CTLA-4 [2,5]. CTLA-4 is another B7 ligand that is expressed later by T cells which results in T cell inactivation following activation by B7 molecules. The family of B7-like costimulatory molecules is rapidly expanding as demonstrated by the recent discoveries of B7h/B7RP-1, B7-H1/PDL-1, and B7-DC/PDL-2, which bind ICOS T cell activating molecule and PD1 inhibitory molecule, respectively, and B7H3, which binds an unknown activating receptor [6]. Another class of costimulatory molecules belongs to the TNF/TNF receptor family. OX-40 ligand triggers OX-40+ T cells and results in long-term expansion of CD4+ T cells [7]. LIGHT can stimulate the receptor HVEM present on CD4+ and CD8+ T cells. The 4-1BB ligand (4-1BBL) binds to 4-1BB present on activated T cells and induces NF-KB, c-jun, and P38 signaling pathways which sustain B-7/CD28-mediated activation [8]. 4-1 BBL prevents activation-induced apoptosis of CD8+ T cells, increases T cell effector function, and enhances clonal proliferation and survival of CD8+ cytotoxic T cells [9,10]. 4-1BBL is expressed on dendritic cells, monocytes, and other myeloid cells. The 4-1BB molecule is expressed by activated CD4+ and CD8+ T cells but also by NK cells, NK-T cells, and CD4+CD25+ regulatory T cells [8].
Tumor cells are generally poor APC due to a defect in costimulatory molecules and/or peptide presentation through MHC molecules [11,12]. 4-1 BBL improves the efficacy of antigen presentation in various tumor models [13]. In particular, administration of an agonist anti-4-1BB monoclonal antibody induced a strong anti-tumor immunity in mice [14,15].
Multiple myeloma (MM) is a B cell neoplasia affecting the late stage of B cell differentiation. Although high-dose chemotherapy and autologous stem cell transplantation have improved the rate of complete remission, some multiple myeloma cells (MMC) escape treatment and all MM patients relapse [16]. The development of immunotherapy designed to enhance cytotoxic T lymphocyte (CTL) eradication or control of residual tumor cells may be one hopeful approach to improve MM treatment. However, MMC are poor APC. They express CD40 molecules that are nonfunctional in terms of B7 induction after triggering with CD40 ligand [17]. Interestingly, transduction of MMC with B7-1 retrovirus can confer them immunogenicity [17]. However, no long-term expansion of anti-tumor CTL could be obtained. Here, we demonstrate that transduction of B7-1 and 4-1 BBL in human myeloma cell lines (HMCLs) made it possible to stimulate long-term anti-myeloma CTL.
Patients, Materials and Methods
Patient and healthy donor samples
Seven newly-diagnosed patients with MM (median age: 64.5 years) were included in this study. There were 3 IgGκ, 2 IgGλ, 1 IgAκ, and 1 Bence-Jones λMM. Bone-marrow aspirates were collected for the determination of plasma cell labeling index (PCLI), a marker of disease activity, and excess cells were used for this study after written informed consent. Bone marrow mononuclear cells (BMMC) were isolated by Ficoll-Hypaque centrifugation (Biowhittaker, Walkersville, MD, USA). HMCLs have been obtained by growing MMC from patients with extramedullary proliferation with IL-6 and GM-CSF, as reported [18]. For 3 HMCLs (XG-5, XG-13, and XG-19), the autologous and nonmalignant B-lymphoblastoid cell line (EBV-5, EBV-13 and EBV-19) were established by in vitro EBV infection of peripheral blood B cells, as described [19]. Peripheral blood T cells from the patient from whom XG-19 was derived were also collected and frozen. Peripheral blood from 5 healthy donors was obtained after informed consent.
Antibodies and cytokines
Monoclonal antibodies (mAbs) conjugated to FITC or phycoerythrin (PE) were used for staining of cells and phenotypic analyses. Anti-4-1BB (PE); anti-4-1BBL (PE); anti-CD27 (PE); anti-CD40 (FITC); anti-CD70 (PE); anti-OX40 (PE); anti-OX40L (PE) and anti-HLA-A2 (FITC) were purchased from Pharmingen (San Diego, CA, USA). Anti-CD3 (PE); anti-CD4 (PE); anti-CD8β (PE); anti-CD16 (PE); anti-CD28 (FITC); anti-CD40L (PE); anti-CD54 (PE); anti-CD56 (PE); anti-CD58 (PE); anti-CD80 (FITC/PE); anti-CD86 (FITC/PE); anti-HLA-ABC (FITC); anti-HLA-DR (FITC) and isotype-matched mouse mAbs (FITC/PE) were from BeckmanCoulter (Villepinte, France). Unconjugated anti-CD16, anti-CD56, and anti-TCRγ/δ were also from BeckmanCoulter. Anti-HLA-ABC (anti-MHC-I; w6.32) was from Diaclone (Besançon, France). Interleukin (IL)-2, IL-6, and IL-12 were purchased from R&D Systems (Abingdon, United Kingdom).
Cell lines
Fifteen IL-6-dependent HMCLs - XG-1, XG-2, XG-3, XG-4, XG-5, XG-6, XG-7, XG-10, XG-11, XG-12, XG-13, XG-14, XG-16, XG-18, XG-19, and five autonomously- growing HCMLs, RPMI 8226, LP1, L363, OPM-2 and U266, were used in this study. IL-6-dependent cell lines had been previously established in our laboratory [18] and autonomously-growing HMCLs and the K562 cell line were purchased from ATCC (Rockville, MD, USA). They were cultured in RPMI 1640 supplemented with 10% FCS, 2 mM L-glutamine, and 2 ng/ml of human recombinant IL-6 for the IL-6- dependent cell lines.
Cell purification
Purification of syndecan-1+ MMC was performed with anti-syndecan-1 Milteny microbeads (Tebu, Le Perray-en-Yvelines, France) as reported previously [20]. Purified myeloma cells contained >95% plasma cells. T lymphocytes were purified by depletion of non-T cells with mAbs (Immunotech) and magnetic beads (Dynal, Oslo, Norway) according to the manufacturer’s instructions. Briefly, monocytes and B cells were first depleted using CD14-and CD19-coated beads. Then, non-α/βT cells were removed by incubation with a cocktail of anti-CD16 and anti-TCRγ/δ mAbs and goat anti-mouse Ig beads. After 2 rounds of purification, the purity of resting CD3+ cells was always higher than 98%. Cells were frozen in 50% FCS, 10% DMSO at 20 × 106 cells per vial.
Phenotypic analysis
Expression of cell surface molecules on parental HMCLs, transduced HMCLs, and lymphocytes was determined using immunofluorescence staining according to standard procedures. For primary BMMC, double staining of MMC was performed using an anti-syndecan-1 mAb (MI15) mAb conjugated to FITC or biotin. All fluorescence analyses were carried out on a FACScan® apparatus (Becton Dickinson, San Jose, CA, USA). Negative controls were done with corresponding isotype-matched mouse mAbs recognizing no human antigens.
Generation of dendritic cells
Immature dendritic cells (DC) were generated from leukapheresis products of MM patients, as previously described [21]. Briefly, 8 × 106 G-CSF-mobilized leukapheresis cells were plated in 2 mL of X-VIVO15 medium (BioWittaker, Walkersville, MD) per well in six-well flat-bottomed plates (Nunc, Roskilde, Denmark). Nonadherent cells were discarded by gentle rinsing after a 2-h incubation at 37°C in 5% CO2. Adherent cells were cultured in X-VIVO15 medium with 2% human albumin, 100 ng/mL of GM-CSF (LEUKINE®, Berlex, Montville, NJ) and 25 ng/mL of IL-4 (Cellgenix, Freiburg, Germany) for 5 days.
Activation of myeloma cells
HMCLs were stimulated with CD40L-transduced L cells (1 × 105/ml) with or without 10 U/ml IL-2 and with or without 2 ng/ml IL-12. As a control of the specificity of CD40 stimulation, we used non-transduced L cells (1 × 105/ml). After 2 days of culture, expression of B7-1, B7-2 and 4-1 BBL was determined on myeloma cells. Immature DC were included as a positive control using the same stimulation protocol.
Obtaining HMCLs transduced with B7-1 and/or 4-1 BBL cDNA
The 5192 RV and the E293 packaging cell line were a gift from Dr. Methali (Transgene, Strasbourg, France). The full length human B7-1 cDNA and 4-1 BBL cDNA were subcloned into the neomycin-selectable 5192 retroviral vector (RV) with EcoRI/Xhol cloning sites for B7-1 and EcoRI/Bgl2 sites for 4-1 BBL, then transduced into the E293 packaging cell line using Lipofectamine® (Gibco BRL, Paisley, UK). The packaging cell lines transfected with the empty RV (ERV), B7-1-RV, 4-1 BBL-RV, or B7-1-RV and 4-1 BBL-RV were cultured at a high cell density and the supernatants were filtered and stored at −70°C. HMCL cells were plated at 106 cells/well in 6-well dishes and exposed to retroviral supernatant for 2 hours in the presence of 8 μg/ml of polybrene (Sigma, St Louis, MO, USA). After 2 cycles of infection, transduced cells were selected by outgrowth with 600 μg/ml of G418 (Gibco BRL) over a period of 10 days and resistant clones were analyzed for surface expression of B7-1 and/or 4-1BBL and sorted using a Vantage® cell sorter apparatus (Becton Dickinson). The stably-transduced HMCLs (named ERV XG, B7-1 XG, 4-1 BBL XG, and B7-1/4-1 BBL XG) were subsequently maintained with 600 μg/ml of G418.
Mixed lymphocyte reaction (MLR)
To evaluate HMCL-induced T cell proliferation, allogeneic and autologous MLR were performed using mitomycin C (Sigma)-treated (50 μg/ml) parental and transduced HMCLs cocultured at 1 × 103, 3 × 103 or 1 × 104 cells/well with 105 T cells/well in 96-well round bottom plates in RPMI 1640 and 5% heat-inactivated human AB serum. When indicated, anti-MHC-class I (w6.32) mAb was added at 10 μg/ml. After 5 days of coculture, cells were pulsed with 1 μCi of tritiated thymidine (3H-TdR, specific activity 25 Ci/ml; Amersham, Buckingham, UK) for 16 hours, harvested, and counted. All microculture tests were carried out in triplicate.
Generation of autologous anti-tumor T cells
From one patient with plasma cell leukemia, peripheral blood mononuclear cells comprising 21.5% CD3+T cells and 8.45% syndecan-1+ tumor cells were frozen at diagnosis. An HMCL (XG-19) and 4 transduced XG-19 subclones were obtained (ERV XG-19, B7-1 XG-19, 4-1 BBL XG-19, and B7-1/4-1BBL XG-19). Purified T lymphocytes (CD3+; 5 × 105/ml) were stimulated with mitomycin-treated (50 μg/ml) parental XG-19 or transduced XG-19 cells at a 10:3 effector:target (E:T) ratio, in RPMI 1640 supplemented with 5% human AB serum for 7 days. Dead cells were eliminated by Ficoll-Hypaque centrifugation. Viable cells were then cultured at 5 × 105/ml and restimulated every week with mitomycin-treated XG-19 or transduced XG-19 cells in the presence of IL-2 (10 U/ml) (R&D). IL-2 was added twice a week.
Cytotoxicity assay
Cell-mediated cytoxicity was determined using a standard 4-h 51Cr-release assay. Target cells (from XG-19 HMCL, allogeneic HMCLs, autologous EBV-19, allogeneic EBV cell lines, and K562) were incubated with 50 μCi Na251CrO4 (Amersham Biosciences Europe, Orsay, France) for 1h30 at 37°C. After three washes, target cells (5000 cells/well) were cocultured with CD8+ T cells at various E:T ratios in 96-well V-bottom plates. All experiments were performed in triplicate. After a 4h-incubation at 37°C, supernatants were harvested and radioactivity was measured with a gamma counter. The percentage of specific lysis was determined as: (Experimental 51Cr release − Spontaneous 51Cr release)/(Maximum 51Cr release − Spontaneous 51Cr release) × 100. The ratio (Spontaneous release/Maximum release) was always below 10%.
T cell cloning
The responder T cells were seeded at different cell concentrations (10, 3, 1, 0.3 cells/well) in 96-microwell round-bottom culture plates along with allogeneic PBMC (10 × 106/plate, 30 Gy irradiated), allogeneic EBV-infected cell line (106 cells/plate, 80 Gy irradiated), and autologous HMCL (XG-19, 106 cells/plate, 50 Gy irradiated) in the presence of rhIL-2 (10 IU/ml). The culture media were changed every week and T cells were reboosted with allogeneic PBMC, EBV cell line, and autologous HMCL every 3–4 weeks. The T cell clones, taken 2 weeks after the last stimulation, were used in cytotoxicity and Elispot assays.
T cell repertoire study
T cell clone total RNA was prepared by a modification of the Chomczyski and Sacchi method [22] with the Trizol reagent (GIBCO-BRL). RT-PCR were performed with 24 different TCRBV subfamily-specific primers and one Cβ consensus primer, followed by a runoff reaction with a fluorescent Cβ primer, as described [23].
IFN-γElispot assay
IFN-γ production by activated T cells was evaluated using an IFN-γ Elispot kit (Diaclone, Besançon, France) according to the manufacturer’s instructions. Briefly, 96-well multiscreen plates were coated overnight with 100 μl/well of 10 μg/ml primary anti-IFN-γ mAb in PBS, then washed three times with PBS containing 0.25% Tween20 (Sigma) and blocked with PBS containing 5% FCS for 2 h at 37°C. 104 T cells/well were incubated with 3 × 103 stimulating cells in a 200 μl reaction volume. Experiments were performed in sextuplicate. To determine whether the IFN-γ production was restricted by HLA class I, target cells were preincubated with 10 μg/ml of anti-MHC class I mAb (w6.32) (Becton Dickinson) for 30 min. Following a 48h incubation, the cells were removed from the plate and a second biotinylated anti-IFN-γ mAb was added to wells, at 1 μg/ml. Spots were revealed with streptavidin alkaline phosphatase conjugate and were counted with a computer controlled microscope (Zeiss-Vision, Eching, Germany).
Results
Myeloma cells do not express 4-1BBL and 4-1BB molecules
We first looked for MMC expression of the 4-1BBL costimulatory molecule. As shown in Table 1, 8 HMCLs lacked 4-1BBL and 8 HMCLs expressed it at a low level unlike EBV-transformed cell lines. The 16 HMCLs did not express the 4-1BB T cell activation molecule but did express CD28, a T cell activation molecule, in agreement with our previous report [24]. We confirmed that these HMCLs fail to express B7-1 and express a low density of B7-2. Most of the cell lines expressed CD70/CD27L but not the CD27 T and B cell activation molecule. In addition, these cell lines failed to express HLA class II but expressed HLA class I molecules and adhesion molecules (CD54, CD58) known to be important in T cell/APC interaction ([17] and unpublished results). We also investigated the 4-1BBL expression on purified MMC from 7 newly diagnosed patients. Results are shown in Table 2. Patients’ MMC did not express 4-1 BBL or B7-1. They expressed a low density of B7-2. Contrary to HMCLs, primary MMC did not express CD70 but did express CD27.
Table 1.
Expression of 4-1 BBL/4-1 BB, B7/CD28, and CD70/CD27 molecules on HMCLs
| HMCL | Percentage of labeled myeloma cells (mean fluorescence intensity) | |||||||
|---|---|---|---|---|---|---|---|---|
| 4-1BBL | 4-1BB | B7-1 | B7-2 | CD28 | CD70 | CD27 | CD40 | |
| XG-1 | 0 | 0 | 0 | 30 (22) | 100 (120) | 0 | 0 | 0 |
| XG-2 | 0 | 0 | 0 | 35 (25) | 100 (60) | 96 (384) | 0 | 100 (2884) |
| XG-3 | 0 | 0 | 0 | 0 | 72 (29) | 93 (87) | 0 | 9 (21) |
| XG-4 | 0 | 0 | 0 | 0 | 96 (68) | 59 (175) | 0 | 28 (53) |
| XG-5 | 29 (32) | 0 | 0 | 0 | 98 (262) | 100 (237) | 0 | 16 (29) |
| XG-6 | 0 | 0 | 0 | 32 (25) | 86 (45) | 0 | 85 (119) | 85 (43) |
| XG-7 | 67 (51) | 0 | 0 | 23 (29) | 100 (461) | 100 (673) | 0 | 30 (65) |
| XG-10 | 99 (123) | 0 | 0 | 98 (37) | 99 (250) | 100 (333) | 95 (106) | 40 (23) |
| XG-11 | 0 | 0 | 0 | 50 (34) | 51 (51) | 54 (391) | 0 | 10 (19) |
| XG-12 | 88 (37) | 0 | 0 | 22 (23) | 100 (492) | 81 (172) | 35 (17) | 0 |
| XG-13 | 0 | 0 | 0 | 25 (26) | 100 (66) | 57 (36) | 0 | 70 (55) |
| XG-14 | 37 (18) | 0 | 0 | 92 (62) | 25 (23) | 94 (75) | 17 (27) | 70 (73) |
| XG-16 | 7 (25) | 0 | 0 | 93 (62) | 55 (77) | 50 (108) | 0 | 49 (39) |
| XG-18 | 0 | 0 | 0 | 54 (15) | 44 (33) | 80 (149) | 0 | 5 (15) |
| XG-19 | 72 (11) | 0 | 0 | 68 (15) | 100 (538) | 51 (29) | 93 (191) | 100 (200) |
| RPMI 8226 | 56 (19) | 0 | 0 | 20 (34) | 100 (153) | 96 (75) | 0 | 52 (60) |
| EBV-16 | 100 (76) | 0 | 100 (140) | 100 (59) | 0 | 100 (334) | 20 | 100 (275) |
| EBV-19 | 100 (45) | 0 | 100 (92) | 100 (93) | 0 | 100 (151) | 24.5 | 100 (325) |
Cells were harvested during the exponential growth phase and were stained with the indicated PE-conjugated mAb or PE-conjugated control mAb and the fluorescence analyzed with a FACSscan device. In brackets are the mean fluorescence intensities obtained with the mAb for an intensity set between 4-6 with a control mAb.
Table 2.
Expression of costimulatory molecules on patients’ purified myeloma cells
| Patient N° | Percentage of labeled myeloma cells | |||||||
|---|---|---|---|---|---|---|---|---|
| 4-1 BBL | 4-1 BB | B7-1 | B7-2 | CD28 | CD70 | CD27 | CD40 | |
| 1 | 5.5 | <0.5 | <0.5 | 20 | 10 | <0.5 | 79 | 100 |
| 2 | <0.5 | <0.5 | <0.5 | 25 | 10 | <0.5 | 86 | 100 |
| 3 | <0.5 | <0.5 | <0.5 | 80 | 6 | <0.5 | 34 | 90 |
| 4 | <0.5 | <0.5 | <0.5 | 25 | 28 | <0.5 | 55 | 75 |
| 5 | <0.5 | <0.5 | <0.5 | 87 | 2 | <0.5 | 90 | 100 |
| 6 | <0.5 | <0.5 | <0.5 | 91 | 2 | <0.5 | 100 | 100 |
| 7 | <0.5 | <0.5 | <0.5 | 34 | 5 | <0.5 | <0.5 | 100 |
Patients’ myeloma cells were purified and stained with the indicated PE-conjugated mAb or PE-conjugated control mAb. The fluorescence was analyzed with a FACSscan device. Data are the percentages of labeled cells.
Stimulation of myeloma CD40, with or without IL-2 and IL-12 did not induce B7-1 and 4-1BBL on myeloma cells
MMC expressed CD40 molecules (Table 1 and 2) but a coactivation with a CD40L transfectant in the presence of IL-2 or IL-2 and IL-12 did not induce B7-1 (Table 3), contrary to a previous report [25]. In fact, in this report, the induction of B7-1 in MMC after CD40L and IL-2 stimulation was found in only one out of 5 samples and at a marginal level. As a control, CD40L transfectant can upregulate B7-1 and B7-2 on human dendritic cells in 3 separate experiments (Table 3). The CD40L transfectant did not induce 4-1 BBL on HMCLs. This CD40L transfectant did not also induce 4-1 BBL expression on human dendritic cells. Such a 4-1 BBL upregulation was previously shown for murine dendritic cells [26] but we could not confirm these data.
Table 3.
Lack of induction of 4-1BBL and B7-1 on myeloma cells activated by a CD40L transfectant
| Myeloma cells | Stimulation | Percentage of labeled cells | ||
|---|---|---|---|---|
| 4-1 BBL | B7-1 | B7-2 | ||
| XG-2 | No | 0 | 0 | 32 (25) |
| CD40L | 0 | 0 | 6 (60) | |
| CD40L + IL-2 | 0 | 0 | 5 (102) | |
| CD40L + IL-2 + IL-12 | 0 | 0 | 9 (61) | |
| XG-6 | No | 0 | 0 | 32 (25) |
| CD40L | 0 | 0 | 26 (13) | |
| CD40L + IL-2 | 0 | 0 | 19 (15) | |
| CD40L + IL-2 + IL-12 | 0 | 0 | 14 (25) | |
| XG-7 | No | 0 | 0 | 23 (29) |
| CD40L | 0 | 0 | 22 (28) | |
| CD40L + IL-2 | 0 | 0 | 15 (33) | |
| CD40L + IL-2 + IL-12 | 0 | 0 | 27 (23) | |
| Purified primary myeloma cells | No | 0 | 0 | 23 (29) |
| CD40L | 0 | 0 | 94 (42) | |
| CD40L + IL-2 | 0 | 0 | 92 (44) | |
| CD40L + IL-2 + IL-12 | 0 | 0 | 88 (32) | |
| Dendritic cells | No | 0 | 82 (45) | 66 (164) |
| CD40L | 0 | 84 (65) | 79 (273) | |
| CD40L + IL-2 | 0 | 73 (66) | 86 (293) | |
| CD40L + IL-2 + IL-12 | 0 | 80 (83) | 83 (339) | |
Cells were stained with the indicated PE-conjugated mAb or PE-conjugated control mAb and the fluorescence analyzed with a FACSscan device. In brackets are the mean fluorescence intensities obtained with the mAb for an intensity set between 4–6 with a control mAb. Data with dendritic cells are for one of 3 reproducible experiments.
Obtaining HMCLs that expressed B7-1 and/or 4-1BBL by retroviral gene transfer
As indicated in Fig. 1, HMCLs expressing a large density of B7-1, 4-1 BBL, or B7-1 and 4-1BBL were obtained after retroviral transduction, G418 selection, and cell sorting. Induction of B7-1 and/or 4-1 BBL did not affect the growth of myeloma cells that expressed CD28. It remained strictly dependent on the addition of exogenous IL-6 despite a continuous triggering of CD28 in B7-1 transfectants (results not shown). This is in agreement with the reported lack of growth stimulating activity of CD28 triggering in MMC [24]. In addition, the expression of 13 main immune molecules was unaffected by 4-1 BBL or B7-1 transduction (Fig. 2). In particular, no change in the density of HLA class I, CD54, or CD58 were found. We observed a decrease in CD28 density in B7-1 transfectant probably due to a masking or downregulation of CD28 epitope.
Figure 1. High expression of B7-1 and 4-1BBL in HMCLs transduced with B7-1 or 4-1BBL retro viruses.
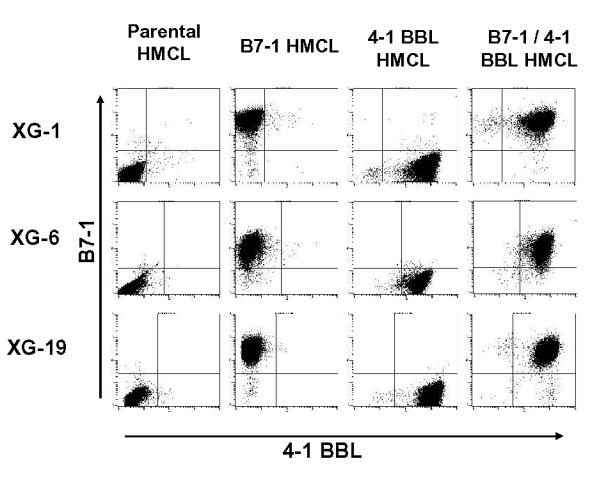
XG-1, XG-6, and XG-19 cells were transduced by one or two retroviral vectors containing B7-1 or 4-1BBL cDNA, were FACS sorted, and expanded with G418. All transduced HMCLs expressed highly B7-1 and/or 4-1BBL.
Figure 2. The expression of immune molecules was not affected by retroviral transduction.
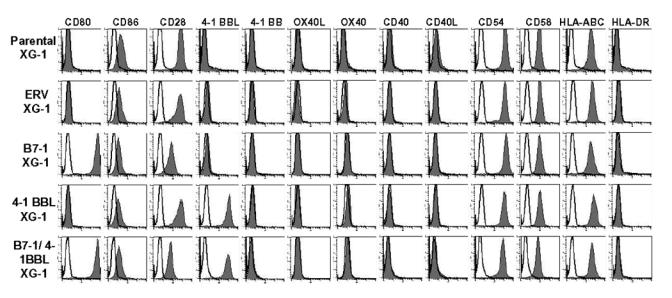
The histograms represent the expression levels of various costimulatory molecules indicated in parental XG-1 and transduced XG-1 HMCLs: ERV- XG-1, B7-1 XG-1, 4-1BBL XG-1, and B7-1/4-1BBL XG-1 HMCLs. Expression of the various costimulatory molecules (black histograms) was monitored by FACS analysis. Isotype-matched control mAbs were used (open histograms).
4-1 BBL and/or B7-1 HMCLs strongly stimulate allogeneic T cells
The 3 parental or ERV-transduced HMCLs (XG-1, XG-6 and XG-19) weakly stimulated the proliferation of allogeneic purified CD3+ cells. Data for the XG-1 HMCL are shown in Fig.3A. This allogeneic stimulation was dramatically increased by transduction with 4-1 BBL and/or B7-1 RV (Fig. 3A). Although the XG-19 HMCL expressed 4-1 BBL weakly, this expression was likely too weak to promote an efficient allogeneic T cell proliferation (data not shown). Altogether, the co-transduction with 4-1 BBL and B7-1 RV yielded a greater alloimmunogenicity in myeloma cells than with 4-1 BBL or B7-1 RV (P ≤ .05). The stimulatory effect of 4-1 BBL and/or B7-1 HMCLs was decreased by an anti-MHC class I mAb indicating that it was mediated though MHC class I alloantigen presentation.
Figure 3. Proliferation of lymphocytes to parental or gene modified HMCLs.
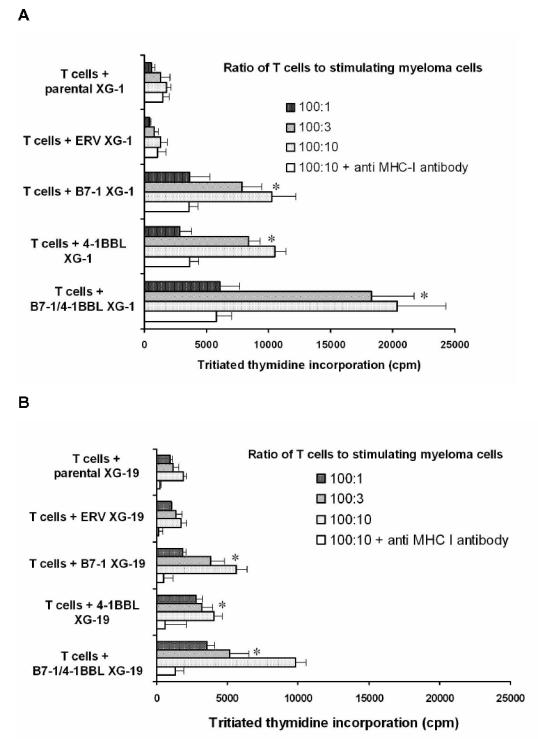
105/well allogeneic purified CD3+ cells from healthy donors (A) or autologous purified CD3+ cells from a patient with plasma cell leukemia (B) were stimulated by mitomycin-treated parental, empty retrovirus (ERV), B7-1, 4-1 BBL, or B7-1/4-1BBL XG-1 (A) or XG-19 (B). HMCLs at various responder-stimulator ratios ranging from 100:1 to 100:10 for 5 days. When indicated, 10 μg/ml of anti-MHC class I mAb were added. Cell proliferation was assayed by tritiated thymidine incorporation and results are the mean ± SD of the proliferation determined on sextuplicate culture wells. * indicates that the mean value is significantly different (P ≤ .05) for that obtained with the parental or ERV HMCL. Results are representative of 3 independent experiments.
4-1 BBL and B7-1 transduction made it possible to generate autologous anti-myeloma cytotoxic T cell lines
Peripheral T cells were available from a patient with plasma cell leukemia from whom the XG-19 HMCL and the nonmalignant EBV cell line (EBV-19) were obtained. Similarly to data obtained with allogeneic T cells, parental or ERV-XG-19 cells weakly stimulated purified autologous CD3+ T cells, despite the fact that these HMCLs expressed low levels of B7-2 and 4-1BBL (Table 1). A significant (P≤ .05) increase in proliferation of autologous T cells was obtained stimulating with 4-1 BBL and/or B7-1 transduced XG-19 cells (Fig. 3B), emphasizing a need of a high costimulatory molecule concentration to get a maximum T cell stimulation. This autologous T cell stimulation was inhibited by an anti-MHC class I mAb that had no effect on the proliferation induced by anti-CD3 mAb and IL-2 (results not shown). Actually, only autologous CD8+ T cells, and no CD4+ T cells, were stimulated according to the lack of MHC class II expression by XG-19 HMCL (results not shown). We looked for the possibility of obtaining anti-myeloma cell T cell lines using either 4-1BBL/B7-1, 4-1 BBL, B7-1, ERV, or parental XG-19 cells. As shown in Fig. 4, starting from purified CD3+ T cells, the 4-1BBL/B7-1 XG-19 HMCL strongly stimulated purified autologous CD8+ T cells in the presence of exogenous IL-2. The B7-1- or the 4-1BBL-XG-19 HMCLs also stimulated T cells but more weakly. No T cell growth was observed with the parental or ERV XG-19 HMCLs. On day 30, CD8+ T cells were able to efficiently kill the parental XG-19 HMCL as well as the transduced XG-19 HMCLs at a low 1:1 effector:target ratio (data not shown). They were unable to kill the EBV-19 autologous lymphoblastoid cell line and the NK target K562 cells (Fig. 5) nor the autologous CD34+ cells (data not shown). Interestingly, these T cell lines killed HMCLs obtained from different patients indicating that shared myeloma antigens were presented by these various HMCLs. Such T cell lines were reproducibly obtained from the patient’s peripheral T cells on three separate occasions.
Figure 4. Growth of autologous lymphocytes stimulated by parental or B7-1 and/or 4-1BBL XG-19 HMCLs.
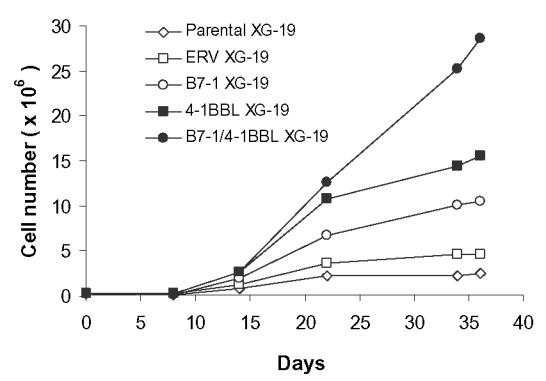
Purified T lymphocytes (CD3+; 5 × 105/ml) from a patient with a plasma cell leukemia were weekly stimulated with mitomycin-treated (50 μg/ml) parental XG-19 HMCL, or with B7-1 and/or 4-1BBL XG-19 HMCLs at a responder-stimulator ratio of 100:30 in RPMI 1640 supplemented with 5% human AB serum. IL-2 (10 U/ml) was added after three days and then twice a week. Results are the T cell number in culture wells of one experiment representative of 3.
Figure 5. Cytotoxic activity of CD8+ T cells stimulated with the autologous B7-1/4-1BBL XG-19 HMCL.
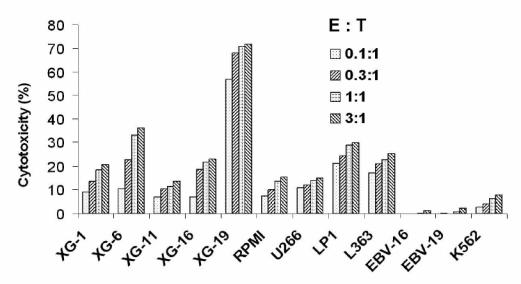
Cell-mediated toxicity was determined after a 4 hour stimulation using a standard 51Cr-release assay at various E:T ratios. Allogeneic HMCLs, parental XG-19, K562, and EBV cell lines were used as target cells. EBV-16 and EBV-19 nonmalignant lymphoblastoid cell lines were autologous to XG-16 and XG-19 HMCLs, respectively.
Anti-myeloma T cell clones
One of the three T cell lines was cloned in limiting dilution using B7-1/4-1BBL XG-19 HMCL, allogeneic EBV, and PBMC as feeder cells. After 3–4 weeks, 42 clones could be amplified from culture microplates at 1 cell/well. All T cell clones were able to kill the parental XG-19 but not the EBV-19 autologous lymphoblastoid cell line nor the NK target K562 cells. For 26 clones, we performed cytotoxicity assays including allogeneic HMCLs as targets. We could distinguish 2 groups of T cell clones according to their cytotoxicity profile: the first group, called “XG-19 restricted”, contained 16 clones which killed only the autologous XG-19 HMCL but not the other HMCLs. Monoclonality of one of these clones was checked with its immunoscope profile (data not shown) and its cytotoxic capacity is shown in Fig. 6a. However, no blockage of the cytotoxicity activity was observed adding an anti-MHC class I mAb. This could be explained by a high affinity of the TCR for peptide-MHC class I complex. The second group of 10 clones, called “pan myeloma”, also killed other HMCLs and the cytotoxic profile of one of these clones (II-1B/A-10) is shown in Fig. 6b. This clone displayed only a single peak using PCR amplification of TCRBV transcript size pattern (data not shown). Clone II-1B/A-10 killed XG-19, XG-4, XG-7, XG-5, XG-13, L363, and the patient’s primary MMC from whom the XG-19 HMCL was obtained (Fig. 6b). Killing was not affected by adding unlabelled cold K562 cells (10 K562 cells for 1 chromium labeled target cell). Clone II1B/A10 did not kill EBV cell lines (EBV-5, EBV-13) that were obtained from B cells of patients from whom XG-5 and XG-13 HMCLs were obtained. It did not kill the Burkitt, Daudi, and Raji cell lines. The HLA class I typing of the HMCLs and EBV cell lines together with their killing degree by the T cell clone are shown in Table 4. No common HLA class I allele could be found among the 4 strongly killed HMCLs (XG-4, XG-5, XG-7 and XG-13). However, a same MHC class I epitope can be efficiently presented by different MHC class I alleles [27,28]. Another explanation is that this 4h-killing is not triggered by TCR recognition of MHC/peptide complexes but induced by other MHC independent mechanisms as NKG2D/MICA-B, in agreement with a lack of blockage of the cytotoxic activity by an anti-MHC class I mAb. However, using gene expression profiling, no differences in the expression of MICA or MICB were found between the 4 highly killed HMCLs and the other poorly killed HMCLs (data not shown). In addition, these T cell clones produced IFN-γ in response to stimulation by the parental XG-19, unlike EBV-19 cell line, that was blocked by an anti-MHC class I mAb (Fig.7).
Figure 6. Cytotoxic activity of CD8+ T cell clones.
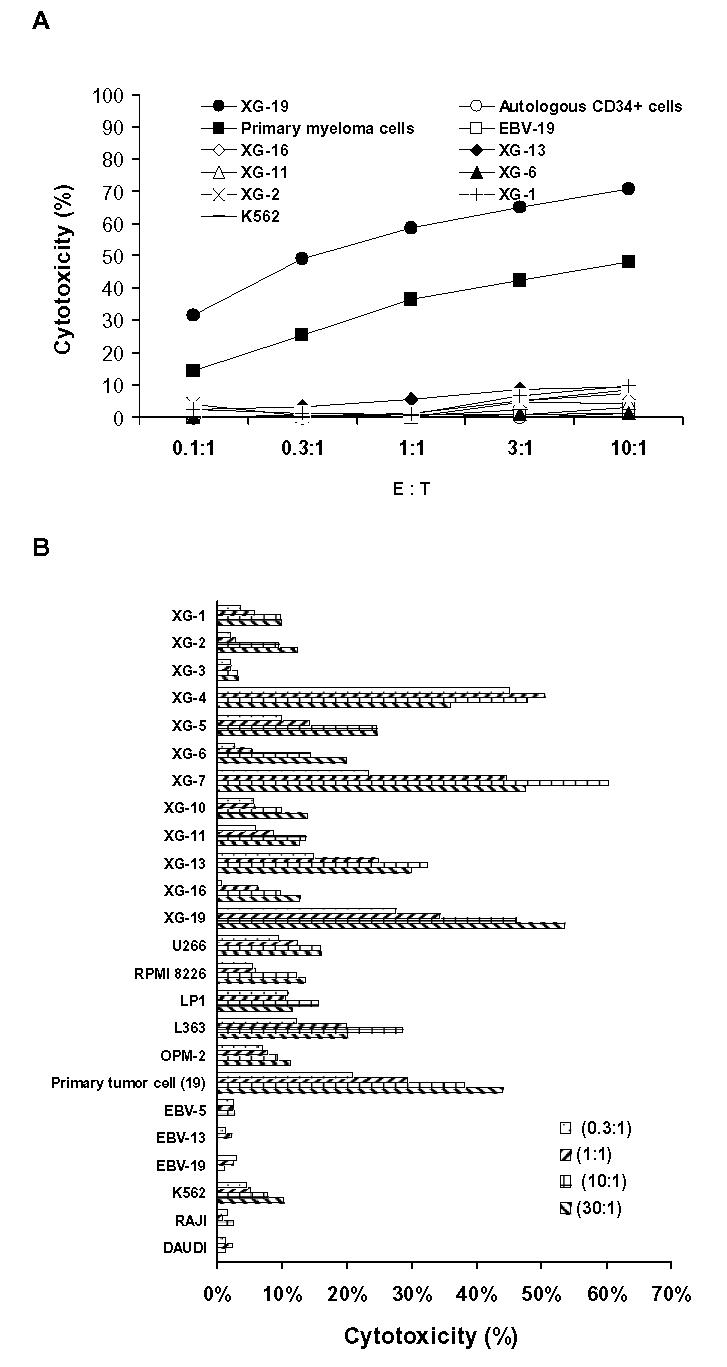
One T cell line stimulated with the autologous B7-1/4-1BBL XG-19 HMCL was cloned in limiting dilution. (A) Cytotoxic profile of a representative “XG-19-restricted” T-cell clone. (B) Cytotoxic profile of the “pan myeloma” T cell clone, named II-1B/A10.
Table 4.
HLA typing of cell lines
| Cell line | HLA- A | HLA-B | HLA-Cw | % lysis (10:1) |
|---|---|---|---|---|
| XG-1 | 02-29 | 40-44 | 0201-0601 | 10% |
| XG-2 | 01-29 | 08-44 | 07-1601 | 10% |
| XG-3 | 02-32 | 35-44 | 03-04 | 3% |
| XG-4 | 02-32 | 15-50 | 03-04 | 48% |
| XG-5 | 02-02 | 44-49 | 05-07 | 25% |
| XG-6 | 02-02 | 15-37 | 0303-0602 | 14% |
| XG-7 | 02-24 | 07-40 | 20-07 | 60% |
| XG-10 | 01-24 | 14-51 | 08-14 | 10% |
| XG-11 | 02-26 | 18-38 | 02-12 | 14% |
| XG-13 | 32-68 | 08-40 | 02-07 | 32% |
| XG-16 | 02-29 | 40-49 | 02-07 | 10% |
| XG-19 | 01-02 | 08-27 | 01-07 | 46% |
| U266 | 02-03 | 70-40 | 0304-0701 | 16% |
| RPMI8226 | 30-68 | 15-15 | 02-03 or 03-1511 | 12% |
| LP1 | 26-30 | 18-35 | 04-07 | 16% |
| OPM2 | 24-24 | 07-40 | 04-07 | 9% |
| L363 | 02-31 | 07-40 | 03-07 | 29% |
| EBV-5 | 02-02 | 44-49 | 05-07 | 3% |
| EBV-13 | 32-68 | 08-40 | 02-07 | 32% |
| EBV-19 | 01-02 | 08-27 | 01-07 | 46% |
For each cell line, alleles shared with XG-19 are in bold.
Figure 7. IFNγ secretion of a CD8+ T cell clone triggered by XG-19 cells is MHC class I restricted.
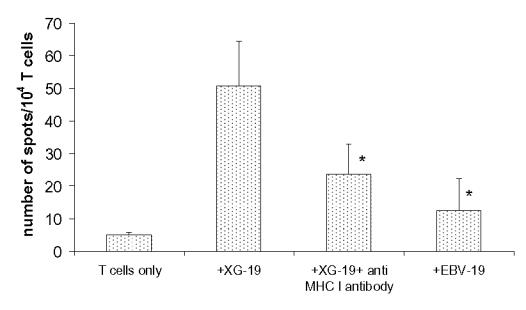
The IFNγ Elispot assay was performed with the clone whose cytotoxic profile is shown in Fig. 6A. 104 T cells were cultured with 3×103 target cells for 48h. * indicates that the number of spots is significantly reduced (P ≤ .01) compared to that elicited by stimulation with XG-19 cells.
Discussion
Tumors may escape immune surveillance through several mechanisms: a) absence of recognizable tumor antigens; b) low level of MHC class I molecules; c) poor costimulatory molecule expression; d) secretion of immunosuppressive substances. In patients with MM, the tumor cells express normal levels of MHC class I molecules [17] and also express identified tumor-associated antigens as MUC-1, HM-1.24, or cancer-testis antigens [29,30]. In this work, we showed that 8/16 HMCLs and primary myeloma cells did not express B7-1 nor 4-1 BBL but express B7-2 at a low level. 8/16 HMCLs slightly expressed 4-1 BBL at a level insufficient to induce strong T cell activation, as it is shown for XG-19. CD40 is expressed by MMC but CD40 activation could not yield B7-1 or 4-1 BBL in MMC, extending our previous findings [17]. In addition, HMCLs transduced by both 4-1 BBL and B7-1 retroviruses were very efficient at stimulating allogeneic or autologous T lymphocytes, unlike the parental HMCLs. Thus, the expression of B7-1 and 4-1 BBL at a high level is sufficient to confer on MMC a MHC class I-restricted and long-term capacity of stimulating T cells.
The autologous cytotoxic T cell lines stimulated by the B7-1/4-1BBL-transduced XG-19 HMCL efficiently killed the parental XG-19 HMCL as well as the patient’s primary myeloma cells from which XG-19 was derived. These cytotoxic T cell lines were highly specific to myeloma cells since they did not kill the autologous lymphoblastoid cell line, the NK target K562 cells, or Burkitt cell lines. The fact that cytotoxic T cell lines kill the parental HMCL indicates that the T cells were not directed against retroviral proteins. The 4-1BBL/4-1BB mediated signal has generally been defined as an amplifier of the B7/CD28 signal, but it is not clear if it can initiate an immune response alone. Here, we found that the HMCLs transduced with a 4-1 BBL retrovirus only can stimulate allogeneic or autologous T cells, but less efficiently than the B7-1/4-1BBL HMCLs. An optimal growth of T cell lines was obtained with the autologous HMCL transduced with both B7-1 and 4-1BBL retroviruses. A synergistic action of the two molecules has already been demonstrated in studies with anti-tumor vaccines in mice [31,32]. In particular, mice injected with a B cell lymphoma cell line expressing B7-1 and B7-2 were not protected against a new tumor challenge whereas the variant expressing both B7-1 and 4-1 BBL conferred long-term anti-tumor immunity [32].
Adoptive transfer of anti-tumor T cells, stimulated and amplified ex vivo using tumor cells transduced with B7-1 and 4-1 BBL, may be a potential approach for myeloma immunotherapy. Major clinical responses have been observed after adoptive transfer of antigen-specific CD8+ T cell clones and tumor-infiltrating T cells in patients with melanoma, demonstrating the interest of tumor-specific T cells in the treatment of cancer [33–35]. In MM, Cignetti et al have shown that lentiviral transduction of primary myeloma cells with B7-1 and CD40L was feasible and turned MMC into efficient APC able to activate specific cytotoxic CD8+ cells, in an autologous context [36]. The current data indicate that the target of CD40L transduction was not the MM CD40 molecules since triggering of myeloma CD40 molecules could not yield B7-1 or 4-1 BBL molecules. The induction of 4-1BBL in myeloma cells by gene transfer might enhance more efficiently memory cytotoxic T cell responses, as demonstrated for anti-viral cytotoxic T cells [37]. A putative protocol to obtain anti-myeloma cell T lymphocytes would be to perform one or two stimulations by the B7-1/4-1BBL- transduced MMC followed by stimulations with MMC transduced with 4-1BBL only in order to avoid a negative signal mediated by B7-1/CTLA-4 engagement. Conversely, using myeloma cells as APC, expanded T cells will not contain CD4+ T cells. This may result in a lack of help and in an eventually weak immune reaction, once T cells are infused [38]. One possibility could be to induce MHC class II expression in MMC through the MHC class II transactivator (CIITA) transduction. Indeed, normal and malignant plasma cells do not express MHC class II molecules because BLIMP 1 – a plasma cell transcription factor – downregulates CIITA expression [39,40]. Of interest, this downregulation can be reverted transducing CIITA in a plasmacytoma cell model [41]. But we have no insurance that MHC class II+ MMC could present exogenous tumor antigens as do professional antigen presenting cells. This should be investigated in vitro. However, we may expect that in vitro generated CD8 effectors will initiate killing of tumor cells in vivo. This killing can make it possible tumor antigen release with “danger” signals, which could recruit local dendritic cells capable of antigen cross-presentation, further activation of CD4+ T cells, and reactivation of TIL by overcoming the inhibitory tumor environment. Such a mechanism was suggested in patients with melanoma, vaccinated with MAGE-A1/3 antigens to explain that expansion of non MAGE-A1/3 T cell clones (anti-MAGE-C2) were found in lymph nodes and metastasis [42].
However, major limitations in using MMC as APC are the obtaining of sufficient numbers of primary myeloma cells and the difficulty of thawing them. In addition, obtaining tumor cell lines from primary myeloma cells is very difficult, contrary to other cancers, in particular melanoma. Here, we show the existence of shared tumor antigens in different HMCLs that could make it possible to bypass the difficulty of obtaining a large number of primary tumor cells by using allogeneic myeloma cell lines. One well characterized T cell clone killed not only the autologous XG-19 cells and patient’s primary myeloma cells, but 5 HLA-A2+ allogeneic HMCLs as well.
Alternatively, bone marrow memory T cells (likely enriched in anti-tumor T cells as demonstrated by Feuerer et al [43] and because bone marrow is the tumor bed) could be expanded using microbeads coated with anti-CD3, anti-CD28, and anti-4-1BBL agonistic mAbs. Indeed, Li et al demonstrated that 4-1BB ligation resulted in high proliferation of tumor-draining lymph node T cells and enhanced IFNγ and GM-CSF release in response to tumor antigen, and that effector cells generated through 4-1 BB and CD28 costimulation mediated potent in vivo tumor regression in mice [44]. However, 4-1 BB ligation may have a dual role since administration of anti-4-1 BB mAbs in mice has been shown to abrogate T cell dependent humoral immunity [45] or to generate CD8+ T cells with potent suppressive function [46]. Finally, using several partially HLA-matched HMCLs transduced with costimulatory molecules as a whole tumor vaccine could be envisaged in MM vaccination strategies.
In summary, our results showed that a T cell repertoire recognizing tumor antigens persisted in the peripheral blood of patients with multiple myeloma even at the terminal stage, and that this T cell repertoire could be activated and amplified by autologous tumor cells transduced with the costimulatory molecules B7-1 and 4-1BBL.
References
- 1.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 3.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified la molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3712. [PubMed] [Google Scholar]
- 4.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 5.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 6.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 7.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 8.Kwon B, Lee HW, Kwon BS. New insights into the role of 4-1BB in immune responses: beyond CD8+ T cells. Trends Immunol. 2002;23:378–380. doi: 10.1016/s1471-4906(02)02263-9. [DOI] [PubMed] [Google Scholar]
- 9.Cannons JL, Lau P, Ghumman B, et al. 4-1 BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 10.Cheuk AT, Mufti GJ, Guinn BA. Role of 4-1 BB:4-1 BB ligand in cancer immunotherapy. Cancer Gene Ther. 2004;11:215–226. doi: 10.1038/sj.cgt.7700670. [DOI] [PubMed] [Google Scholar]
- 11.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 12.Schultze J, Nadler LM, Gribben JG. B7-mediated costimulation and the immune response. Blood Rev. 1996;10:111–127. doi: 10.1016/s0268-960x(96)90040-5. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrom KE, Hellstrom I. Therapeutic vaccination with tumor cells that engage CD137. J Mol Med. 2003;81:71–86. doi: 10.1007/s00109-002-0413-8. [DOI] [PubMed] [Google Scholar]
- 14.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1 BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 15.Martinet O, Divino CM, Zang Y, et al. T cell activation with systemic agonistic antibody versus local 4-1 BB ligand gene delivery combined with interleukin-12 eradicate liver metastases of breast cancer. Gene Ther. 2002;9:786–792. doi: 10.1038/sj.gt.3301687. [DOI] [PubMed] [Google Scholar]
- 16.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 17.Tarte K, Zhang XG, Legouffe E, et al. Induced expression of B7-1 on myeloma cells following retroviral gene transfer results in tumor-specific recognition by cytotoxic T cells. J Immunol. 1999;163:514–524. [PubMed] [Google Scholar]
- 18.Zhang XG, Gaillard JP, Robillard N, et al. Reproducible obtaining of human myeloma cell lines as a model for tumor stem cell study in human multiple myeloma. Blood. 1994;83:3654–3663. [PubMed] [Google Scholar]
- 19.Commes T, Klein B, Jourdan M, et al. The defect in peripheral blood B-cell activation in patients with multiple myeloma is not due to a deficiency in the production of B-cell growth and differentiation factors. J Clin Immunol. 1989;9:65–73. doi: 10.1007/BF00917129. [DOI] [PubMed] [Google Scholar]
- 20.Sun RX, Lu ZY, Wijdenes J, et al. Large scale and clinical grade purification of syndecan-1+ malignant plasma cells. J Immunol Methods. 1997;205:73–79. doi: 10.1016/s0022-1759(97)00056-2. [DOI] [PubMed] [Google Scholar]
- 21.Tarte K, Fiol G, Rossi JF, Klein B. Extensive characterization of dendritic cells generated in serum-free conditions: regulation of soluble antigen uptake, apoptotic tumor cell phagocytosis, chemotaxis and Tcell activation during maturation in vitro. Leukemia. 2000;14:2182–2192. doi: 10.1038/sj.leu.2401925. [DOI] [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Ferrand C, Robinet E, Contassot E, et al. Retrovirus-mediated gene transfer in primary T lymphocytes: influence of the transduction/selection process and of ex vivo expansion on the T cell receptor beta chain hypervariable region repertoire. Hum Gene Ther. 2000;11:1151–1164. doi: 10.1089/10430340050015202. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XG, Olive D, Devos J, et al. Malignant plasma cell lines express a functional CD28 molecule. Leukemia. 1998;12:610–618. doi: 10.1038/sj.leu.2400971. [DOI] [PubMed] [Google Scholar]
- 25.Pope B, Brown RD, Gibson J, Yuen E, Joshua D. B7-2-positive myeloma: incidence, clinical characteristics, prognostic significance, and implications for tumor immunotherapy. Blood. 2000;96:1274–1279. [PubMed] [Google Scholar]
- 26.Futagawa T, Akiba H, Kodama T, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 27.Missale G, Redeker A, Person J, et al. HLA-A31 - and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J Exp Med. 1993;177:751–762. doi: 10.1084/jem.177.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidney J, del Guercio MF, Southwood S, et al. Several HLA alleles share overlapping peptide specificities. J Immunol. 1995;154:247–259. [PubMed] [Google Scholar]
- 29.Pellat-Deceunynck C. Tumour-associated antigens in multiple myeloma. Br J Haematol. 2003;120:3–9. doi: 10.1046/j.1365-2141.2003.03760.x. [DOI] [PubMed] [Google Scholar]
- 30.Hundemer M, Schmidt S, Condomines M, et al. Identification of a new HLA-A2-restricted T-cell epitope within HM1.24 as immunotherapy target for multiple myeloma. Exp Hematol. 2006;34:486–496. doi: 10.1016/j.exphem.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melero I, Bach N, Hellstrom KE, Aruffo A, Mittler RS, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1 BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol. 1998;28:1116–1121. doi: 10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Guinn BA, DeBenedette MA, Watts TH, Berinstein NL. 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol. 1999;162:5003–5010. [PubMed] [Google Scholar]
- 33.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meidenbauer N, Marienhagen J, Laumer M, et al. Survival and tumor localization of adoptively transferred Melan-A-specific T cells in melanoma patients. J Immunol. 2003;170:2161–2169. doi: 10.4049/jimmunol.170.4.2161. [DOI] [PubMed] [Google Scholar]
- 35.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cignetti A, Vallario A, Follenzi A, et al. Lentiviral transduction of primary myeloma cells with CD80 and CD154 generates antimyeloma effector T cells. Hum Gene Ther. 2005;16:445–456. doi: 10.1089/hum.2005.16.445. [DOI] [PubMed] [Google Scholar]
- 37.Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004;101:1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 39.Piskurich JF, Lin KI, Lin Y, Wang Y, Ting JP, Calame K. BLIMP-I mediates extinction of major histocompatibility class II transactivator expression in plasma cells. Nat Immunol. 2000;1:526–532. doi: 10.1038/82788. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh N, Gyory I, Wright G, Wood J, Wright KL. Positive regulatory domain I binding factor 1 silences class II transactivator expression in multiple myeloma cells. J Biol Chem. 2001;276:15264–15268. doi: 10.1074/jbc.M100862200. [DOI] [PubMed] [Google Scholar]
- 41.Silacci P, Mottet A, Steimle V, Reith W, Mach B. Developmental extinction of major histocompatibility complex class II gene expression in plasmocytes is mediated by silencing of the transactivator gene CIITA. J Exp Med. 1994;180:1329–1336. doi: 10.1084/jem.180.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Germeau C, Ma W, Schiavetti F, et al. High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens. J Exp Med. 2005;201:241–248. doi: 10.1084/jem.20041379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feuerer M, Beckhove P, Garbi N, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Carr A, Ito F, Teitz-Tennenbaum S, Chang AE. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res. 2003;63:2546–2552. [PubMed] [Google Scholar]
- 45.Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med. 1999;190:1535–1540. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myers L, Takahashi C, Mittler RS, Rossi RJ, Vella AT. Effector CD8 T cells possess suppressor function after 4-1BB and Toll-like receptor triggering. Proc Natl Acad Sci U S A. 2003;100:5348–5353. doi: 10.1073/pnas.0837611100. [DOI] [PMC free article] [PubMed] [Google Scholar]


